Abstract
OBJECTIVE
Blockade of the renin-angiotensin system (RAS) plays an important role in preventing end-organ injury associated with diabetes. The recent development of direct renin inhibitors (DRIs) provides a new approach to block the RAS, but the effects of DRIs on renal and systemic vascular function in uncomplicated type 1 diabetes have not been elucidated.
RESEARCH DESIGN AND METHODS
Renal hemodynamic function (inulin and paraaminohippurate clearance), augmentation index and pulse wave velocity, endothelial dependent vasodilatation (flow-mediated dilation [FMD]), and endothelial independent vasodilatation (response to sublingual nitroglycerin) were evaluated before and after administration of aliskiren (300 mg daily for 30 days) in 10 adult subjects with uncomplicated type 1 diabetes during clamped euglycemia (4–6 mmol/l) and hyperglycemia (9–11 mmol/l).
RESULTS
In response to the DRI, plasma renin activity decreased (from 0.40 to 0.13 ng · ml−1 · h−1, P < 0.05) and plasma renin increased (from 5.2 to 75.0 ng/l, P < 0.05). Peripheral and central blood pressures decreased, and effective renal plasma flow and glomerular filtration rate increased during clamped euglycemia and hyperglycemia (P < 0.05). The carotid augmentation index during clamped euglycemia decreased (from 26 ± 6 to 20 ± 5%, P < 0.05) as did pulse wave velocity during clamped hyperglycemia (from 7.8 ± 0.6 to 6.8 ± 0.5 m/s, P < 0.05). In response to the DRI, FMD increased during both clamped euglycemia (from 1.92 ± 1.13 to 5.55 ± 0.81%) and hyperglycemia (from 1.86 ± 0.98 to 5.63 ± 0.62) as did the vasodilatory response to sublingual nitroglycerin.
CONCLUSIONS
DRIs exert a renal vasodilatory effect and improve parameters of systemic vascular function, suggesting that blockade of the RAS with this new class of agents has important functional effects in subjects with uncomplicated type 1 diabetes.
Diabetes renal complications are, in part, mediated by activation of the renin-angiotensin system (RAS), which leads to maladaptive renal and systemic hemodynamic responses including renal hyperfiltration, increased arterial stiffness, and endothelial dysfunction (1–3). More recently, a new class of RAS inhibitors, called direct renin inhibitors (DRIs), has become available. DRIs block the generation of angiotensin I from angiotensinogen, thereby preventing the generation of angiotensin II (one of the main effectors of the RAS). DRIs also mitigate the cellular effects of prorenin and renin on the prorenin receptor, thereby accounting for the prominent hemodynamic effects of DRIs in animals and humans (4). From a functional perspective, the addition of a DRI to an angiotensin receptor blocker (ARB) or ACE inhibitor (ACEI) provides additional antihypertensive effects (4). In humans with type 2 diabetes and clinical evidence of diabetic nephropathy, DRIs such as aliskiren (Rasilez; Novartis Pharmaceuticals Canada) exert antiproteinuric effects that are additive to those of ARBs, suggesting that DRIs may specifically enhance blockade of the intrarenal RAS in humans (5). Use of a DRI is therefore an attractive strategy to block the RAS in conditions in which the RAS is activated, such as diabetes, and may provide incremental protective hemodynamic effects in humans when added to ACEI or ARB therapy (4).
However, the renal and peripheral vascular hemodynamic effects of DRI monotherapy have not been studied in human subjects with uncomplicated type 1 diabetes. Accordingly, the objective of this pilot study was to examine the renal hemodynamic and peripheral vascular effects of a DRI using aliskiren in subjects with uncomplicated type 1 diabetes, when RAS activation may promote the cellular and hemodynamic changes that contribute to the development of diabetic nephropathy and vascular complications (6).
RESEARCH DESIGN AND METHODS
Five men and five women with uncomplicated type 1 diabetes participated in this pilot study (Table 1). Inclusion criteria were duration of type 1 diabetes ≥10 years, age ≥16 years, blood pressure <140/90, and no history of renal disease or macrovascular disease. No subjects had microalbuminuria (persistent urinary albumin-to-creatinine ratio >2.1 mg/mmol in men or 2.8 mg/mmol in women). Female subjects were studied during the late follicular phase of the menstrual cycle, determined by cycle day and measurement of 17β-estradiol levels. None were using oral contraceptive medications. The local research ethics boards at the University Health Network and Hospital for Sick Children (Toronto, ON, Canada) approved the protocol, and all subjects gave informed consent.
