Abstract
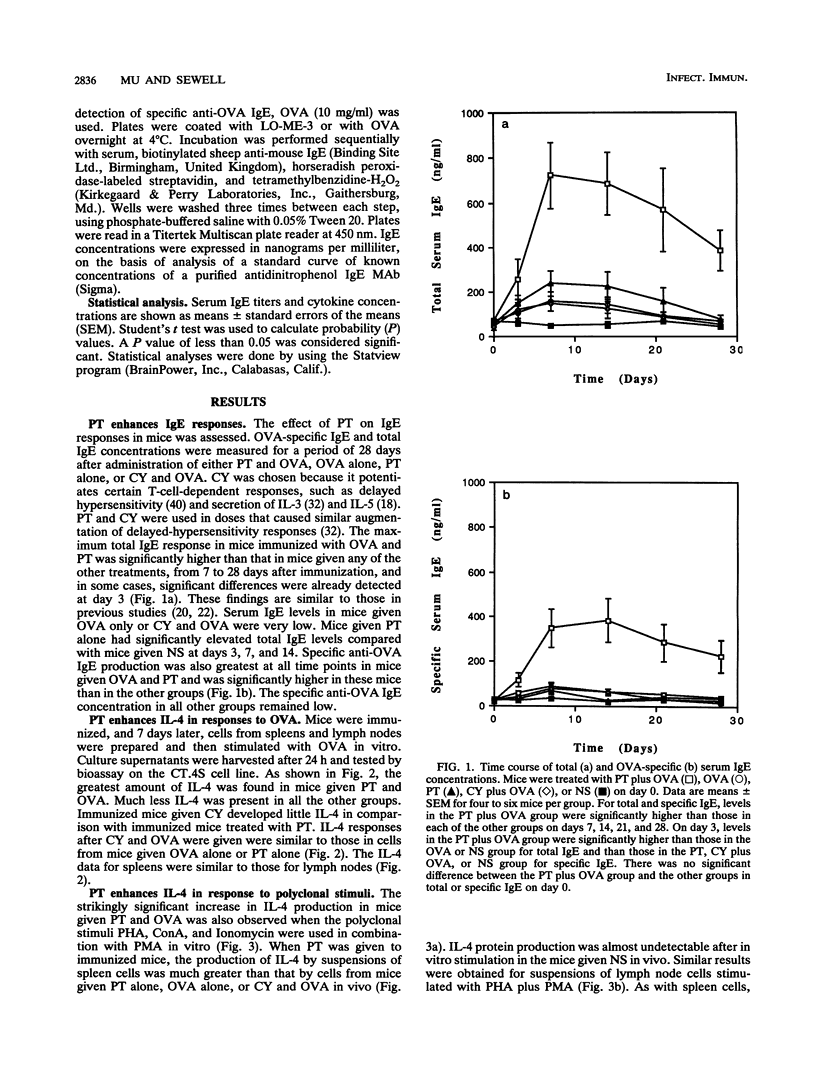
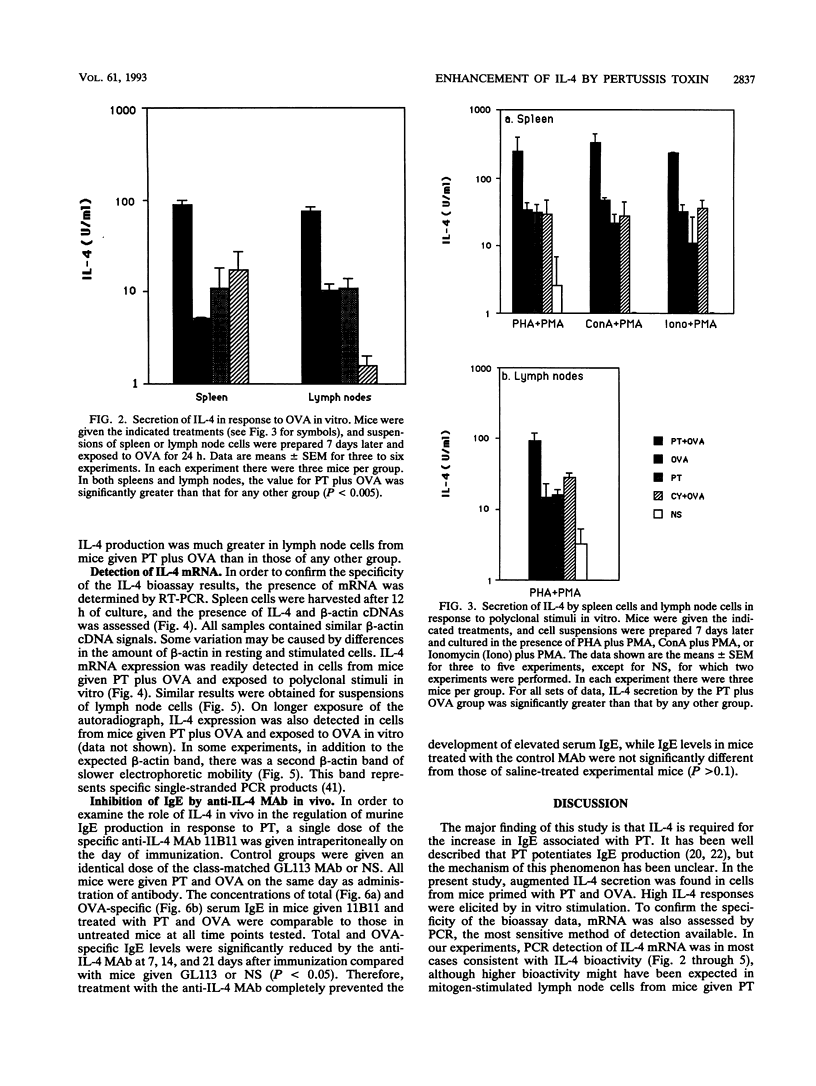
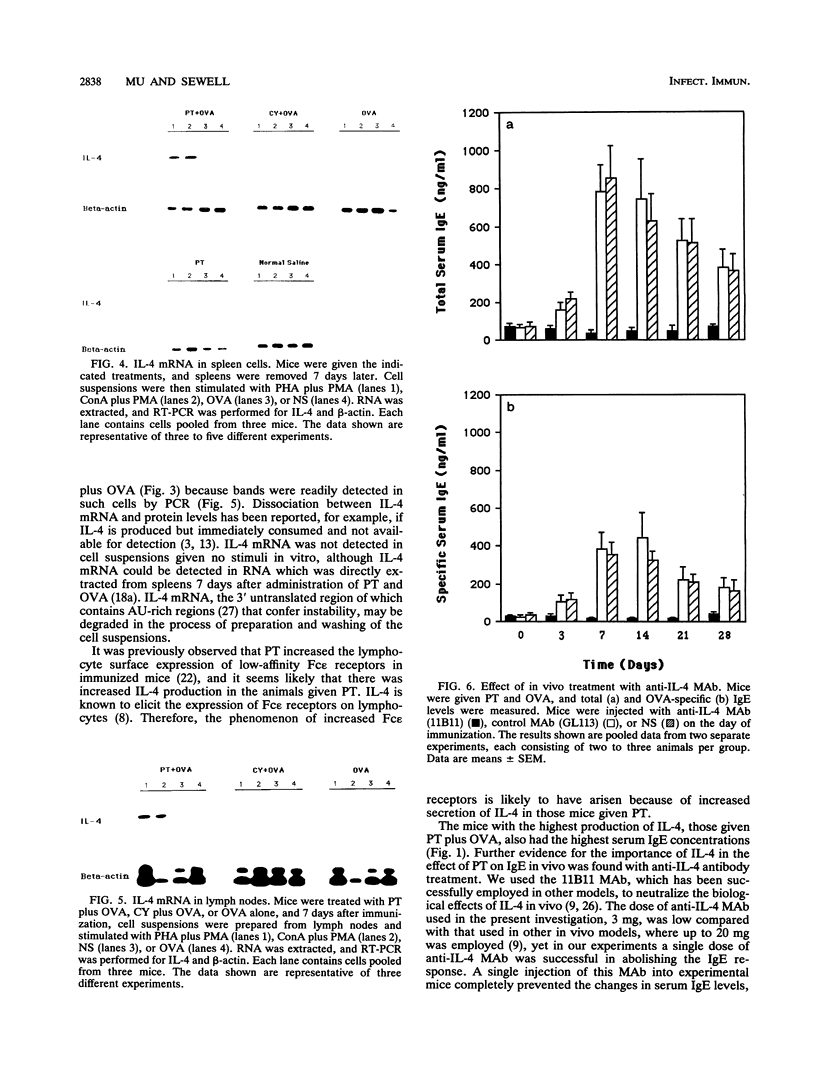
Pertussis toxin (PT), a protein toxin of Bordetella pertussis, has many biological activities, including potent adjuvant capacity and the ability to up-regulate immunoglobulin E (IgE) production. Interleukin-4 (IL-4) is a cytokine which is essential for the IgE response. Accordingly, we examined the effect of PT on IL-4 production. Spleen and lymph node cell suspensions were prepared from immunized mice and cultured with antigen or polyclonal stimuli in vitro. IL-4 secretion was assessed by bioassay, and IL-4 mRNA expression was assessed by reverse transcription and polymerase chain reaction. Significantly larger amounts of IL-4 protein and mRNA were produced in vitro by cells from mice given PT at the time of immunization than by cells from mice given antigen alone, PT alone, or the combination of antigen with cyclophosphamide. Total and antigen-specific serum IgE levels were significantly elevated in immunized mice given PT, compared with the other groups. Thus, there was a relationship between serum IgE and IL-4 production. The administration of a single dose of an anti-IL-4 monoclonal antibody in vivo, at the time of immunization and treatment with PT, abolished the development of the IgE response. These results indicate that PT is a potent stimulus for the production of IL-4, which is required for the adjuvant effect of PT on IgE formation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black W. J., Munoz J. J., Peacock M. G., Schad P. A., Cowell J. L., Burchall J. J., Lim M., Kent A., Steinman L., Falkow S. ADP-ribosyltransferase activity of pertussis toxin and immunomodulation by Bordetella pertussis. Science. 1988 Apr 29;240(4852):656–659. doi: 10.1126/science.2896387. [DOI] [PubMed] [Google Scholar]
- Carding S. R., West J., Woods A., Bottomly K. Differential activation of cytokine genes in normal CD4-bearing T cells is stimulus dependent. Eur J Immunol. 1989 Feb;19(2):231–238. doi: 10.1002/eji.1830190203. [DOI] [PubMed] [Google Scholar]
