Abstract
OBJECTIVE
Diabetes is increasingly common in cystic fibrosis, but little information describing its influence on mortality exists. Using national U.K. data, in this study we document diabetes-specific mortality rates, estimate the impact of diabetes on survival, and estimate population-attributable fractions.
RESEARCH DESIGN AND METHODS
This retrospective cohort study identified 8,029 individuals aged 0–65 years from the U.K. Cystic Fibrosis Registry (1996–2005). A total of 5,892 patients were included in analyses of mortality rates, and 4,234 were included in analyses of risk factors. We calculated age-adjusted mortality rates using Poisson regression, standardized mortality ratios using the population of England and Wales, and relative risks using proportional hazards modeling.
RESULTS
During 17,672 person-years of follow-up, 393 subjects died. The age-adjusted mortality rate was 1.8 per 100 person-years (95% CI 1.6–2.0). The age-adjusted mortality rates per 100 person-years were 2.0 (1.8–2.4) in female subjects and 1.6 (1.4–1.9) in male subjects, and 4.2 (3.4–5.1) in individuals with diabetes vs. 1.5 (1.3–1.7) in those without diabetes. Independent risk factors for death included diabetes (hazard ratio 1.31 [95% CI 1.03–1.67], female sex (1.71 [1.36–2.14]) plus poorer pulmonary function, lower BMI, Burkholderia cepacia infection, absence of Staphylococcus aureus infection, allergic bronchopulmonary aspergillosis, liver disease, prior organ transplantation, and corticosteroid use.
CONCLUSIONS
Individuals with cystic fibrosis die earlier if they have diabetes, which, if delayed or better treated, might reasonably extend survival; this hypothesis merits testing.
Cystic fibrosis is the most common autosomal recessive disease leading to premature death in white populations. Because of improvements in care, both survival, with a life expectancy to the mid-30s, and, as a consequence, the prevalence of complications have increased dramatically (1–3). The influence of birth year and sex on mortality has been described in the British cystic fibrosis population (2,4), but little is documented about the association between complications and specifically diabetes and mortality.
The majority of patients with cystic fibrosis die of respiratory complications. In patients with cystic fibrosis, there is a high incidence of diabetes (5), which has been shown to increase the risk of death in the U.S. (6). Yet, little information exists worldwide to document the absolute mortality rates associated with diabetes in cystic fibrosis. Using national registry data, in this study we estimate the impact of diabetes on survival in adults and children with cystic fibrosis in Britain, taking into account recognized and potential risk factors for death. We document mortality rates, estimate the risk increase associated with diabetes, and calculate the population-attributable fraction (PAF) for diabetes associated with death.
RESEARCH DESIGN AND METHODS
This retrospective cohort study identified 8,029 individuals aged 0–65 years registered on the U.K. Cystic Fibrosis Registry from 1996 to 2005. The registry, administered by the U.K. Cystic Fibrosis Trust, records information about the health and treatment of patients from birth (7). Data are collected from 50 British cystic fibrosis care centers in a standardized anonymous fashion after patient consent. Data gathering is an ongoing effort, and the registry is updated annually.
Of 8,029 patients with cystic fibrosis, 6,678 had baseline data comprising registration followed by at least one clinic visit and an annual review within the same calendar year. We considered the first visit as the start of follow-up. We excluded 786 individuals without further follow-up, leaving 5,892 patients for analyses of mortality rates. Of these, we included 4,234 individuals with complete data in analyses of risk factors for mortality. Individuals with complete data were older because of the difficulties in testing pulmonary function in young children and were more likely to be white.
End point and potential risk factors
Information on whether a patient had died and the date of death were taken from the cystic fibrosis registry. The registry did not contain information about individuals' identities, and we could not obtain death certificates.