Table 1.
Baseline clinical characteristics
| n | 10 |
| Sex (male/female) | 5/5 |
| Age (years) | 43.1 ± 3.9 |
| A1C (%) | 7.93 ± 0.40 |
| Diabetes duration (years) | 23.2 ± 2.2 |
| BMI (kg/m2) | 26.01 |
| Albumin excretion rate (μg/min) | 0.233 ± 0.053 |
| 24-h Na+ intake (mmol/day) | 199 ± 25 |
| Estimated protein intake (g · kg−1 · day−1) | 1.03 ± 0.15 |
| Weight (kg) | 79 ± 5 |
Data are means ± SEM.
Subjects adhered to a high-sodium (>150 mmol/day) and moderate-protein (<1.5 g/kg/day) diet during the 7-day period before each experiment, as described previously (Table 1). Euglycemic (4–6 mmol/l) and hyperglycemic (9–11 mmol/l) conditions were maintained on two consecutive days for ∼6 h preceding and during all investigations, a period of time demonstrated to be sufficient for detection of changes in vascular function (7). In all phases of the experiment, blood glucose was maintained by a modified glucose clamp technique, as described previously (8). A 16-gauge peripheral venous cannula was inserted into the left antecubital vein for infusion of glucose and insulin and a second cannula was inserted for blood sampling more distally. Blood glucose was measured every 10–15 min, and the insulin infusion was adjusted to maintain the desired glycemic level. All experiments were performed in the same warm (25°C), temperature-controlled room and in a dark, quiet environment after 10 min of rest in the supine position. Renal and systemic vascular function studies were performed at baseline and then repeated in an identical fashion after 30 days of aliskiren therapy (300 mg orally/day).
Assessment of arterial stiffness and endothelial function
After the glucose clamp, peripheral blood pressure was measured in the right brachial artery with an automated DINAMAP sphygmomanometer (Critikon, Tampa, FL). Right carotid artery waveforms were recorded with a high-fidelity micromanometer (SPC-301; Millar Instruments), and with use of the validated transfer function, corresponding central aortic pressure waveform and central blood pressure data were generated (SphygmoCor; AtCor Medical Systems, Sydney, Australia). Mean arterial pressure and heart rate were determined using the integral software. Augmentation index, an estimate of systemic arterial stiffness, was calculated as the difference between the second systolic peak and inflection point, expressed as a percentage of the central pulse pressure corrected to an average heart rate of 75 bpm. The aortic pulse wave velocity was measured using the same device by sequentially recording electrocardiogram-gated right carotid and femoral artery waveforms. The interoperator variability and reproducibility of the augmentation index have been validated to be 0.4 ± 6.4%. Our group has published and validated the use of the SphygmoCor device (9).
Brachial artery endothelial function was determined by recording diameter changes in the brachial artery in response to increased blood flow generated during reactive hyperemia (flow-mediated dilatation [FMD]) and glyceryl trinitrate-induced dilatation (GTN). In brief, the right brachial artery was scanned 2–5 cm above the antecubital fossa using high-resolution B-mode vascular ultrasound (Vividi, 7–15 MHz linear-array transducer; GE/Vingmed, Waukesha, WI). Longitudinal, electrocardiogram-gated, end-diastolic images were acquired over six cardiac cycles, the brachial arterial diameter was determined for each image using integrated software, and the results were averaged. Diameter measurements were taken from the anterior to the posterior interface between the media and adventitia (7). After baseline images were recorded, the blood pressure cuff was inflated around the forearm distal to the elbow to >200 mmHg for 5 min. After cuff deflation, the increase in blood flow was measured (reactive hyperemia) along with the change in vessel diameter (endothelium-dependent dilatation), which was measured for a further 5 min. GTN (400 μg) was then administered sublingually, and the changes were measured over a further 5 min (endothelium-independent dilatation). FMD and GTN were defined as the maximal percent changes in vessel diameter after reactive hyperemia and administration of GTN, respectively. FMD was also reported as “FMD/flow,” defined as the maximal percent change in vessel diameter divided by percent change in flow (peak instantaneous flow − baseline instantaneous flow/baseline instantaneous flow × 100, where baseline instantaneous flow was averaged over 1 min before pressure cuff inflation and peak instantaneous flow was calculated immediately after cuff deflation), to create a stimulus-adjusted response measure (10). A single observer (D.Z.I.C.) obtained all measurements. The intraobserver variability for repeated measurements of arterial diameters at flow-mediated vasodilatation was 0.01 ± 0.0.005 mm (absolute diameter) or 0.26 ± 0.01% (percentage of the absolute value of the brachial artery at flow-mediated vasodilatation), which is similar to that reported previously (7,11).