- Cheever A. W., Finkelman F. D., Caspar P., Heiny S., Macedonia J. G., Sher A. Treatment with anti-IL-2 antibodies reduces hepatic pathology and eosinophilia in Schistosoma mansoni-infected mice while selectively inhibiting T cell IL-5 production. J Immunol. 1992 May 15;148(10):3244–3248. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Lebman D. A., Hiraki D. D., Christiansen J. A., Shrader B., Cherwinski H. M., Savelkoul H. F., Finkelman F. D., Bond M. W. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988 Feb;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Defrance T., Aubry J. P., Rousset F., Vanbervliet B., Bonnefoy J. Y., Arai N., Takebe Y., Yokota T., Lee F., Arai K. Human recombinant interleukin 4 induces Fc epsilon receptors (CD23) on normal human B lymphocytes. J Exp Med. 1987 Jun 1;165(6):1459–1467. doi: 10.1084/jem.165.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Holmes J., Ohara J., Tung A. S., Sample J. V., Paul W. E. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988 Oct 1;141(7):2335–2341. [PubMed] [Google Scholar]
- Finkelman F. D., Snapper C. M., Mountz J. D., Katona I. M. Polyclonal activation of the murine immune system by a goat antibody to mouse IgD. IX. Induction of a polyclonal IgE response. J Immunol. 1987 May 1;138(9):2826–2830. [PubMed] [Google Scholar]
- Hedenskog S., Björkstén B., Blennow M., Granström G., Granström M. Immunoglobulin E response to pertussis toxin in whooping cough and after immunization with a whole-cell and an acellular pertussis vaccine. Int Arch Allergy Appl Immunol. 1989;89(2-3):156–161. doi: 10.1159/000234939. [DOI] [PubMed] [Google Scholar]
- Hu-Li J., Ohara J., Watson C., Tsang W., Paul W. E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S). J Immunol. 1989 Feb 1;142(3):800–807. [PubMed] [Google Scholar]
- Kelso A., Troutt A. B., Maraskovsky E., Gough N. M., Morris L., Pech M. H., Thomson J. A. Heterogeneity in lymphokine profiles of CD4+ and CD8+ T cells and clones activated in vivo and in vitro. Immunol Rev. 1991 Oct;123:85–114. doi: 10.1111/j.1600-065x.1991.tb00607.x. [DOI] [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Miller E., Ashworth L. A., Robinson A., Waight P. A., Irons L. I. Phase II trial of whole-cell pertussis vaccine vs an acellular vaccine containing agglutinogens. Lancet. 1991 Jan 12;337(8733):70–73. doi: 10.1016/0140-6736(91)90735-8. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Bergman R. K., Sadowski P. L. Biological activities of crystalline pertussigen from Bordetella pertussis. Infect Immun. 1981 Sep;33(3):820–826. doi: 10.1128/iai.33.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J. J., Bernard C. C., Mackay I. R. Elicitation of experimental allergic encephalomyelitis (EAE) in mice with the aid of pertussigen. Cell Immunol. 1984 Jan;83(1):92–100. doi: 10.1016/0008-8749(84)90228-4. [DOI] [PubMed] [Google Scholar]