Potential risk factors for death measured at baseline included age, sex, ethnicity, BMI, pulmonary function, diabetes, respiratory infections, class of cystic fibrosis transmembrane conductance regulator (CFTR) alleles, diagnosis of cystic fibrosis by neonatal screening, prior organ transplantation, and other medical interventions as described below. We categorized ethnicity as white or nonwhite. BMI was calculated as weight in kilograms divided by the square of height in meters, and we computed BMI Z scores using a U.K. reference population (8). We coded CFTR alleles into five classes reflecting, for CFTR protein, defective production, processing, regulation, conductance, and quantity (9) and further categorized as high (classes I–III) and low (classes IV–V) risk for mortality (5,10). Pulmonary function was measured as forced expiratory volume at 1 s (FEV1) and forced vital capacity, both expressed as percentage of predicted (11). We considered diabetes present if a patient had at least one of the following: 1) diagnosed diabetes, 2) treatment with insulin or oral hypoglycemic drugs, or 3) values consistent with diabetes on oral glucose tolerance testing per World Health Organization criteria. We considered an individual infected if bacteria or fungi were cultured at baseline from sputum or a throat swab or if a physician had diagnosed chronic infection. Organisms included Pseudomonas aeruginosa, Staphylococcus aureus, Burkholderia cepacia complex, Haemophilus influenzae, methicillin-resistant S. aureus, and Aspergillus fumigatus. Allergic bronchopulmonary aspergillosis (ABPA) was diagnosed clinically. We defined liver disease as one of the following: 1) abnormal liver function tests, 2) cirrhosis with portal hypertension, or 3) use of supplementary bile acids. Pancreatic insufficiency was defined as supplementation with oral pancreatic enzymes. We defined corticosteroid use as any oral or inhaled use in the year before baseline and nutritional supplementation as feedings of any kind, ranging from oral supplements to parenteral feedings.
Statistical analyses
We calculated mortality rates expressed per 100 persons/year in 5,892 individuals as the number of deaths divided by total survival time, i.e., from registration to death or the last clinic visit. Using Poisson regression, we age-adjusted mortality rates separately for male and female subjects and for those with and without diabetes. We stratified mortality rates by 10-year age-groups. We calculated directly standardized mortality rates and standardized mortality ratios (SMRs) using as a standard the 2005 population of England and Wales (12).
We tested for differences in baseline characteristics between those who died and those who survived using a χ2 test for categorical variables and t tests or Kruskal-Wallis tests for normally distributed or nonnormally distributed continuous variables. We identified risk factors for mortality among the 4,234 patients with complete data using Cox proportional regression; the hazard ratio (HR) was used to estimate the relative risk. We evaluated proportional hazards assumptions examining Kaplan-Meier survival curves for categorical variables; none violated the assumptions. We chose variables for multivariate modeling if associated with mortality in univariate Cox regression analyses (P < 0.05) or if a factor had been previously reported to be associated with the risk of death in cystic fibrosis, e.g., sex and S. aureus. We chose the final model using a stepwise approach and likelihood ratios. We tested for two-way interactions between diabetes and each risk factor in the final multivariate model.
We calculated PAF as (13)
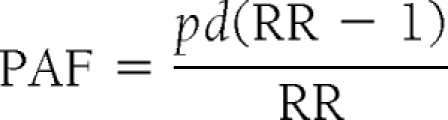 |
where pd is the proportion of deaths of those exposed to a risk factor (e.g., diabetes) and RR is the adjusted HR from multivariate proportional hazards modeling.
We coded percent predicted FEV1, BMI Z score, and age as continuous variables in proportional hazards models and as binary variables around the approximate medians (<70% for FEV1, <−0.23 for BMI Z score, and >16 years for age) to calculate PAFs. Analyses were performed using Access (Microsoft), R (R Development Core Team, 2007), and STATA (StataCorp).
RESULTS
Study population
Of the 5,892 individuals, 3,155 (54%) were male. The median age at baseline was 13.4 years (interquartile range 6.9–21.8). Of 4,234 individuals with data on risk factors, an equivalent proportion were male. The median age was 16.0 and 97% were white.
Mortality rates and standardized mortality ratios
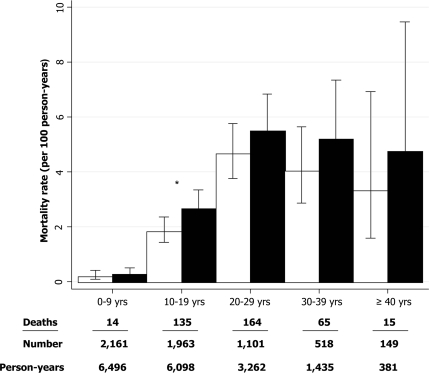
The median follow-up time was 2.9 years (interquartile range 1.9–4.0). In 17,672 person-years, 393 subjects died. The mortality rates increased with increasing age (Fig. 1). The crude annual mortality rate was 2.2 (95% CI 2.0–2.5) per 100 person-years. The age-adjusted mortality rate for the cohort was 1.8 per 100 person-years (1.6–2.0). Female subjects had a higher age-adjusted mortality rate at 2.0 (1.8–2.4) per 100 person-years than did male subjects at 1.6 (1.4–1.9) per 100 person-years.
