Abstract
OBJECTIVE
To determine the effects of daily walnut consumption on endothelial function, cardiovascular biomarkers, and anthropometric measures in type 2 diabetic individuals.
RESEARCH DESIGN AND METHODS
This study was a randomized, controlled, single-blind, crossover trial. Twenty-four participants with type 2 diabetes (mean age 58 years; 14 women and 10 men) were randomly assigned to one of the two possible sequence permutations to receive an ad libitum diet enriched with 56 g (366 kcal) walnuts/day and an ad libitum diet without walnuts for 8 weeks. Subjects underwent endothelial function testing (measured as flow-mediated dilatation [FMD]) and assessment of cardiovascular biomarkers before and after each 8-week treatment phase. The primary outcome measure was the change in FMD after 8 weeks. Secondary outcome measures included changes in plasma lipids, A1C, fasting glucose, insulin sensitivity, and anthropometric measures.
RESULTS
Endothelial function significantly improved after consumption of a walnut-enriched ad libitum diet compared with that after consumption of an ad libitum diet without walnuts (2.2 ± 1.7 vs. 1.2 ± 1.6%; P = 0.04). The walnut-enriched diet increased fasting serum glucose and lowered serum total cholesterol and LDL cholesterol from baseline (10.0 ± 20.5 mg/dl, P = 0.04; −9.7 ± 14.5 mg/dl, P < 0.01; and −7.7 ± 10 mg/dl, P < 0.01, respectively), although these changes were not significant compared with those for an ad libitum diet without walnuts. There were no significant changes in anthropometric measures, plasma A1C, and insulin sensitivity.
CONCLUSIONS
A walnut-enriched ad libitum diet improves endothelium-dependent vasodilatation in type 2 diabetic individuals, suggesting a potential reduction in overall cardiac risk.
Diabetes and its complications are major causes of morbidity and mortality in the U.S. and contribute substantially to health care costs (1). Men and women with type 2 diabetes have three- and fivefold higher cardiovascular mortality, respectively, than the nondiabetic population (2). The Nurses Health Study (3) showed an inverse association between nut consumption and the risk of type 2 diabetes. Although intervention studies with nuts have not demonstrated considerable benefits for diabetic individuals in terms of long- or short-term glycemic control, nuts may help diabetic individuals depress postprandial glycemia, reduce postprandial oxidative stress, and improve blood lipid profiles (4). Epidemiological and clinical trial evidence has consistently demonstrated the beneficial effects of nut consumption on risk factors associated with coronary heart disease (5,6). Numerous studies have shown that diets rich in polyunsaturated fatty acids (PUFAs) can significantly reduce blood LDL cholesterol levels, decrease the total cholesterol–to–HDL cholesterol ratio, and help achieve optimal fat consumption without adverse effects on total fat or energy intake (7,8). Walnuts have a higher content of PUFAs than do other nuts, including α-linolenic acid, which may give walnuts additional antiatherogenic properties. In a review of clinical trials, it was found that walnut consumption in the amount of two to three servings per day consistently decreased total cholesterol and LDL cholesterol (7).
Walnut ingestion has been shown to improve endothelial function in patients with hypercholesterolemia (9,10). Despite the prevalence of endothelial dysfunction in diabetes and evidence that walnut consumption may improve both endothelial function and biomarkers of cardiovascular disease risk in patients with hypercholesterolemia, no studies have investigated the effect of walnut intake on endothelial function in type 2 diabetes. Therefore, this study was performed to investigate the effects of a walnut-enriched diet on endothelial function and other cardiovascular risk factors in type 2 diabetic individuals.