- Munoz J. J., Peacock M. G. Action of pertussigen (pertussis toxin) on serum IgE and on Fc epsilon receptors on lymphocytes. Cell Immunol. 1990 May;127(2):327–336. doi: 10.1016/0008-8749(90)90136-f. [DOI] [PubMed] [Google Scholar]
- Nencioni L., Pizza M., Bugnoli M., De Magistris T., Di Tommaso A., Giovannoni F., Manetti R., Marsili I., Matteucci G., Nucci D. Characterization of genetically inactivated pertussis toxin mutants: candidates for a new vaccine against whooping cough. Infect Immun. 1990 May;58(5):1308–1315. doi: 10.1128/iai.58.5.1308-1315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. Y., Gunning P., Eddy R., Ponte P., Leavitt J., Shows T., Kedes L. Evolution of the functional human beta-actin gene and its multi-pseudogene family: conservation of noncoding regions and chromosomal dispersion of pseudogenes. Mol Cell Biol. 1985 Oct;5(10):2720–2732. doi: 10.1128/mcb.5.10.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochel M., Vohr H. W., Pfeiffer C., Gleichmann E. IL-4 is required for the IgE and IgG1 increase and IgG1 autoantibody formation in mice treated with mercuric chloride. J Immunol. 1991 May 1;146(9):3006–3011. [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Otsuka T., Villaret D., Yokota T., Takebe Y., Lee F., Arai N., Arai K. Structural analysis of the mouse chromosomal gene encoding interleukin 4 which expresses B cell, T cell and mast cell stimulating activities. Nucleic Acids Res. 1987 Jan 12;15(1):333–344. doi: 10.1093/nar/15.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M. The concept of pertussis as a toxin-mediated disease. Pediatr Infect Dis. 1984 Sep-Oct;3(5):467–486. doi: 10.1097/00006454-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Regulation and deregulation of human IgE synthesis. Immunol Today. 1990 Sep;11(9):316–321. doi: 10.1016/s0167-5699(10)80004-0. [DOI] [PubMed] [Google Scholar]
- Sato H., Sato Y. Protective activities in mice of monoclonal antibodies against pertussis toxin. Infect Immun. 1990 Oct;58(10):3369–3374. doi: 10.1128/iai.58.10.3369-3374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell W. A., Andrews P. Inhibition of lymphocyte circulation in mice by pertussis toxin. Immunol Cell Biol. 1989 Oct;67(Pt 5):291–296. doi: 10.1038/icb.1989.43. [DOI] [PubMed] [Google Scholar]