Figure 1.
Mortality rates with 95% CI by age and sex in patients with cystic fibrosis. □, Male patients; ■, female patients. *P < 0.05, difference between sexes.
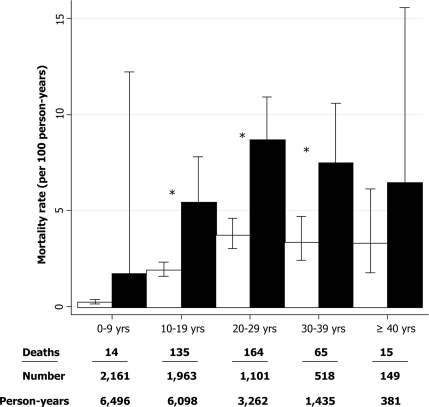
Individuals with diabetes had considerably higher age-adjusted mortality rates at 4.2 (95% CI 3.4–5.1) per 100 person-years than those without diabetes at 1.5 (1.3–1.7) per 100 person-years. Patients with diabetes had higher absolute mortality rates at all ages, with the greatest relative difference in children <10 years old at baseline (P = 0.09) (Fig. 2).
Figure 2.
Mortality rates with 95% CI by age and diabetes in patients with cystic fibrosis. □, Patients without diabetes; ■, patients with diabetes. *P < 0.05, difference between individuals with and without diabetes.
Crude and directly standardized mortality rates expressed per 1,000 person-years and SMRs are presented in Table 1. The SMRs, which measure the risk of death relative to that of the general population of the same age, were 55.5, 39.8, and 84.1 for the total cohort, men, and women, respectively. Female patients with diabetes were >200 times more likely to die relative to their counterparts in the general population.
Table 1.
Crude, directly standardized mortality rates (per 1,000 person-years) and SMRs by sex and diabetes in patients with cystic fibrosis
| Patients (n) | Person-years at risk | Deaths (n) | Crude mortality rate (95% CI) | Directly standardized mortality rate (95% CI)* | SMR (95% CI)* | |
|---|---|---|---|---|---|---|
| Total | 5,892 | 17,672 | 393 | 22.2 (20.1–24.6) | 26.8 (19.0–40.5) | 55.5 (40.8–70.1) |
| Male | 3,155 | 9,425 | 191 | 20.3 (17.6–23.4) | 23.5 (14.9–43.3) | 39.8 (24.7–55.0) |
| Female | 2,737 | 8,247 | 202 | 24.5 (21.3–28.1) | 30.9 (19.0–56.5) | 84.1 (53.4–114.7) |
| Diabetes | 696 | 1,953 | 141 | 72.2 (61.2–85.1) | 48.4 (27.7–106.2) | 123.4 (74.7–172.1) |
| Male | 349 | 970 | 61 | 62.9 (48.9–80.8) | 41.9 (22.2–106.8) | 82.2 (33.6–130.9) |
| Female | 347 | 983 | 80 | 81.4 (65.4–101.3) | 56.1 (24.0–209.8) | 208.7 (99.4–318.0) |
| Without diabetes | 5,196 | 15,718 | 252 | 16.0 (14.2–18.1) | 20.6 (13.3–35.7) | 42.4 (28.1–56.7) |
| Male | 2,806 | 8,454 | 130 | 15.4 (12.9–18.3) | 16.7 (9.5–41.4) | 32.1 (16.9–47.2) |
| Female | 2,390 | 7,264 | 122 | 16.8 (14.1–20.1) | 25.4 (13.9–53.9) | 60.4 (31.3–89.5) |
*Using the 2005 population of England and Wales as a standard population.
