Abstract
OBJECTIVE
Despite increased information on the importance of an inappropriate inflammatory response in the acute Charcot process, there has been no previous attempt to define the specific pathways that mediate its pathogenesis. Here, the role played by monocytes was analyzed.
RESEARCH DESIGN AND METHODS
The immune phenotype of peripheral monocytes was studied by fluorescence-activated cell sorter analysis comparing patients with acute Charcot (n = 10) in both the active and recovered phase, diabetic patients with neuropathy (with or without osteomyelitis), and normal control subjects.
RESULTS
When compared with diabetic control subjects and healthy subjects, monocytes from acute Charcot patients showed a proinflammatory immune phenotype characterized by increased production of proinflammatory cytokines, reduced secretion of anti-inflammatory cytokines, increased expression of surface costimulatory molecules, and increased resistance to serum withdrawal-induced apoptosis. In addition, the pattern of circulating cytokines confirmed activation of proinflammatory cytokines. No modulation of the monocyte phenotype was documented in diabetic control subjects and healthy subjects, thus indicating that the proinflammatory alterations of monocytes are specific and causative of acute Charcot.
CONCLUSIONS
Together, these data provide evidence for the role of proinflammatory changes in the immune phenotype of monocytes in the pathogenesis of acute Charcot. These alterations may explain the abnormally intense and prolonged inflammatory response that characterizes this disorder and may represent a potential therapeutic target for specific pharmacological interventions.
Diabetes is probably the most common cause of denervation-induced destruction of joints (Charcot foot) in the world today. Painlessness and abnormal foot biomechanics play an important part in the pathogenesis of the disorder. Recent attention, however, has focused on several abnormalities, which together suggest a more complex cause. A pilot study by La Fontaine et al. (1) suggests that abnormal calcitonin gene-related peptide and endothelial nitric oxide synthase activity may play a role in the development of Charcot foot. Also, receptor activator of the nuclear factor-κβ ligand (RANKL)-activated peripheral blood monocytes have been found to induce a significant increase in bone resorption in Charcot patients (2,3). Finally, Jeffcoate, Game, and Cavanagh (4) drew attention to the possible link between proinflammatory cytokines and neuroarthropathy in the context of an exaggerated inflammatory response to trauma. The inability of the Charcot patient to control the intensity and the length of the local inflammatory response would lead to increased expression of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) which, in turn, would trigger increased expression of RANKL leading to maturation of osteoclast and subsequent bone changes (5–8). Despite increased information on the importance of an inappropriate inflammatory response in the acute Charcot process, there has been no previous attempt to define the specific pathways that mediate its pathogenesis. Here, the role played by monocytes was analyzed. The immune phenotype of monocytes was assessed by testing spontaneous and induced production of proinflammatory and anti-inflammatory cytokines by measuring the expression of surface molecules (CD40, CD80, and CD86), which enable monocytes to became competent costimulatory cells and to activate T-lymphocytes responses (9–11), and by studying the ability of monocytes to undergo apoptosis, an important homeostatic mechanism that contributes to regulate the intensity and length of the inflammatory response (12). Patients with acute Charcot, in both the active and recovered phase, were compared with diabetic patients with neuropathy (with or without osteomyelitis) and normal control subjects.
RESEARCH DESIGN AND METHODS
We studied 10 consecutive diabetic patients (4 with type 1 diabetes and 6 with type 2 diabetes) without macrovascular complications referred to the University of Rome's diabetic foot clinic for unexplained and relatively painless increased swelling of a foot and ankle absent of active foot ulceration and/or signs of soft tissue infection, with no history of recent (within 6 months) immunosuppressive treatment including corticosteroids and anti–TNF-α compounds, fever, evidence of infectious diseases, inflammatory disorders, or any kind of malignancy, and presenting the magnetic resonance diagnostic characteristics of stress injuries: swelling, local warmth, and clinical instability due to ligamentous injury/occult trauma (at this stage, radiographic changes are absent or minimal, but the typical bone marrow edema is present at magnetic resonance imaging); (8 age- and sex-matched diabetic patients with polyneuropathy without macrovascular complications and clinical and radiological evidence of a history of Charcot foot; 8 age- and sex-matched diabetic patients with polyneuropathy and clinical and radiological documented foot osteomyelitis without clinical and radiological evidence of a history of Charcot foot; and 8 age- and sex-matched healthy subjects). Blood samples by peripheral venipuncture from Charcot patients were first obtained within 24 h of meeting enrollment criteria and then just after recovery (as determined by clinical and magnetic resonance imaging evaluations). The blood was immediately separated from plasma by centrifugation, stored at +4°C, and processed within 1 h. Serum was obtained for circulating cytokine determination and immediately stored at −80°C. A single blood sample was obtained from diabetic control and healthy control subjects. This study was approved by the ethics committee of the University of Rome “Policlinico Tor Vergata” hospital. Informed consent was obtained from all subjects.