RESEARCH DESIGN AND METHODS
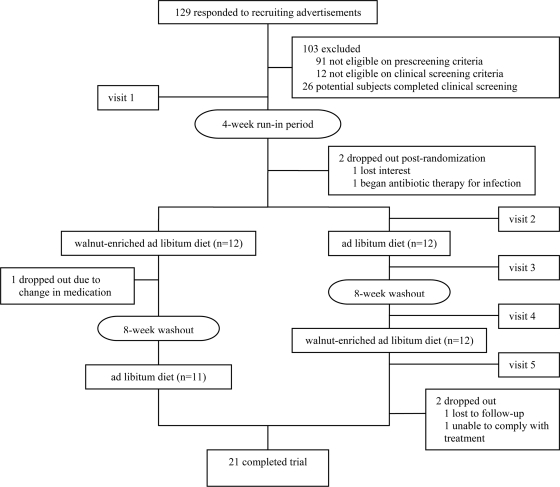
Twenty-four participants with type 2 diabetes were recruited from the Lower Naugatuck Valley in Connecticut through flyers and newspaper advertisements. Those responding (n = 129) were prescreened using a semistructured telephone interview. Eligible participants had a clinical diagnosis of type 2 diabetes for at least 1 year but no more than 5 years, were nonsmokers, were aged 30–75 years, had serum glucose levels and medication doses that had been stable for 3 months, and were not currently receiving insulin therapy. Exclusion criteria included current use of vasoactive medications or supplements, current eating disorder, known atherosclerotic vascular disease, sleep apnea, pregnancy, restricted diet, allergy to walnuts or other nuts, and use of lipid-lowering or antihypertensive medications unless stable condition with medication for at least 3 months, and willingness to refrain from taking medication for 12 h before assessment. Those passing the telephone screening (n = 38) underwent a clinical screening examination consisting of height, weight, BMI, and blood pressure measurements and laboratory testing including serum lipids, fasting serum glucose, fasting serum insulin, and plasma A1C. The study protocol and consent form were approved by the Griffin Hospital (Derby, CT) Institutional Review Board. Signed informed consent was obtained from all study participants, and all participants received monetary compensation for their participation. Subject participation and flow are shown in Fig. 1.
Figure 1.
Flow chart for subject participation.
This study was a randomized, controlled, single-blind, crossover clinical trial. All participants first underwent a 4-week run-in period to allow for diet and weight stabilization, during which participants discontinued eating walnuts and taking vasoactive over-the-counter medications and supplements. After the run-in period, participants (n = 24) were randomly assigned to one of two possible permutations of a walnut-enriched ad libitum diet and an ad libitum diet without walnuts for 8 weeks. Treatment assignments were separated by an 8-week washout period.
During the walnut-enriched diet treatment period, participants consumed 56 g of shelled, unroasted English walnuts per day. Participants were given an 8-week supply of walnuts and were instructed to consume the walnuts as a snack or with a meal. Participants were asked to bring their empty packages at the end of the walnut-enriched diet period. Participants completed 3-day diet records during the study. The 3-day diet records were done once during the original run-in period, once during the dietary period, and once during the washout period. Participants were instructed to maintain their baseline medications, supplements, and physical activity levels throughout the study. To ensure that weight remained constant during the walnut-enriched diet period, participants were counseled by a registered dietitian on strategies for isocalorically substituting walnuts for other foods in the diet but were instructed to otherwise continue with their usual dietary patterns. Compliance with treatment and dietary patterns was assessed from the diet records.
During each visit, participants underwent endothelial function testing in the morning after a minimum 8-h overnight fast. Weight, height, blood pressure, serum lipids, fasting serum glucose, fasting serum insulin, and plasma A1C were also measured. Baseline data were collected for all outcome measurements at the beginning of the first treatment period.