Assessment of renal hemodynamic function
After the assessment of arterial stiffness and endothelial function, a third intravenous line was inserted into the right arm and was connected to a syringe infusion pump for administration of inulin and paraaminohippurate (PAH). After collection of blood for inulin and PAH blank, a priming infusion containing 25% inulin (60 mg/kg) and 20% PAH (8 mg/kg) was administered. Thereafter, inulin and PAH were infused continuously at a rate calculated to maintain their respective plasma concentrations constant at 20 and 1.5 mg/dl. After a 90-min equilibration period, blood was collected for inulin, PAH, and hematocrit. Blood was further collected every 30 min for 60 min for inulin and PAH, and glomerular filtration rate (GFR) and effective renal plasma flow (ERPF) were estimated by steady-state infusion of inulin and PAH, respectively (8).
Sample collection and analytical methods
Blood samples collected for inulin and PAH determinations were immediately centrifuged at 3,000 rpm for 10 min at 4°C. Plasma was separated, placed on ice, and then stored at −70°C before the assay. Inulin and PAH were measured in serum by colorimetric assays using anthrone and N-(1-naphthyl)ethylenediamine, respectively. The mean of two baseline clearance periods represent GFR and ERPF, expressed per 1.73 m2. Renal blood flow (RBF) was derived using ERPF/[1 − hematocrit] and renal vascular resistance derived by dividing the mean arterial pressure by the RBF. All renal hemodynamic measurements were adjusted for body surface area.
Active plasma renin was measured by a two-site immunoradiometric assay in which two monoclonal antibodies to human active renin are used. One antibody was coupled to biotin, and the second was radiolabeled for detection. The sample containing active renin was incubated simultaneously with both antibodies to form a complex. The radioactivity of this complex was directly proportional to the amount of immunoreactive renin present in the sample. Plasma renin activity was measured with a radioimmunoassay kit (GammaCoat Plasma Renin Activity 125I RIA Kit, CA-1533; DiaSorin, Stillwater, MN). Plasma renin activity determination involves an initial incubation of plasma to generate angiotensin I, followed by quantitation of angiotensin I by radioimmunoassay. In the GammaCoat Plasma Renin Activity 125I RIA Kit, the antibody is immobilized onto the lower inner wall of the GammaCoat tube. After incubation of standards, unknown samples, and 125I-labeled angiotensin I in the GammaCoat tube, the reaction mixture is removed by aspiration, and the bound tracer is counted in a gamma counter. A standard curve is constructed, and the concentration of angiotensin I in the unknown sample is obtained by interpolation.
The urinary albumin excretion rate was determined from three timed overnight urine collections. The urinary albumin concentration was determined by immunoturbidimetry. A1C was measured by high-performance liquid chromatography.
Statistical analysis
Changes in renal and systemic hemodynamic function in response to the DRI were assessed using a repeated-measures ANOVA. All statistical analyses were performed using the statistical package SPSS (SPSS for graduate students, version 14.0). The vascular data were obtained and analyzed by a single observer (D.Z.I.C.), who was blinded to the glycemic day and renal hemodynamic measurements.
RESULTS
Baseline clinical characteristics and hemodynamic function
At baseline, subjects were normoalbuminuric and 24-h urine collections demonstrated adherence to the prescribed sodium and protein dietary preparation. The mean serum estradiol level was 138 ± 36.5 pmol/l, consistent with the late follicular phase of the menstrual cycle. Baseline peripheral and central blood pressure profiles, renal function, arterial stiffness, and endothelial function are described in Table 2. Subjects were normotensive and had normal renal function. No subjects exhibited renal hyperfiltration (defined as GFR ≥135 ml/min per 1.73 m2) at baseline.
Table 2.