Compounds
Lipopolysaccharide (LPS) from Escherichia coli 0111/B4 was purchased from Sigma Chemical (St. Louis, MO). Recombinant granulocyte-macrophage colony–stimulating factor was obtained from Sandoz Research Institute (East Hanover, NJ) and contained 5.4 × 106 chronic myelogenous leukemia units per mg of glycoprotein.
Limulus amebocyte lysate test
All the compounds and media used in this study were analyzed for endotoxin contamination by the limulus amebocyte lysate test (QCL-1000; BioWhittaker, Walkersville, MD). All the samples analyzed were found free of endotoxin contamination (<0.1 EU/ml).
Antibodies
For fluorescence-activated cell sorter (FACS) analysis, the following monoclonal antibodies were used: anti-CD40, anti-CD80, anti-CD86, anti–TNF-α, anti–IL-1β, anti–IL-6, anti–IL-4, and anti–IL-10 (PharMingen, San Diego, CA). Staining was performed with fluorescein isothiocyanate–, phycoethrin-, and Quantum Red–conjugated antibodies.
ELISA immunoassay
The analyses of TNF-α, IL-1β, IL-6, IL-4, and IL-10 were done with commercially available ELISA test kits (R&D Systems, Minneapolis, MN). According to the manufacturer's specifications, these ELISAs are specific for the relative interleukin. All the samples were determined in duplicate in a single analytical set. Intra-series variation coefficient was <20%.
Cell stimulation
Peripheral blood from control subjects or patients was enriched for peripheral blood mononuclear cells (PBMNCs) by centrifugation over Ficoll Hypaque. The cells were cultured in RPMI-1640 medium supplemented with 20% heat-inactivated FCS, 2 mmol/l l-glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin, referred to as complete medium. The cells were kept at 37°C in a humidified atmosphere of 5% CO2 in air, in 96-well V-bottom plates (Corning, Corning, NY) at a concentration of 5 × 105 cells/well/250 μl. For the determination of intracellular cytokine production by FACS, PBMNCs from patients and control subjects were cultured at a concentration of 5 × 105 cells/well/250 μl for 18 h in 96-well V-bottom plates (Corning) in complete medium in the presence or in the absence of 100 ng/ml LPS. Thirty minutes after stimulation, 1 μg/ml of the protein transport inhibitor brefeldin A (Sigma Chemical) was added. At the end of the incubation period, the cells were analyzed by FACS for intracellular cytokine production.
Surface marker and intracellular cytokine staining
After incubation, the cells were washed and stained for surface markers. Then, cells were either analyzed by FACS to determine cell surface antigen expression or suspended in Cytofix/Cytoperm solution (Pharmingen), stained for intracellular cytokines, and then analyzed by FACS.
FACS
Flow cytometry was performed using a FACScan flow cytometer and analyzed with Cell Quest software (Becton Dickinson). For each analysis, 104 monocytes were gated according to scatter characteristics designed to include only viable cells.
Assessment of hypodiploid DNA formation
Hypodiploid DNA formation (a reliable marker of apoptosis) was assessed by propidium iodide assay as previously described (13). In brief, PBMNCs (5 × 105) were incubated in RPMI-1640 alone for 72 h. At the end of the incubation period, the cells were resuspended in 1.5-ml propidium iodide solution containing 250-μg DNase-free RNase A. The propidium iodide fluorescence of individual nuclei was measured by FACS.