Vascular reactivity testing: brachial artery reactivity studies
Endothelial function was measured noninvasively in the right brachial artery by a high-frequency ultrasound scanning machine (Sonos 4500; Phillips Medical Systems, Andover, MA) in accordance with published guidelines (11) and our previous endothelial function studies. In brief, subjects were required to rest in a quiet, temperature-controlled, softly lit room for 15 min before scanning was initiated. The right brachial artery was imaged longitudinally, 2–5 cm above the antecubital fossa, by an experienced registered vascular technologist who was blinded to the treatment assignments. A resting scan was performed, and arterial flow velocity was measured. An occluding cuff placed on the upper arm was inflated to a pressure of 50 mmHg for 5 min and rapidly deflated to induce reactive hyperemia. Brachial artery scans were acquired on magnetic optical disk continuously between 30 and 180 s after cuff deflation, including a repeated flow-velocity measurement during the first 15 s after cuff release. Brachial artery diameters were analyzed by commercially available software (Brachial Analyzer; Medical Imaging Application, Iowa City, IA). Dilatation from baseline was measured at 50–80 s after cuff deflation to assess endothelium-dependent vasodilatation. To test intraobserver reliability, a random sample of 20 brachial artery reactivity studies was read by the same registered vascular technologist for a blinded second reading. The resulting intraobserver reliability coefficient was 0.96.
Outcome measures
Endothelial function.
Endothelial function was measured as flow-mediated dilation (FMD), the percent change of brachial artery diameter from before cuff inflation to 60 s after cuff release. In addition to brachial diameter at 60 s after cuff release, flow after cuff deflation within the first 15 s was used as an indicator of stimulus strength, hyperemic flow being the stimulus for endothelial reactivity. To account for potential variability in stimulus strength, FMD was divided by flow at 15 s after cuff deflation to create a stimulus-adjusted response measure.
Cardiovascular biomarkers.
Fasting serum lipids, fasting serum glucose, fasting serum insulin, and plasma A1C were measured at the Griffin Hospital laboratory using standard procedures at each visit. Homeostasis model assessment of insulin resistance (HOMA-IR) values were calculated (HOMA calculator version 2.2.1) from fasting serum glucose and serum insulin levels to gauge the degree of insulin resistance.
Anthropometric measures.
To measure weight, participants were asked to remove their heavy outer garments (jacket, coat, etc.) and shoes, and stand in the center of the platform with weight distributed evenly on both feet. BMI was calculated using the weight and height measurements. Waist circumference was measured at the umbilicus with the measurement tape surrounding the abdomen horizontal to the floor.
Diet record analysis.
Diet records were analyzed using basic nutrition and diet analysis software (The Food Processor II, version 7.0; ESHA Research, Salem, OR).
Statistical analysis
Repeated-measures ANOVA was used to assess differences in intraindividual responses across treatments. Paired t tests were also used to compare baseline mean values of all outcome measures among participants by group assignment. A Tukey test was used for post hoc analysis of the repeated-measures ANOVA to correct for the experiment-wise error rate. The combined effect of independent variables (age, race, BMI, hypertension, dyslipidemia, and treatment sequence) and treatment assignment on all outcome measures was assessed with multivariable models with the use of ANOVA. All analyses of end points were based on the intention-to-treat principle. Statistical significance was set at two-tailed α < 0.05. Data were analyzed with the use of SAS software for Windows (version 9.1; SAS Institute, Cary, NC). Results are expressed as means ± SD in text and tables. The sample size was determined to allow for ∼20% attrition and noncompliance and to provide ≥80% power to detect a minimal difference of 2.5% in FMD between treatments with maximum allowable type I error of 5%.
RESULTS
Subject characteristics
Twenty-one of the 24 participants who began treatment completed the study. Three participants dropped out because of changes in medications or poor compliance with the treatment protocol. The mean age of participants who completed the study was 58 years; 58% of subjects were women. The mean baseline diameter of the brachial arteries was 4.2 mm, and the mean absolute change in diameter was 0.3 mm. Mean baseline endothelial function measured as FMD was 8.6%. Demographic and baseline characteristics are shown in Table 1.
Table 1.