Risk factors associated with mortality in cystic fibrosis
The characteristics and results of univariate analyses of the individuals who died or survived are shown in supplementary Table 1 (available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-1215/DC1). In univariate Cox regression analyses evaluating 325 deaths in 12,930 person-years, increasing age, diabetes, decreasing pulmonary function, and a high-risk CFTR class were all associated with an increased risk of death. Diabetes was associated with a fourfold increase in risk of death relative to that in those without diabetes (HR 4.04 [95% CI 3.23–5.07], P < 0.001). In univariate analyses, infection with P. aeruginosa, B. cepacia complex, H. influenzae, or A. fumigatus; use of corticosteroids; the presence of ABPA; use of nutritional supplements; and decreased BMI Z score were all associated with an increased risk of death. Other characteristics that differed between subjects who died and those who survived were a history of liver disease, pancreatic insufficiency, and a history of organ transplantation. Patients with cystic fibrosis whose disease had been detected by neonatal screening had a lower risk of death compared with those not detected through screening, as did individuals infected with S. aureus relative to uninfected individuals. Not significantly associated with death in univariate analyses were sex (P = 0.057) and infection with methicillin-resistant S. aureus.
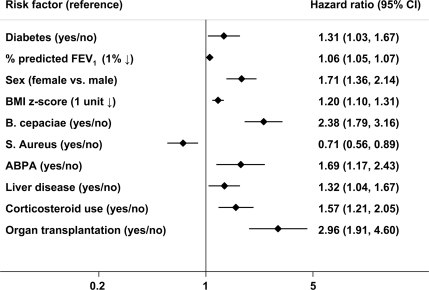
Given the relationship between complications, we evaluated the independent role of each risk factor in multivariate modeling. The final age-adjusted model showed an increased risk of death associated with each of the following: diabetes, decreasing pulmonary function, female sex, decreasing BMI Z score, infection with B. cepacia complex, diagnosis of ABPA, history of liver disease, prior organ transplantation, and the use of corticosteroids (Fig. 3). Infection with S. aureus was independently associated with a decreased risk of death. Diabetes was associated with a 31% (95% CI 3–67) increase in risk of death independent of other complications of cystic fibrosis. Female patients were 71% more likely to die than male patients, with all other factors being equal.
Figure 3.
Risk factors for death in cystic fibrosis. Cox proportional hazards modeling adjusted for age and all risk factors in the figure. n = 4,234.
Including high- and low-risk genotype classes and pancreatic insufficiency in the multivariate model described above did not render insignificant any factor or change the HR for death associated with diabetes. We found no interactions.
PAF of death in cystic fibrosis
The estimated PAFs for mortality are shown in supplementary Table 2. With continuous variables coded as binary variables, nutritional supplementation was associated with an increased risk of death. Of modifiable factors contributing to mortality, poor pulmonary function defined as a predicted FEV1 <70% accounted for the greatest proportion of risk at 78%. Low BMI (BMI Z score <−0.23) accounted for the next largest component of risk. The PAF for diabetes was 14% (95% CI 8–19).
CONCLUSIONS
This report highlights the specific contribution of diabetes to mortality in Britain's population with cystic fibrosis. Using nationally representative and systematically collected data, in this report we estimate the absolute, relative, and attributable risk of death associated with diabetes. Because some of the factors are potentially modifiable, then, if they are also causal, this provides hope and identifies research targets to further extend the lives of individuals with cystic fibrosis.
In Britain, where the incidence of diabetes in cystic fibrosis approximates 2–7% per year (5), we estimate that diabetes accounts for some 14% of deaths. That the components from PAF modeling add up to >100%, although not an intuitive finding, assumes that risk factors interrelate and that each risk factor is the first to be eliminated (14). This increased risk associated with diabetes is not explained by previously identified risk factors for death including poor pulmonary function (15), other complications (6), sex (16,17), or genotype (18), all of which are also associated with an increased risk of diabetes (5). It is likely that the metabolic derangements associated with diabetes nonetheless predispose individuals to infection, worsen pulmonary function, and make maintenance of body weight difficult.
The present study showed a disproportionately increased, albeit insignificant, relative risk of death associated with diabetes in children aged <10 years relative to individuals aged >10 years. Research in France has shown that individuals with cystic fibrosis who develop abnormal glucose metabolism as children have higher mortality rates than those who developed dysglycemia as adults (19). In our study, of the children <10 years, only a very small proportion would be expected to have autoimmune-mediated diabetes (type 1), given the low prevalence in the U.K. general population (20). Because screening for diabetes in cystic fibrosis in the U.K. is encouraged for adults during periods of clinical stability but not for children aged <12 years, diabetic children at presentation may have more severe hyperglycemia. We could not address degree of hyperglycemia, duration of diabetes, or age of its onset in these analyses.