Statistics
The normality of variable distribution was assessed by the Kolmogorov-Smirnov goodness-of-fit test. Comparison between distribution of two variables for a single group was performed by either the Student paired t test or Mann-Whitney U test, as appropriate. All P values are two-tailed. P values <0.05 were considered significant. Statistical analyses were performed using SPSS version 10.0 (SPSS, Chicago, IL) statistical package.
RESULTS
Patients
The mean age was similar between patients with acute Charcot (6 male and 4 female subjects) and diabetic control patients with (5 male and 3 female subjects) or without (6 male and 2 female subjects) oteomyelitis (53 ± 2.8 vs. 59 ± 2.9 and 59 ± 7 years, P ≥ 0.05), as was the mean age between the former and healthy control participants (4 male subjects and 4 female subjects) (53 ± 2.8 vs. 47 ± 2.7 years, P > 0.05). The mean duration of diabetes in acute Charcot case subjects and diabetic control subjects with or without oseomyelitis was 31 ± 5.1, 27 ± 4.6, and 36 ± 2.9 years, respectively. All diabetic patients, both acute Charcot case subjects and control subjects, were on insulin therapy. The mean A1C was 7.4 ± 1% in acute Charcot case subjects and 7.1 ± 0.5% and 7.6 ± 2.2% in diabetic control subjects with or without osteomyelitis, respectively.
Increased proinflammatory and reduced anti-inflammatory cytokine production by monocytes from acute Charcot patients
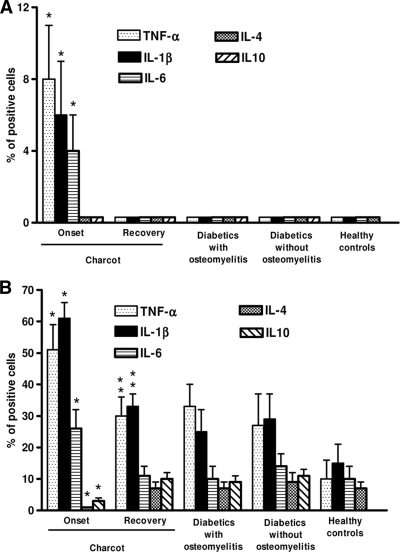
Blood monocytes from case and control subjects were evaluated for spontaneous (Fig. 1A) and inducible (Fig. 1B) TNF-α, IL-1β, IL-6, IL-4, and IL-10 production. As shown by FACS analysis for intracellular cytokine production, monocytes from diabetic control and healthy control subjects did not spontaneously produce detectable amounts of TNF-α, IL-1β, IL-6, IL-4, and IL-10. As opposed, a limited but detectable production of TNF-α, IL-1β, and IL-6, but not IL-4 and IL-10, was observed in cells from patients with acute Charcot. When activated by LPS, monocytes from patients with acute Charcot produced significantly more TNF-α, IL-1β, and IL-6, but less IL-4 and IL-10, than monocytes from diabetic control and healthy control subjects. Interestingly, both spontaneous and inducible cytokine production by monocytes from acute Charcot case subjects significantly decreased after recovery from the acute phase.
Figure 1.
Spontaneous cytokine production by monocytes from acute Charcot case subjects and control subjects. Spontaneous (A) and inducible (monocytes stimulated for 18 h by 100 ng/ml LPS) (B) cytokine production was assessed by FACS, as intracellular accumulation on a single-cell basis. Appropriate controls with isotype-matched irrelevant mAbs were carried out and consistently showed <1% of positive cells. For each analysis, 104 monocytes were gated according to scatter characteristics designed to include only viable cells. Fluorescence data were expressed as percentage of positive cells after subtraction of background isotype-matched values. The data represent the means ± SD (error bars). *P < 0.05, with respect to control subjects. **P > 0.005, with respect to diabetic control and healthy subjects.
Circulating cytokines in patients with acute Charcot
Circulating cytokines in acute Charcot case subjects were analyzed during the acute phase of the process and after recovery. As shown in Table 1, the concentrations of TNF-α, IL-1β, and IL-6 were slightly but significantly higher than those found in control subjects and decreased after recovery to values similar to those found in control subjects.