Blood pressure, arterial stiffness, and endothelial function responses to a DRI in type 1 diabetes
| Parameter | Before DRI |
After DRI |
||
|---|---|---|---|---|
| Euglycemia | Hyperglycemia | Euglycemia | Hyperglycemia | |
| Heart rate (bpm) | 63 ± 2 | 61 ± 3 | 64 ± 3 | 62 ± 2 |
| Blood pressure | ||||
| Peripheral SBP (mmHg) | 120 ± 4 | 123 ± 4 | 107 ± 3* | 111 ± 4† |
| Peripheral DBP (mmHg) | 70 ± 2 | 72 ± 2 | 62 ± 3* | 68 ± 3† |
| Renal hemodynamic function | ||||
| ERPF | 558 ± 27 | 606 ± 29 | 665 ± 24* | 704 ± 35† |
| GFR | 105 ± 3 | 117 ± 5 | 114 ± 3* | 124 ± 2† |
| Filtration fraction | 0.19 ± 0.01 | 0.20 ± 0.001 | 0.18 ± 0.01 | 0.18 ± 0.01 |
| RBF | 914 ± 58 | 984 ± 65 | 1,049 ± 59* | 1,102 ± 76† |
| Renal vascular resistance | 0.082 ± 0.013 | 0.086 ± 0.010 | 0.080 ± 0.013 | 0.078 ± 0.007 |
| Applanation tonometry | ||||
| Central SBP (mmHg) | 104 ± 3 | 109 ± 4 | 97 ± 3* | 103 ± 3† |
| Augmentation index (%) | 21.7 ± 16.6 | 22.0 ± 5.0 | 16.6 ± 4.8* | 18.7 ± 4.6 |
| Aortic PWV (m/s) | 7.4 ± 0.4 | 7.8 ± 0.6 | 6.7 ± 0.4 | 6.8 ± 0.5† |
| Endothelial function | ||||
| Flow (% change) | 74.58 ± 5.63 | 81.01 ± 7.32 | 75.54 ± 9.69 | 69.59 ± 8.19 |
| FMD (% change) | 1.92 ± 1.13 | 1.86 ± 0.98 | 5.55 ± 0.81* | 5.63 ± 0.62† |
| FMD/flow | 0.030 ± 0.017 | 0.036 ± 0.015 | 0.084 ± 0.015* | 0.094 ± 0.016† |
| GTN (% change) | 9.52 ± 2.01 | 11.22 ± 1.36 | 14.22 ± 1.49* | 15.85 ± 1.46† |
Data are means ± SEM.
*P < 0.05 vs. pre-DRI parameter during clamped euglycemia.
†P < 0.05 vs. pre-DRI parameter during clamped hyperglycemia. DBP, diastolic blood pressure; flow, % change in instantaneous flow; SBP, systolic blood pressure; PWV, pulse wave velocity.
Renal and systemic hemodynamic responses to a DRI
As expected, circulating plasma renin activity decreased (from 0.40 to 0.13 ng · ml−1 · h−1, P = 0.049) and plasma renin increased (from 5.2 to 75.0 ng/l, P = 0.016) after 30 days of DRI administration. Peripheral blood pressure decreased by 13/8 mmHg during clamped euglycemia and 12/4 mmHg during clamped hyperglycemia compared with baseline measurements (P < 0.05), whereas heart rate did not change (Table 2). DRI administration also resulted in significant declines in central blood pressure during clamped euglycemia and clamped hyperglycemia (P < 0.05). During clamped euglycemia and hyperglycemia, ERPF and GFR increased proportionately (P < 0.05), so that net filtration fraction did not change.
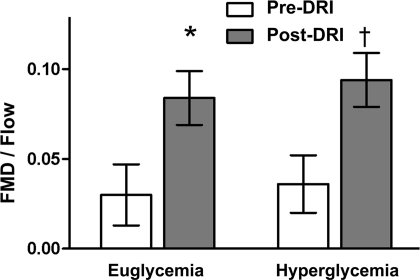
Administration of a DRI resulted in both a significant reduction in the carotid augmentation index during clamped euglycemia and a decline in pulse wave velocity during clamped hyperglycemia (all P < 0.05). The DRI also produced alterations in endothelial function. Although the flow stimulus did not change significantly with the DRI, FMD increased during both clamped euglycemia (from 1.92 ± 1.13 to 5.55 ± 0.81%) and hyperglycemia (from 1.86 ± 0.98 to 5.63 ± 0.62) (Table 2). Compared with baseline, FMD corrected for the flow stimulus (FMD/flow) and the response to GTN were also increased during clamped euglycemia and hyperglycemia (Table 2, Fig. 1).