- Sewell W. A., Munoz J. J., Vadas M. A. Enhancement of the intensity, persistence, and passive transfer of delayed-type hypersensitivity lesions by pertussigen in mice. J Exp Med. 1983 Jun 1;157(6):2087–2096. doi: 10.1084/jem.157.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell W. A., de Moerloose P. A., Hamilton J. A., Schrader J. W., Mackay I. R., Vadas M. A. Potentiation of delayed-type hypersensitivity by pertussigen or cyclophosphamide with release of different lymphokines. Immunology. 1987 Aug;61(4):483–488. [PMC free article] [PubMed] [Google Scholar]
- Sher A., Coffman R. L., Hieny S., Cheever A. W. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J Immunol. 1990 Dec 1;145(11):3911–3916. [PubMed] [Google Scholar]
- Snapper C. M., Finkelman F. D., Stefany D., Conrad D. H., Paul W. E. IL-4 induces co-expression of intrinsic membrane IgG1 and IgE by murine B cells stimulated with lipopolysaccharide. J Immunol. 1988 Jul 15;141(2):489–498. [PubMed] [Google Scholar]
- Spangrude G. J., Braaten B. A., Daynes R. A. Molecular mechanisms of lymphocyte extravasation. I. Studies of two selective inhibitors of lymphocyte recirculation. J Immunol. 1984 Jan;132(1):354–362. [PubMed] [Google Scholar]
- Steinman L., Weiss A., Adelman N., Lim M., Zuniga R., Oehlert J., Hewlett E., Falkow S. Pertussis toxin is required for pertussis vaccine encephalopathy. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8733–8736. doi: 10.1073/pnas.82.24.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper R. I., Levinson D. A., Stanger B. Z., Campos-Torres J., Abbas A. K., Leder P. IL-4 induces allergic-like inflammatory disease and alters T cell development in transgenic mice. Cell. 1990 Aug 10;62(3):457–467. doi: 10.1016/0092-8674(90)90011-3. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Parker D., Poulter L. W. Functional aspects of the selective depletion of lymphoid tissue by cyclophosphamide. Immunology. 1972 Oct;23(4):493–501. [PMC free article] [PubMed] [Google Scholar]
- Valentine J. E., Boyle M. J., Sewell W. A., Gough N. M. Presence of single-stranded DNA in PCR products of slow electrophoretic mobility. Biotechniques. 1992 Aug;13(2):222–224. [PubMed] [Google Scholar]