Table 1.
Circulating cytokines in acute Charcot cases
| Cytokine | Cytokine concentrations in sera (pg/ml) |
||||
|---|---|---|---|---|---|
| Acute Charcot |
Diabetic control subjects |
Healthy subjects | |||
| Onset | Recovered | With osteomyelitis | Without osteomyelitis | ||
| TNF-α | 5.2 ± 3.2* | 1.5 ± 0.9 | 2.8 ± 2.3 | 2.1 ± 1 | 2.6 ± 1.2 |
| IL-1β | 0.6 ± 0.3* | <0.125 | <0.125 | <0.125 | <0.125 |
| IL-6 | 15.3 ± 7.4* | 7.4 ± 4.2 | 3 ± 2.9 | 5.2 ± 2.7 | 6.7 ± 3.5 |
| IL-4 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 |
| IL-10 | <0.78 | <0.78 | <0.78 | <0.78 | <0.78 |
Data are means ± SD. TNF-α and IL-6 were detectable in 100% of the samples; IL-1β was detectable in 3 out of 9 acute Charcot patients at the onset time point. The lowest standards were as follows: TNF-α, 0.5 pg/ml; IL-1β, 0.125 pg/ml; IL-6, 0.156 pg/ml; IL-4, 0.25 pg/ml; IL-10, 0.78 pg/ml.
*P < 0.05, with respect to control subjects.
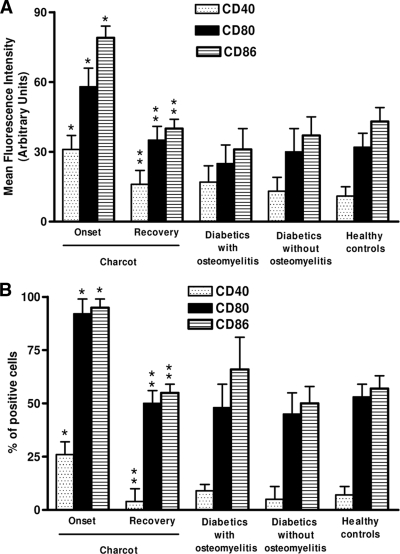
Upmodulation of surface molecules in monocytes from Charcot patients
As shown in Fig. 2, diabetic control and healthy control subjects did not statistically differ with respect to CD40, CD80, and CD86 expression (P > 0.05). In contrast, monocytes from acute Charcot patients showed a statistically significant increase of both percentage of positive cells and intensity of expression of these molecules with respect to diabetic control and healthy control subjects. CD40, CD80, and CD86 expression markedly decreased in patients with acute Charcot after recovery, reaching values that were not different from those observed in diabetic control and healthy control subjects.
Figure 2.
Upmodulation of surface molecules in monocyte-macrophages from Charcot patients. The expression of CD40, CD80, and CD86 was analyzed by FACS on CD14+ gated cells in fresh explanted PBMCs. Appropriate controls with isotype-matched irrelevant mAbs were carried out and consistently showed <1% of positive cells. For each analysis, 104 monocytes were gated according to scatter characteristics designed to include only viable cells. Fluorescence data were expressed as mean channel fluorescence (A) and as percentage of positive cells (B) after subtraction of background isotype-matched values. The data represent the means ± SD (error bars). *P < 0.05, with respect to control subjects; **P > 0.05, with respect to diabetic control and healthy subjects.