Demographic and baseline values before to initial treatment assignment
| Age (years) | 58.1 ± 9.2 |
| Women | 14 (58.3) |
| Men | 10 (41.7) |
| Diabetes medication use* | 20 (83.3) |
| Lipid-lowering medication use† | 13 (54.2) |
| Blood pressure–lowering medication use‡ | 17 (70.8) |
| Baseline diameter (mm) | 4.2 ± 0.8 |
| Absolute change in diameter (mm) | 0.3 ± 0.1 |
| FMD (%) | 8.6 ± 4.3 |
| Stimulus-adjusted response measure | 0.08 ± 0.05 |
| BMI (kg/m2) | 32.5 ± 5.0 |
| Weight (kg) | 89.0 ± 15.5 |
| Waist circumference (cm) | 103.1 ± 11.3 |
| Systolic blood pressure (mmHg) | 133.2 ± 14.0 |
| Diastolic blood pressure (mmHg) | 77.7 ± 7.3 |
| Mean blood pressure (mmHg) | 105.4 ± 9.9 |
| Fasting serum glucose (mg/dl) | 129.7 ± 34.4 |
| Serum insulin (mIU/ml) | 15.6 ± 12.4 |
| Insulin resistance | 2.1 ± 1.5 |
| Plasma A1C (%) | 6.7 ± 0.9 |
| Serum total cholesterol (mg/dl) | 183.4 ± 38.9 |
| Serum triglycerides (mg/dl) | 123.6 ± 67.0 |
| Serum HDL cholesterol (mg/dl) | 56.0 ± 14.7 |
| Serum LDL cholesterol (mg/dl) | 102.9 ± 32.4 |
| Serum cholesterol–to-HDL cholesterol ratio | 3.4 ± 1.0 |
Data are means ± SD or n (%). n = 24.
*Included metformin (n = 18), glipizide (n = 3), chlorpropamide (n = 1), sitagliptin (n = 3), pioglitazone (n = 5), and glyburide (n = 3).
†Included atorvastatin (n = 5), pravastatin (n = 2), simvastatin (n = 2), fenofibrate (n = 1), lovastatin (n = 1), rosuvastatin (n = 1), and ezetimibe/simvastatin (n = 1).
‡Included lisinopril (n = 9), verapamil (n = 1), irbesartan (n = 1), atenolol (n = 1), metoprolol (n = 2), valsartan (n = 1), enalapril (n = 1), hydrochlorothiazide/moexipril (n = 1), nadolol (n = 1), and olmesartan (n = 1).
Endothelial function
FMD improved significantly after consumption of the walnut-enriched diet as compared with consumption of an ad libitum diet without walnuts (2.2 ± 1.7 vs. 1.2 ± 1.6%, P = 0.04). No significant change was seen in the stimulus-adjusted response measure (P > 0.05).
Serum lipids
After consumption of a walnut-enriched diet, serum total cholesterol and LDL cholesterol decreased significantly from baseline (−9.7 ± 14.5 mg/dl, P < 0.01 and −7.7 ± 10.0 mg/dl, P < 0.01, respectively). However, the improvement did not reach statistical significance compared with consumption of an ad libitum diet without walnuts (P > 0.05). Serum triglycerides and HDL cholesterol did not improve after consumption of a walnut-enriched diet (Table 2).
Table 2.