Registry data from the U.S. show that diabetes, controlling for other factors, is associated with a 55% increase in mortality risk in cystic fibrosis (6); we estimate a more modest 30% increase in risk. If we acknowledge imprecision in estimates and the potential misclassification of diabetes, a real difference may exist. This may represent in the U.K., relative to the U.S., earlier detection of diabetes, less detection bias (diagnosing diabetes in sicker patients with more frequent medical attention), or better glycemic control (for which evidence linking better control to mortality in cystic fibrosis is sparse). A difference could represent different statistical models; the U.S. model does not include organ transplantation or use of corticosteroids, both risk factors for diabetes (5) and, in this study, for death.
These findings imply that delaying the onset of diabetes, possibly through avoidance of corticosteroids, or assuring earlier and better treatment for diabetes in those with cystic fibrosis may lengthen survival. Yet no evidence for prevention of diabetes in cystic fibrosis exists, and trials of treatment are few (21). A randomized trial of lowering blood glucose has shown improvements in BMI, but not in pulmonary function (22). In the general population, weight loss and exercise can prevent or delay onset of type 2 diabetes (23), but these factors have minimal relevance to cystic fibrosis–related diabetes, in which β-cell dysfunction and decreased insulin production predominate. In type 1 diabetes, trials of early treatment to prevent diabetes have failed (24,25).
For other risk factors for death, the present study shows, consistent with others (16), that female patients die earlier than male patients. The increased risk of death in women does not appear to be due to a higher prevalence of complications; rather it indicates that female patients with a given set of comorbidities fared worse than same-aged male patients with the same problems. We confirm that lower BMI, pulmonary complications, and infection with B. cepacia complex are associated with an increased risk of death in cystic fibrosis and that infection with S. aureus is associated with a decreased risk of death (6). Increasing age was not by itself associated with death in cystic fibrosis, reflecting, perhaps, the fact that death rates stabilized after age 20 years (Figs. 1 and 2). Our models incorporated age-dependent variables (percent predicted FEV1 and BMI Z score); when modeling was done instead with absolute values for FEV1 and BMI, increasing age was associated with an increased death rate. Separate analyses for children and adults (age <16 vs. ≥16 years) showed that in multivariate modeling, pulmonary function was associated with mortality in both groups, whereas BMI was associated with mortality only in adults. We do not know of another study showing that corticosteroids are associated with an increase in the risk of death independent of other complications. For oral corticosteroids, there is evidence of a short-term benefit in pulmonary function, but the balance of risk in the long term is not clear. Physicians may simply offer corticosteroids to patients with the greatest risk of dying.
The high SMRs we report reconfirm that mortality in the cystic fibrosis population in Britain far exceeds that of the general population (4). For individuals with diabetes, these values are higher at nearly 120 times those for the general population. Our findings from directly standardized mortality rates show that the cystic fibrosis population in the U.K., with a median age of 13 years, has a mortality rate similar to that of those aged 70–74 years in the general population of England and Wales (12).
The strengths of this study are its size and its systematically collected longitudinal data. Potential limitations include ascertainment of death from the registry rather than from death records and possible misclassification of individuals with diabetes. Underdetection of diabetes is probably lessened by universal and free access to health care in Britain. If unwell individuals with stress hyperglycemia were labeled as diabetic, this would suggest that the value for risk associated with diabetes we report underestimates the truth. Likewise, if factors associated with the risk of death were more apt to be detected in the sickest patients, the relative risks we report would overestimate the true associations but would not bias the overall mortality rates.
In summary, this report documents recent mortality statistics for adults and children with cystic fibrosis in Britain. In this study, we identify complications including diabetes that, if delayed or better treated, might reasonably extend the lives of individuals with cystic fibrosis in the U.K. Advances in life expectancy to date support this opportunity, whereas the PAF quantifies potential gains.