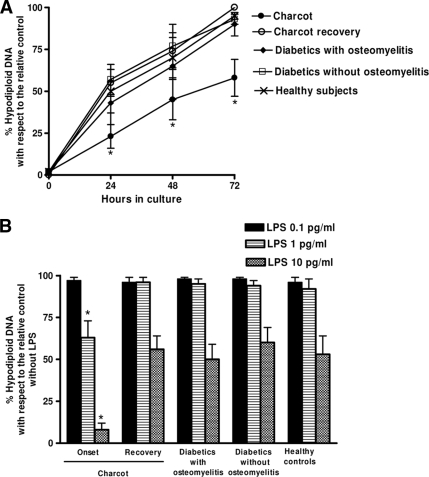
Increased resistance of monocytes from acute Charcot case subjects to serum withdrawal-induced apoptosis
As shown in Fig. 3A, just after separation, <1% of monocytes were nonviable as determined by Trypan blue assay, and no hypodiploid DNA was detectable in case and control subjects. The percentage of hypodiploid DNA increased over time in cultured cells in both case and control subjects. After 72 h of culture, the percentage of hypodiploid DNA was significantly lower in cells from acute Charcot case subjects as compared with cells from diabetic and healthy control subjects. Titration experiments (Fig. 3B) showed that the addition of LPS at concentrations ≤1 pg/ml reduced hypodiploid DNA formation by >30% in monocytes from acute Charcot patients but had a very limited effect in cells from recovered Charcot case subjects and control subjects. Moreover, >99% and <50% reduction of hypodiploid DNA formation could be obtained with 10 pg/ml LPS in monocytes from acute Charcot patients and recovered Charcot case subjects and control subjects, respectively.
Figure 3.
Kinetics of hypodiploid DNA formation in monocyte-macrophages. PBMCs were cultured in medium with no added serum and hypodiploid DNA formation was determined at indicated intervals (A). PBMCs were cultured in medium with no added serum with different LPS concentrations, and hypodiploid DNA formation was determined at 72 h (B). The red fluorescence due to propidium iodide staining of the DNA was registered on a logarithmic scale at >620 nm. The forward and side scatter of particles were simultaneously measured. Cell debris was excluded from analysis by appropriately raising the forward scatter threshold. The residual cell debris had a very low DNA fluorescence emission and a low side scatter signal. At least 104 cells of each sample were analyzed. *P < 0.05, with respect to recovered acute case subjects and control subjects.
CONCLUSIONS
We show here that in patients with acute Charcot, peripheral monocytes acquire a proinflammatory immune phenotype characterized by increased production of proinflammatory cytokines, reduced secretion of anti-inflammatory cytokines, increased expression of costimulatory surface molecules, and increased resistance to apoptosis.
Monocytes play a pivotal role in the development and maintenance of the inflammatory response. These cells are the major source of proinflammatory (TNF-α, IL-1β, and IL-6) as well as anti-inflammatory cytokines (IL-4 and IL-10) (14–16). Alterations in the correct timing, intensity, and balance of expression of proinflammatory versus anti-inflammatory cytokines by monocytes result in pathologic modulation of the inflammatory response. Thus, the activation of inflammatory and suppression of anti-inflammatory cytokines that we have found in patients with acute Charcot is consistent with the abnormally intense and prolonged inflammatory response that characterizes the acute phase of this disease. A growing body of evidence is now supporting the possibility that this inflammatory response plays a pivotal pathogenetic role in the changes in bone and joints that develop in this disorder (4). Indeed, TNF-α and IL-1β, released during the inflammatory process, trigger increased expression of RANKL (5,6). This leads to activation of NF-κB and maturation of osteoclasts (7,8). The effect of IL-6 on bone formation/resorption is more controversial. Indeed, several reports support the possibility that IL-6 could in fact induce an osteocytic phenotype (17). As opposed, there is evidence that IL-6 can stimulate osteoclasts differentiation and bone resorption by an indirect mechanism, increasing interactions between osteoblasts and osteoclasts (18). At variance with Devaraj et al. (19) and Kulseng et al. (20), who reported increased proinflammatory cytokine production by monocytes from diabetic patients, with respect to healthy subjects we did not find differences between diabetic control and healthy subjects. It is possible that these seemingly conflicting data are due to variations related to the experimental procedures used to detect cytokine production in cultured monocytes. Both Devaraj et al. and Kulseng et al. measured cytokine production by ELISA testing of supernatants from unfractionated cell cultures. Here we used a FACS-based technique that allowed us to identify producer cells by cell surface markers and cytokine production simultaneously (13). It is likely that this technique is more suitable to study a primarily cell-based phenomenon than simple measurement of cytokine production by a bulk-release method such as ELISA testing of supernatants from unfractionated cell cultures.