Change in outcome measures from baseline
| Walnut-enriched ad libitum | Ad libitum diet without walnuts | P | |
|---|---|---|---|
| FMD (%) | 2.2 ± 1.7* | 1.2 ± 1.6* | 0.04 |
| Stimulus-adjusted response measure | 0.01 ± 0.05 | 0.04 ± 0.14 | 0.37 |
| BMI (kg/m2) | −0.0 ± 1.4 | 0.3 ± 0.9 | 0.44 |
| Weight (kg) | 0.1 ± 3.2 | 0.7 ± 2.2 | 0.46 |
| Waist circumference (cm) | −0.0 ± 6.1 | 0.3 ± 4.1 | 0.87 |
| Systolic blood pressure (mmHg) | 4.0 ± 9.2 | −4.9 ± 11.7 | 0.01 |
| Diastolic blood pressure (mmHg) | 1.6 ± 4.6 | −2.5 ± 6.4 | 0.02 |
| Mean blood pressure (mmHg) | 2.8 ± 6.2 | −3.7 ± 8.4 | 0.01 |
| Fasting serum glucose (mg/dl) | 10.0 ± 20.5* | 2.9 ± 21.5 | 0.27 |
| Serum insulin (mIU/ml) | 3.6 ± 10.4 | −3.4 ± 8.0 | 0.02 |
| Insulin resistance | 0.2 ± 0.9 | −0.2 ± 0.7 | 0.10 |
| Plasma A1C (%) | −0.0 ± 0.3 | −0.0 ± 0.3 | 0.85 |
| Serum total cholesterol (mg/dl) | −9.7 ± 14.5* | −4.5 ± 23.0 | 0.38 |
| Serum triglycerides (mg/dl) | −1.9 ± 48.3 | 8.2 ± 43.4 | 0.48 |
| Serum HDL cholesterol (mg/dl) | −0.8 ± 6.5 | 1.8 ± 7.2 | 0.22 |
| Serum LDL cholesterol (mg/dl) | −7.7 ± 10.0* | −7.8 ± 20.6 | 0.97 |
| Serum cholesterol–to-HDL cholesterol | −0.2 ± 0.4 | −0.2 ± 0.5 | 0.73 |
Data are means ± SD. n = 22. P values were obtained from repeated-measures ANOVA.
*P < 0.05 from paired Student t test.
Fasting serum glucose, serum insulin, plasma A1C, HOMA-IR
The walnut-enriched diet significantly increased fasting serum glucose from baseline (10.0 ± 20.5 mg/dl, P = 0.04), but the difference was not significant (P > 0.05) compared with that for the ad libitum diet without walnuts. Fasting serum insulin, plasma A1C, and HOMA-IR did not change significantly (P > 0.05) after consumption of the walnut-enriched diet (Table 2).
Blood pressure
The ad libitum diet without walnuts (control) significantly lowered blood pressure compared with the walnut-enriched diet (systolic blood pressure −4.9 ± 11.7 vs. 4.0 ± 9.2 mmHg, P = 0.01; diastolic blood pressure −2.5 ± 6.4 vs. 1.6 ± 4.6 mmHg, P = 0.02).
Anthropometric measures
BMI, weight, and waist circumference did not change after consumption of the walnut-enriched diet (Table 2).
Dietary intake and compliance
Dietary intakes of PUFAs, n-3 fatty acid, and n-6 fatty acid increased significantly with the walnut-enriched diet compared with those with an ad libitum diet without walnuts (PUFAs 33 ± 5.4 vs. 9.2 ± 4.2 g, P < 0.01; n-3 fatty acid 5.84 ± 0.86 vs. 0.81 ± 0.45 g, P < 0.01; and n-6 fatty acid 26.02 ± 4.43 vs. 6.17 ± 3.10 g, P < 0.01). Dietary intake of other nutrients remained stable throughout the study (Table 3). According to participants' reports and recounts of empty walnut packages, 96% of study subjects complied with walnut ingestion (defined as consumption of ≥80% of the assigned amount of walnuts) during treatment.
Table 3.