Figure 1.
Effect of a DRI on endothelial function in type 1 diabetes (means ± SEM). *P = 0.04 vs. baseline during clamped euglycemia. †P = 0.0001 vs. baseline during clamped hyperglycemia.
CONCLUSIONS
Blockade of the RAS is a critical approach to the management of patients with type 1 diabetes (12). The objective of this pilot study was therefore to examine the renal and peripheral vascular hemodynamic effects of DRI monotherapy using aliskiren in subjects with uncomplicated type 1 diabetes. Our major findings were that aliskiren 1) decreases peripheral and central blood pressures, independent of glycemic status, 2) has a significant renal vasodilatory effect, regardless of ambient glycemia, and 3) increases arterial compliance and endothelial function as measured by FMD and GTN responsiveness.
DRIs may block the RAS through a variety of mechanisms. First, in addition to conversion from angiotensin I by ACE, angiotensin II can be generated from angiotensin I via non-ACE, chymostatin-sensitive pathways, which are not affected by ACEIs, but are blocked by DRIs (13). Second, circulating levels of angiotensin peptides, including angiotensin II, increase after ARB treatment, leading to angiotensin type 2 receptor activation and possibly to vascular proliferative effects (14). This effect is not seen with DRIs (14). Third, ACEI and ARB therapies increase circulating levels of renin and prorenin. These circulating factors bind to the (pro)renin receptor [(P)RR], increasing its catalytic activity and the generation of both angiotensin I and angiotensin II (12). In contrast, DRIs may inhibit (P)RR activation (12). Fourth, direct binding of renin and/or prorenin to the (P)RR may stimulate signaling pathways associated with cell injury, ultimately leading to an increase in the expression of profibrotic mediators by mesangial cells (12). This signaling cascade may be blocked using a DRI (12). These mechanisms of action may be important when the additive effect of a DRI to an ACEI or ARB is examined, because DRIs may mitigate compensatory pathways that limit the long-term efficacy of ACEI- or ARB-based monotherapies (4).
Our first major observation was the significant peripheral antihypertensive effect of DRI monotherapy in humans with type 1 diabetes during clamped euglycemia and hyperglycemia. ACEIs and ARBs have well-described antihypertensive effects in healthy humans and in those with type 1 diabetes (15,16). In human subjects with essential hypertension, DRI monotherapy is associated with a 12.7/9.7 mmHg reduction in blood pressure (17); the 13/8 mmHg reduction in systemic blood pressure that we observed in normotensive subjects with uncomplicated type 1 diabetes was similar to this previous report in nondiabetic subjects. Moreover, we observed a significant central blood pressure reduction after DRI therapy during clamped euglycemia and hyperglycemia. These changes in central blood pressures were similar in magnitude to the previously reported effects of ACEIs (18). Whether the effect of ACEI or ARB therapy combined with a DRI would have an additive antihypertensive effect on central blood pressure clearly requires future direct comparison studies. The antihypertensive effect that we observed during clamped hyperglycemia is of critical importance, as hyperglycemia can be a vasoconstrictive and hypertensive stimulus (19). Because patients with diabetes are frequently exposed to hyperglycemia, the ability of a DRI to control blood pressure during ambient hyperglycemic conditions is an important feature of this drug.
Our second major observation was that in subjects ingesting a carefully controlled high-sodium diet, ERPF increased by 16 and 14% during clamped euglycemia and hyperglycemia, respectively. Previous studies examining the effect of ACEIs and ARBs on renal hemodynamic function have demonstrated a wide spectrum of renal vasodilatory responses; the variability in the renal vascular response to these drugs has been attributed to the influences of sex, renal filtration status, sodium intake, ambient glycemia, and the presence or absence of diabetes (15,16,20). Fisher et al. (20) described the renal hemodynamic effects of DRIs in healthy, salt-depleted individuals. In that study, administration of 300 mg of aliskiren was associated with a 29% increase in ERPF, which was greater than the effect observed in their historical control subjects who were treated with 25 mg of captopril. We have previously observed that dietary sodium restriction can paradoxically increase renal hemodynamic effects in uncomplicated type 1 diabetes, possibly through nonhemodynamic mechanisms (21). Whether the renal hemodynamic effect of DRIs can be enhanced in diabetic subjects through the use of dietary sodium depletion is not known. Our results focus on DRI monotherapy, so future studies will be necessary to examine the additive effects of a DRI to an ACEI or ARB in this patient population.