CD40, CD80, and CD86 are involved in antigen presentation to T-cells, CD4+ T-cell activation (e.g., cytokine expression), and proliferation/differentiation (9–11). Thus, the enhanced expression of these molecules on Charcot-derived monocytes suggests that increased costimulatory signals were received by T-cells, which might alter the balance between regulation and inflammation and further increased bone loss. Indeed, activated T-cells are able to produce RANKL and initiate the differentiation program that leads to the formation of mature osteoclasts (21).
Unless activated, monocytes undergo apoptosis. Proinflammatory cytokines, such as IL-1β or TNF-α, protect monocytes from apoptosis (22). This suggests that monocytes recruited to a site of inflammation receive stimuli allowing for survival through activation. Inversely, when inflammation abates, the rate of monocyte apoptosis increases markedly. Thus, apoptosis represents an important homeostatic mechanism that regulates the duration and intensity of inflammation (23). As we show here, monocytes from patients with acute Charcot were significantly more resistant to apoptosis than monocytes from control subjects. Nonetheless, we cannot exclude that contaminating lymphocytes contributed to the measured apoptosis in the monocyte cultures. However, because lymphocytes survive for weeks, a major contribution from this cell type is unlikely (22,23). It is possible that this increased resistance to apoptosis, which may be related to the increased release of IL-1β and TNF-α, may contribute, at least in part, to the inability of Charcot patients to terminate the inflammation in the affected limb.
The proinflammatory alterations we have found in the phenotype of monocytes from acute Charcot patients appear to be specific to this condition. Indeed, both the phenotype of monocytes from diabetic patients with uncomplicated neuropathy and that of monocytes from diabetic patients with neuropathy and osteomyelitis-associated foot inflammation was not different from that of cells from healthy control subjects. This indicates that neither diabetes nor neuropathy or inflammation, per se, is associated with any modulation of the inflammatory response of monocytes. Interestingly, we found that all the modification of the immune phenotype of monocytes disappeared after recovery in patients with acute Charcot. This suggests that the initiating cause that triggers the inflammatory response in patients with acute Charcot acts in an environment where mechanisms that physiologically control the intensity and duration of inflammation are lacking. Calcitonin gene-related peptide (CGRP), a 37-amino acid peptide widely distributed in the central and peripheral nervous systems and mainly in sensory nerves (24), has been shown to inhibit proinflammatory cytokine production and augment the release of IL-10 by monocytes (25). Thus, local reduction of CGRP in the denervated Charcot foot may affect the activation of monocytes with increased production of proinflammatory cytokines and reduced release of anti-inflammatory cytokines.
In conclusion, these data provide evidence for a pathogenetic role of proinflammatory changes in the immune phenotype of monocyte in acute Charcot. These alterations may explain the abnormally intense and prolonged inflammatory response that characterizes this disorder and may represent a potential therapeutic target for specific pharmacological interventions.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.La Fontaine J, Harkless LB, Sylvia VL, Carnes D, Heim-Hall J, Jude E. Levels of endothelial nitric oxide synthase and calcitonin gene-related peptide in the Charcot foot: a pilot study. J Foot Ankle Surg 2008; 47: 424– 429 [DOI] [PubMed] [Google Scholar]
- 2.Mabilleau G, Petrova NL, Edmonds ME, Sabokbar A. Increased osteoclastic activity in acute Charcot's osteoarthropathy: the role of receptor activator of nuclear factor-κβ ligand. Diabetologia 2008; 51: 1035– 1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeffcoate W. Vascular calcification and osteolysis in diabetic neuropathy: is RANKL the missing link? Diabetologia 2004; 47: 1488– 1492 [DOI] [PubMed] [Google Scholar]