Selected nutrient intake
| Walnut-enriched ad libitum | Ad libitum diet without walnuts | P | |
|---|---|---|---|
| Energy (kcal) | 1,765 ± 358 | 1,685 ± 402 | 0.51 |
| Fat (kcal) | 798 ± 220 | 654 ± 268 | 0.07 |
| Fat (% kcal) | 45 ± 9 | 38 ± 10 | 0.01 |
| Saturated fatty acids (kcal) | 192 ± 81 | 211 ± 96 | 0.50 |
| MUFAs (g) | 18 ± 8.7 | 18 ± 8.5 | 0.81 |
| PUFAs (g) | 33 ± 5.4 | 9.2 ± 4.2 | <0.01 |
| n-3 (g) | 5.8 ± 0.9 | 0.8 ± 0.5 | <0.01 |
| n-6 (g) | 26.0 ± 4.4 | 6.2 ± 3.1 | <0.01 |
| Protein (g/day) | 77 ± 18 | 82 ± 22 | 0.45 |
| Protein (% kcal) | 17 ± 3 | 19 ± 4 | 0.06 |
| Carbohydrates (g) | 170 ± 51 | 177 ± 51 | 0.67 |
| Carbohydrates (% kcal) | 39 ± 9 | 43 ± 12 | 0.20 |
| Cholesterol (mg) | 247 ± 133 | 306 ± 200 | 0.28 |
| Soluble fiber (g) | 2.9 ± 1.1 | 2.4 ± 1.8 | 0.27 |
Data are averages ± SD from one 3-day record for each dietary period.
CONCLUSIONS
This study demonstrated that consumption of an ad libitum diet enriched with 56 g walnuts/day for 8 weeks significantly improved endothelial function in this sample of type 2 diabetic individuals. These results are consistent with results of previous trials demonstrating that ingestion of walnuts improved endothelial function in hypercholesterolemic adults (9) and reversed postprandial endothelial dysfunction resulting from a high-fat diet (10).
In addition to having a favorable fatty acid profile consisting of monounsaturated fatty acids (MUFAs) and PUFAs, nuts are also a rich source of bioactive compounds with beneficial effects on cardiovascular artery disease risk, including dietary fiber, folate, and antioxidants. Walnuts differ from other nuts because they contain a high content of α-linolenic acid, which might confer additional antiatherogenic and antiarrhythmic properties. The mechanism by which walnuts may improve endothelial function has been hypothesized to involve increased membrane fluidity of endothelial cells, thus promoting release of nitric oxide (10) or to involve the role of vascular cell adhesion molecule-1, whose levels may be reduced by a walnut-rich diet, leading to attenuated endothelial activation (9). A walnut feeding experiment in an animal model also showed a reduction in the expression of endothelin-1, a potent endothelial activator (12). l-Arginine and antioxidants in walnuts also may play a role in improving vascular reactivity.
Although reductions of total and LDL cholesterol were observed in the walnut-enriched diet compared with baseline, they were not significant compared with the ad libitum diet without walnuts. Previous trials examining the effect of walnut ingestion on lipid profiles reported mixed results. Some trials (13,14) did not observe significant decreases in total cholesterol and LDL cholesterol after ingestion of walnuts. However, some have found improvements in total and LDL cholesterol resulting from the addition of walnuts to the diets of hypercholesterolemic or normolipidemic patients (15). Given the variability in the results of these trials, it remains unclear whether walnut consumption improves cholesterol levels and at what dose and in which population a benefit may be conferred. Possible confounding factors in this study include the concomitant use of lipid-lowering medications, elevated body weight, and the variability inherent in an ad libitum diet.
This study showed that a walnut-enriched ad libitum diet did not affect body weight or BMI. This finding is consistent with previous clinical studies (16,17). Epidemiological evidence consistently indicates that nut consumers have lower BMIs than nonconsumers (18).
The walnut-enriched diet significantly increased fasting serum glucose compared with baseline levels, although this increase was not significant compared with that for an ad libitum diet without walnuts. Furthermore, the walnut-enriched diet did not significantly affect fasting serum insulin, plasma A1C, or HOMA-IR compared with those at both baseline and with an ad libitum diet without walnuts. These results are fairly consistent with those from previous clinical studies in diabetic subjects (8,19). Although epidemiological studies have indicated that higher intakes of MUFAs and PUFAs are associated with improvements in insulin sensitivity (3), clinical evidence, including this trial, has thus far not been able to support the hypothesis. However, acute feeding studies have demonstrated the ability of nuts to lower postprandial glycemia when eaten with carbohydrates (20,21).