Our third major observation was the significant effect of the DRI on arterial stiffness and endothelial function. In the present set of experiments using a typical therapeutic dose of the DRI, we observed reductions in the carotid augmentation index (23.5%) and pulse wave velocity (12.8%) that were similar to the effects of ACEIs that have been reported by others (22). In addition to effects on arterial stiffness, we observed significant positive effects on endothelial function. The effect of ACEI therapy on endothelial function is variable; in obese, nondiabetic human subjects, and in some studies involving subjects with type 1 diabetes, ACEIs had no effect on endothelial function (23,24). In hypertensive individuals, ACEIs improve flow-mediated dilatation by 30–40% (24). In the present study, we observed a 65% increase in endothelial function whether or not FMD was corrected for the flow stimulus. The DRI also improved GTN responsiveness, independent of glycemic status, suggesting that this class of agents increases nitric oxide bioavailability, similar to the effect of ACEIs. As reviewed elsewhere, the physiological impact related to the compensatory rise in plasma renin after a DRI is not known (4). Although the small sample size precludes any definitive conclusions, this pilot study suggests that the positive blood pressure and renal hemodynamic effects of DRI therapy extend to arterial stiffness and endothelial function in subjects with uncomplicated type 1 diabetes.
This pilot study has important limitations. First, the sample size was small and may have limited our ability to detect some differences in renal hemodynamic parameters (such as renal vascular resistance) and arterial stiffness (such as the carotid augmentation index during clamped hyperglycemia). We attempted to minimize the effect of the small sample size by using homogeneous study groups and by careful prestudy dietary preparation. We also decreased variability by using a study design that allowed each subject to act as his or her own control.
In summary, DRIs have significant beneficial hemodynamic effects in the renal and systemic circulations, leading to reductions in peripheral and central blood pressures, renal vasodilatation, and augmented arterial compliance and endothelial function in subjects with uncomplicated type 1 diabetes. The magnitude of these effects is similar to that seen in previous studies using ACEIs and ARBs. This work highlights the need for future physiological and clinical studies in humans that examine the effect of dual RAS blockade using a DRI and ACEI or ARB therapy.
Acknowledgments
This work was supported by a generous operating grant from the Canadian Diabetes Association (to D.Z.I.C., J.A.M., and B.Z.). D.Z.I.C. is currently supported by a Kidney Foundation of Canada Scholarship. J.W.S. is the CIHR/AMGEN Canada Kidney Research Chair at the University Health Network, University of Toronto. H.N.R. is the recipient of a KRESCENT New Investigator Award, and her work is supported by the Physicians' Services Foundation, courtesy of the physicians of Ontario.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Brooks B, Molyneaux L, Yue DK. Augmentation of central arterial pressure in type 1 diabetes. Diabetes Care 1999; 22: 1722– 1727 [DOI] [PubMed] [Google Scholar]
- 2.Lansang MC, Osei SY, Coletti C, Krupinski J, Hollenberg NK. Hyperglycaemia-induced intrarenal RAS activation: the contribution of metabolic pathways. J Renin Angiotensin Aldosterone Syst 2002; 3: 19– 23 [DOI] [PubMed] [Google Scholar]
- 3.Arcaro G, Zenere BM, Saggiani F, Zenti MG, Monauni T, Lechi A, Muggeo M, Bonadonna RC. ACE inhibitors improve endothelial function in type 1 diabetic patients with normal arterial pressure and microalbuminuria. Diabetes Care 1999; 22: 1536– 1542 [DOI] [PubMed] [Google Scholar]
- 4.Brown MJ. Aliskiren. Circulation 2008; 118: 773– 784 [DOI] [PubMed] [Google Scholar]