Supplementary Material
Acknowledgments
P.C. received funding from the Thai government.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 69th annual meeting of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
We thank the U.K. Cystic Fibrosis Trust and the specialist cystic fibrosis teams who contributed data.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Cystic Fibrosis Foundation Patient Registry 2007 Annual Data Report, Bethesda, MD [Google Scholar]
- 2.Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947–2003. Eur Respir J 2007; 29: 522– 526 [DOI] [PubMed] [Google Scholar]
- 3.Bellis G, Cazes MH, Parant A, Gaimard M, Travers C, Le Roux E, Ravilly S, Rault G. Cystic fibrosis mortality trends in France. J Cyst Fibros 2007; 6: 179– 186 [DOI] [PubMed] [Google Scholar]
- 4.Dodge JA, Morison S, Lewis PA, Coles EC, Geddes D, Russell G, Littlewood JM, Scott MT. Incidence, population, and survival of cystic fibrosis in the UK, 1968–95. UK Cystic Fibrosis Survey Management Committee. Arch Dis Child 1997; 77: 493– 496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adler AI, Shine BS, Chamnan P, Haworth CS, Bilton D. Genetic determinants and epidemiology of cystic fibrosis-related diabetes: results from a British cohort of children and adults. Diabetes Care 2008; 31: 1789– 1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol 2001; 153: 345– 352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta G, Sims E, Culross F, McCormick J, Mehta A. Potential benefits of the UK Cystic Fibrosis Database. J R Soc Med 2004; 97: 60– 71 [PMC free article] [PubMed] [Google Scholar]
- 8.Pan H, Cole T. Users Guide to lmsGrowth London, Medical Research Council UK, 2007 [Google Scholar]
- 9.Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Ann Intern Med 2003; 67: 471– 485 [DOI] [PubMed] [Google Scholar]
- 10.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. CFTR genotype as a predictor of prognosis in cystic fibrosis. Chest 2006; 130: 1441– 1447 [DOI] [PubMed] [Google Scholar]
- 11.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Spirometric reference values from a sample of the U.S. population. Am J Respir Crit Care Med 1999; 159: 179– 187 [DOI] [PubMed] [Google Scholar]
- 12.Office for National Statistics. Mortality Statistics: General. DH1—Annual Review of the Registrar General on Deaths in England and Wales Newport, South Wales, Office for National Statistics, 2005 [Google Scholar]
- 13.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health 1998; 88: 15– 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe AK, Powell KE, Flanders WD. Why population attributable fractions can sum to more than one. Am J Prev Med 2004; 26: 243– 249 [DOI] [PubMed] [Google Scholar]
- 15.Koch C, Rainisio M, Madessani U, Harms HK, Hodson ME, Mastella G, McKenzie SG, Navarro J, Strandvik BInvestigators of the European Epidemiologic Registry of Cystic Fibrosis. Presence of cystic fibrosis-related diabetes mellitus is tightly linked to poor lung function in patients with cystic fibrosis: data from the European Epidemiologic Registry of Cystic Fibrosis. Pediatr Pulmonol 2001; 32: 343– 350 [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld M, Davis R, FitzSimmons S, Pepe M, Ramsey B. Gender gap in cystic fibrosis mortality. Am J Epidemiol 1997; 145: 794– 803 [DOI] [PubMed] [Google Scholar]
- 17.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis related diabetes: current trends in prevalence, incidence and mortality. Diabetes Care 2009; 32: 1626– 1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet 2003; 361: 1671– 1676 [DOI] [PubMed] [Google Scholar]
- 19.Bismuth E, Laborde K, Taupin P, Velho G, Ribault V, Jennane F, Grasset E, Sermet I, de Blic J, Lenoir G, Robert JJ. Glucose tolerance and insulin secretion, morbidity, and death in patients with cystic fibrosis. J Pediatr 2008; 152: 540– 545 [DOI] [PubMed] [Google Scholar]
- 20.Harvey JN, Craney L, Kelly D. Estimation of the prevalence of diagnosed diabetes from primary and secondary care source data: analysis comparison of record linkage with capture-recapture. J Epidemiol Community Health 2002; 56: 18– 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onady G, Stolfi A. Insulin and oral agents for managing cystic fibrosis-related diabetes. Cochrane Database Syst Rev 2005; 3: CD004730. [DOI] [PubMed] [Google Scholar]
- 22.Moran A, Pekow P, Grover P, Zorn M, Slovis B, Pilewski J, Tullis E, Liou TG, Allen H. Insulin therapy to improve BMI in cystic fibrosis related diabetes without fasting hyperglycemia: results of the CFRDT Trial. Diabetes Care 2009; 32: 1783– 1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Näntö-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, Korhonen S, Erkkola R, Sipilä JI, Haavisto L, Siltala M, Tuominen J, Hakalax J, Hyöty H, Ilonen J, Veijola R, Simell T, Knip M, Simell O. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 2008; 372: 1746– 1755 [DOI] [PubMed] [Google Scholar]
- 25.Diabetes Prevention Trial—Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002; 346: 1685– 1691 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.