Interestingly, blood pressure decreased significantly in those consuming the ad libitum diet without walnuts, compared with baseline values and values for those consuming the walnut-enriched diet. Few studies have evaluated the effects of walnuts or other nuts on blood pressure, but most of these have found either a beneficial effect or no effect (22–24). It is possible that the changes in blood pressure observed in this trial could have influenced the improvement in endothelial function. It is unclear why, in this study, an ad libitum diet without walnuts, in essence, the subjects' habitual diet, was associated with reduced blood pressure.
This study has several limitations. The ad libitum diet without walnuts was not standardized, which limits our ability to determine effects found exclusively with walnuts. Using subjects as their own controls in the crossover study design should have mitigated this problem, however. The ad libitum diet without walnuts was not standardized on purpose to ensure the generalizability of our results to the broader population of diabetic individuals, most of whom do not follow a clinically controlled diet. Participants were allowed to maintain the ad libitum diet and stable medication doses and only received diet consultation to adjust their caloric intake to prevent weight gain during the walnut intervention phase. Therefore, we believe that the improved endothelial function with sustained walnut consumption that we observed would be sustained in conditions approximating a real-world setting. As this is the first walnut feeding study without a standardized control diet, we feel that this study will add a new dimension to the existing literature on the health benefits of walnuts. Limitations to the generalizability of our study include the small sample size, lack of geographic variation, and a predominance of white women in our sample. In addition, we do not know how physical activity or genetic factors may factor into our results, nor do we know the effect of varying duration of diabetes and medication use among our participants. It is also worth noting that there was a considerable range of responses to the addition of walnuts to the diet, with some participants experiencing little to no improvement or even a reduction in endothelial function and others experiencing substantial improvement.
Despite its limitations, this study provides evidence that regular walnut consumption improves endothelial function in type 2 diabetic individuals and may therefore help reduce cardiovascular disease risk in this high-risk population. Because subjects did not follow a restricted diet, these results may have broader applicability to the general population than previous diet-controlled trials. Future researchers should continue to investigate the role of walnuts and other nuts on endothelial function and blood lipids in populations at risk for cardiovascular disease.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Centers for Disease Control and Prevention. Preventing diabetes and its complications [article online], 2008. Available from http://www.cdc.gov/nccdphp/publications/factsheets/Prevention/diabetes.htm Accessed 22 July 2009
- 2.Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 2003; 108: 1527– 1532 [DOI] [PubMed] [Google Scholar]
- 3.Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 2002; 288: 2554– 2560 [DOI] [PubMed] [Google Scholar]
- 4.Jenkins DJ, Hu FB, Tapsell LC, Josse AR, Kendall CW. Possible benefit of nuts in type 2 diabetes. J Nutr 2008; 138: 1752S– 1756S [DOI] [PubMed] [Google Scholar]
- 5.Albert CM, Gaziano JM, Willett WC, Manson JE. Nut consumption and decreased risk of sudden cardiac death in the Physicians' Health Study. Arch Intern Med 2002; 162: 1382– 1387 [DOI] [PubMed] [Google Scholar]