- 5.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK, AVOID Study Investigators Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 2008; 358: 2433– 2446 [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int 1996; 49: 1774– 1777 [DOI] [PubMed] [Google Scholar]
- 7.Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, Kugiyama K, Ogawa H, Yasue H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol 1999; 34: 146– 154 [DOI] [PubMed] [Google Scholar]
- 8.Cherney DZ, Miller JA, Scholey JW, Bradley TJ, Slorach C, Curtis JR, Dekker MG, Nasrallah R, Hébert RL, Sochett EB. The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with type 1 diabetes. Diabetes 2008; 57: 688– 695 [DOI] [PubMed] [Google Scholar]
- 9.Mustata S, Chan C, Lai V, Miller JA. Impact of an exercise program on arterial stiffness and insulin resistance in hemodialysis patients. J Am Soc Nephrol 2004; 15: 2713– 2718 [DOI] [PubMed] [Google Scholar]
- 10.Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Normalization of flow-mediated dilation to shear stress area under the curve eliminates the impact of variable hyperemic stimulus. Cardiovasc Ultrasound 2008; 6: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchi Y. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation 1995; 92: 3431– 3435 [DOI] [PubMed] [Google Scholar]
- 12.Nguyen G, Danser AH. Prorenin and (pro)renin receptor: a review of available data from in vitro studies and experimental models in rodents. Exp Physiol 2008; 93: 557– 563 [DOI] [PubMed] [Google Scholar]
- 13.Sepehrdad R, Frishman WH, Stier CT, Jr, Sica DA. Direct inhibition of renin as a cardiovascular pharmacotherapy: focus on aliskiren. Cardiol Rev 2007; 15: 242– 256 [DOI] [PubMed] [Google Scholar]
- 14.Plovsing RR, Wamberg C, Sandgaard NC, Simonsen JA, Holstein-Rathlou NH, Hoilund-Carlsen PF, Bie P. Effects of truncated angiotensins in humans after double blockade of the renin system. Am J Physiol Regul Integr Comp Physiol 2003; 285: R981– R991 [DOI] [PubMed] [Google Scholar]
- 15.Miller JA, Cherney DZ, Duncan JA, Lai V, Burns KD, Kennedy CR, Zimpelmann J, Gao W, Cattran DC, Scholey JW. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol 2006; 17: 2554– 2560 [DOI] [PubMed] [Google Scholar]
- 16.Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol 2006; 17: 1703– 1709 [DOI] [PubMed] [Google Scholar]
- 17.Pool JL, Schmieder RE, Azizi M, Aldigier JC, Januszewicz A, Zidek W, Chiang Y, Satlin A. Aliskiren, an orally effective renin inhibitor, provides antihypertensive efficacy alone and in combination with valsartan. Am J Hypertens 2007; 20: 11– 20 [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF, Izzo JL, Jr, Lacourcière Y, Ouellet JP, Neutel J, Qian C, Kerwin LJ, Block AJ, Pfeffer MA. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation 2002; 105: 2955– 2961 [DOI] [PubMed] [Google Scholar]
- 19.Miller JA. Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J Am Soc Nephrol 1999; 10: 1778– 1785 [DOI] [PubMed] [Google Scholar]
- 20.Fisher ND, Jan Danser AH, Nussberger J, Dole WP, Hollenberg NK. Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation 2008; 117: 3199– 3205 [DOI] [PubMed] [Google Scholar]
- 21.Miller JA. Renal responses to sodium restriction in patients with early diabetes mellitus. J Am Soc Nephrol 1997; 8: 749– 755 [DOI] [PubMed] [Google Scholar]
- 22.Manolis AJ, Iraklianou S, Pittaras A, Zaris M, Tsioufis K, Psaltiras G, Psomali D, Foussas S, Gavras I, Gavras H. Arterial compliance changes in diabetic normotensive patients after angiotensin-converting enzyme inhibition therapy. Am J Hypertens 2005; 18: 18– 22 [DOI] [PubMed] [Google Scholar]
- 23.Mullen MJ, Clarkson P, Donald AE, Thomson H, Thorne SA, Powe AJ, Furuno T, Bull T, Deanfield JE. Effect of enalapril on endothelial function in young insulin-dependent diabetic patients: a randomized, double-blind study. J Am Coll Cardiol 1998; 31: 1330– 1335 [DOI] [PubMed] [Google Scholar]
- 24.Schalkwijk CG, Smulders RA, Lambert J, Donker AJ, Stehouwer CD: ACE-inhibition modulates some endothelial functions in healthy subjects and in normotensive type 1 diabetic patients. Eur J Clin Invest 2000; 30: 853– 860 [DOI] [PubMed] [Google Scholar]