- 4.Jeffcoate WJ, Game F, Cavanagh PR. The role of proinflammatory cytokines in the cause of neuropathic osteoarthropathy (acute Charcot foot) in diabetes. Lancet 2005; 366: 2058– 2061 [DOI] [PubMed] [Google Scholar]
- 5.Lam J, Abu-Amer Y, Nelson CA, Fremont DH, Ross FP, Teitelbaum SL. Tumor necrosis factor superfamily cytokines and the pathogenesis of inflammatory osteolisis. Ann Rheum Dis 2002; 62( Suppl. 2): ii82– ii83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Wu HF, Ang ES, Yip K, Woloszyn M, Zheng MH, Tan RX. NF-κβ modulators in osteolytic bone diseases. Cytokine Growth Factor Rev 2009; 20: 7– 17 [DOI] [PubMed] [Google Scholar]
- 7.Hofbauer LC, Heufelder AE. The role of receptor activator of nuclear factor-κβ ligand and osteoprotegerin in the pathogenesis and treatment of metabolic bone diseases. J Clin Endocrinol Metab 2000; 85: 2355– 2363 [DOI] [PubMed] [Google Scholar]
- 8.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003; 423: 337– 342 [DOI] [PubMed] [Google Scholar]
- 9.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol 2001; 19: 23– 45 [DOI] [PubMed] [Google Scholar]
- 10.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell 1995; 80: 707– 718 [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Wilson JM. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science 1996; 273: 1862– 1864 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Mejia ME, Doseff AI. Regulation of monocytes and macrophages cell fate. Front Biosci 2009; 14: 2413– 2431 [DOI] [PubMed] [Google Scholar]
- 13.Sinistro A, Almerighi C, Ciaprini C, Natoli S, Sussarello E, Di Fino S, Calò-Carducci F, Rocchi G, Bergamini A. Downregulation of CD40 ligand response in monocytes from sepsis patients. Clin Vaccine Immunol 2008; 15: 1851– 1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiener PA, Moran-Davis P, Rankin BM, Wahl AF, Aruffo A, Hollenbaugh D. Stimulation of CD40 with purified soluble gp39 induces proinflammatory responses in human monocytes. J Immunol 1995; 155: 4917– 4925 [PubMed] [Google Scholar]
- 15.Gautam SC, Chikkala NF, Hamilton TA. Anti-inflammatory action of IL-4: negative regulation of contact sensitivity to trinitrochlorobenzene. J Immunol 1992; 148: 1411– 1415 [PubMed] [Google Scholar]
- 16.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 1991; 174: 1209– 1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chipoy C, Berreur M, Couillaud S, Pradal G, Vallette F, Colombeix C, Rédini F, Heymann D, Blanchard F. Downregulation of osteoblast markers and induction of the glial fibrillary acidic protein by oncostatin M in osteosarcoma cells require PKCdelta and STAT3. J Bone Miner Res 2004; 19: 1850– 1861 [DOI] [PubMed] [Google Scholar]
- 18.Palmqvist P, Persson E, Conaway HH, Lerner UH. IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-κβ ligand, osteoprotegerin, and receptor activator of NF-κβ in mouse calvariae. J Immunol 2002; 169: 3353– 3362 [DOI] [PubMed] [Google Scholar]
- 19.Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 2006; 55: 774– 779 [DOI] [PubMed] [Google Scholar]
- 20.Kulseng B, Skjåk-Braek G, Følling I, Espevik T. TNF production from peripheral blood mononuclear cells in diabetic patients after stimulation with alginate and lipopolysaccharide. Scand J Immunol 1996; 43: 335– 340 [DOI] [PubMed] [Google Scholar]
- 21.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999; 397: 315– 323 [DOI] [PubMed] [Google Scholar]
- 22.Mangan DF, Welch GR, Wahl SM. Lipopolysaccharide, tumor necrosis factor-α and IL-1β prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol 1991; 146: 1541– 1546 [PubMed] [Google Scholar]
- 23.McConkey DJ, Hartzell P, Amador-Pérez JF, Orrenius S, Jondal M. Calcium-dependent killing of immature thymocytes by stimulation via the CD3/T cell receptor complex. J Immunol 1989; 143: 1801– 1806 [PubMed] [Google Scholar]
- 24.Poyner DR. Calcitonin gene-related peptide: multiple actions, multiple receptors. Pharmacol Ther 1992; 56: 23– 51 [DOI] [PubMed] [Google Scholar]
- 25.Feng Y, Tang Y, Guo J, Wang X. Inhibition of LPS-induced TNF-α production by calcitonin gene-related peptide (CGRP) in cultured mouse peritoneal macrophages. Life Sci 1997; 61: 281– 287 [DOI] [PubMed] [Google Scholar]