- 6.Kris-Etherton PM, Zhao G, Binkoski AE, Coval SM, Etherton TD. The effects of nuts on coronary heart disease risk. Nutr Rev 2001; 59: 103– 111 [DOI] [PubMed] [Google Scholar]
- 7.Feldman EB. The scientific evidence for a beneficial health relationship between walnuts and coronary heart disease. J Nutr 2002; 132: 1062S– 1101S [DOI] [PubMed] [Google Scholar]
- 8.Tapsell LC, Gillen LJ, Patch CS, Batterham M, Owen A, Baré M, Kennedy M. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care 2004; 27: 2777– 2783 [DOI] [PubMed] [Google Scholar]
- 9.Ros E, Núñez I, Pérez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation 2004; 109: 1609– 1614 [DOI] [PubMed] [Google Scholar]
- 10.Cortés B, Núñez I, Cofán M, Gilabert R, Pérez-Heras A, Casals E, Deulofeu R, Ros E. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J Am Coll Cardiol 2006; 48: 1666– 1671 [DOI] [PubMed] [Google Scholar]
- 11.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery. A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 16: 257– 265 [DOI] [PubMed] [Google Scholar]
- 12.Kris-Etherton PM, Hu FB, Ros E, Sabaté J. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr 2008; 138: 1746S– 1751S [DOI] [PubMed] [Google Scholar]
- 13.Chisholm A, Mann J, Skeaff M, Frampton C, Sutherland W, Duncan A, Tiszavari S. A diet rich in walnuts favourably influences plasma fatty acid profile in moderately hyperlipidaemic subjects. Eur J Clin Nutr 1998; 52: 12– 16 [DOI] [PubMed] [Google Scholar]
- 14.Morgan JM, Horton K, Reese D, Carey C, Walker K, Capuzzi DM. Effects of walnut consumption as part of a low-fat, low-cholesterol diet on serum cardiovascular risk factors. Int J Vitam Nutr Res 2002; 72: 341– 347 [DOI] [PubMed] [Google Scholar]
- 15.Olmedilla-Alonso B, Granado-Lorencio F, Herrero-Barbudo C, Blanco-Navarro I, Blázquez-García S, Pérez-Sacristán B. Consumption of restructured meat products with added walnuts has a cholesterol-lowering effect in subjects at high cardiovascular risk: a randomised, crossover, placebo-controlled study. J Am Coll Nutr 2008; 27: 342– 348 [DOI] [PubMed] [Google Scholar]
- 16.Sabaté J. Nut consumption and body weight. Am J Clin Nutr 2003; 78: 647S– 650S [DOI] [PubMed] [Google Scholar]
- 17.Sabaté J, Cordero-Macintyre Z, Siapco G, Torabian S, Haddad E. Does regular walnut consumption lead to weight gain? Br J Nutr 2005; 94: 859– 864 [DOI] [PubMed] [Google Scholar]
- 18.Mattes RD, Kris-Etherton PM, Foster GD. Impact of peanuts and tree nuts on body weight and healthy weight loss in adults. J Nutr 2008; 138: 1741S– 1745S [DOI] [PubMed] [Google Scholar]
- 19.Jenkins DJ, Kendall CW, Marchie A, Josse AR, Nguyen TH, Faulkner DA, Lapsley KG, Singer W. Effect of almonds on insulin secretion and insulin resistance in nondiabetic hyperlipidemic subjects: a randomized controlled crossover trial. Metabolism 2008; 57: 882– 887 [DOI] [PubMed] [Google Scholar]
- 20.Josse AR, Kendall CW, Augustin LS, Ellis PR, Jenkins DJ. Almonds and postprandial glycemia—a dose-response study. Metabolism 2007; 56: 400– 404 [DOI] [PubMed] [Google Scholar]
- 21.Jenkins DJ, Kendall CW, Josse AR, Salvatore S, Brighenti F, Augustin LS, Ellis PR, Vidgen E, Rao AV. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr 2006; 136: 2987– 2992 [DOI] [PubMed] [Google Scholar]
- 22.Djoussé L, Rudich T, Gaziano JM. Nut consumption and risk of hypertension in US male physicians. Clin Nutr 2009; 28: 10– 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Lapiscina EH, Pimenta AM, Beunza JJ, Bes-Rastrollo M, Martinez JA, Martinez-Gonzalez MA. Nut consumption and incidence of hypertension: the SUN prospective cohort. Nutr Metab Cardiovasc Dis 14August2009. [ Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Sabaté J, Fraser GE, Burke K, Knutsen SF, Bennett H, Lindsted KD. Effects of walnuts on serum lipid levels and blood pressure in normal men. N Engl J Med 1993; 328: 603– 607 [DOI] [PubMed] [Google Scholar]