Abstract
OBJECTIVE
Familial predisposition to hypertension has been associated with the development of diabetic nephropathy in adults, but there are limited data in adolescents. Our aim was to assess whether parental ambulatory blood pressure (ABP) was associated with ABP and albumin excretion in young offspring with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Twenty-four-hour ABP monitoring was performed in 509 young offspring (mean ± SD age 15.8 ± 2.3 years) with type 1 diabetes, 311 fathers, and 444 mothers. Systolic (SBP) and diastolic blood pressure (DBP) measurements during 24 h, daytime, and nighttime were calculated. Three early morning urinary albumin-to-creatinine ratios (ACRs), A1C, and anthropometric parameters were available for the offspring.
RESULTS
All paternal ABP parameters, except for nighttime SBP, were independently related to the offspring's ABP (24-h SBP β = 0.18, 24-h DBP β = 0.22, daytime SBP β = 0.25, daytime DBP β = 0.23, and nighttime DBP β = 0.18; all P < 0.01). Maternal 24-h DBP (β = 0.19, P = 0.004), daytime DBP (β = 0.09, P = 0.04), and nighttime SBP (β = 0.24 P = 0.001) were related to the corresponding ABP parameter in the offspring. Significant associations were found between the offspring's logACR and maternal ABP. The association with 24-h DBP (β = 0.16, P = 0.02), daytime DBP (β = 0.16 P = 0.02), and nighttime DBP (β = 0.15 P = 0.03) persisted even after adjustment for the offspring's ABP. Mothers of offspring with microalbuminuria had higher ABP than mothers of offspring without microalbuminuria (all P < 0.05).
CONCLUSIONS
In this cohort, parental ABP significantly influenced offspring blood pressure, therefore confirming familial influences on this trait. In addition, maternal ABP, particularly DBP, was closely related to ACR in the offspring, suggesting a dominant effect of maternal genes or an effect of the intrauterine environment on microalbuminuria risk.
Microalbuminuria remains the best predictive marker for the development of overt diabetic nephropathy and represents an independent risk factor for cardiovascular disease (CVD) (1). Evidence indicating that the risk for the development of microalbuminuria and diabetic nephropathy is partly genetic and may relate to the inheritance of genes associated with CVD is accumulating (2). Several studies have shown familial aggregation of renal disease in type 1 diabetes (2,3), and a family history of hypertension, dyslipidemia, insulin resistance, type 2 diabetes, or a cluster of these cardiovascular risk factors has been associated with an increased risk of diabetic nephropathy (2,4).
In particular, several lines of evidence have highlighted the fact that predisposition to hypertension might be a risk factor for the development and progression of diabetic nephropathy in individuals with type 1 diabetes (4–8), and, therefore, the inheritance of blood pressure–related genes might also contribute to abnormal albumin excretion and renal damage. Parental hypertension has been associated with changes in renal hemodynamics (9) and with the development of diabetic nephropathy in the offspring with diabetes (5–8). However, the relationship between family history of hypertension and albuminuria has not been confirmed in all studies (10) and in the majority of studies, confirmation has been based on a single blood pressure assessment in the parents (7,8) or on a questionnaire-based history of parental hypertension. In addition, the effect of parental blood pressure, as was that of other heritable factors, has been mainly investigated in adults with diabetes, whereas it has been seldom studied in children and adolescents (11,12).
Understanding the role of such a familial/genetic effect of blood pressure on renal disease would be particularly important in adolescents with type 1 diabetes, who represent a vulnerable group at risk of vascular complications (13). In particular, the identification of familial/genetic factors predisposing to diabetic nephropathy and the understanding of their interplay with glycemic control and the hormonal and metabolic changes of puberty could help in identifying subjects at higher risk for diabetes complications, who therefore require more intensive treatment to prevent them. The aim of the present study was to assess whether parental ambulatory blood pressure (ABP) was related to variations in the same trait and in albumin excretion rates in young offspring with childhood-onset type 1 diabetes.
RESEARCH DESIGN AND METHODS
The study population was composed of young people with type 1 diabetes and their parents recruited in the Nephropathy Family Study (NFS). The NFS was set up in 2000 as part of the Juvenile Diabetes Research Foundation/Wellcome Trust Diabetes Inflammation Laboratory: Genetic Resource Investigating Diabetes (GRID) study (14). Between 2000 and 2005, 1,066 subjects, aged 10–16 years, who had developed type 1 diabetes before the age of 16 years, were recruited throughout four English regions (East Anglia, Birmingham, Bristol, and Oxford) for detailed phenotypic and genetic study of the early pathogenesis of microalbuminuria. Subjects with insulin-treated diabetes due to other pathological conditions were excluded. Similarly, children with chronic renal disease or other chronic diseases, which are likely to affect renal function, were excluded. The median duration of follow-up is currently 2.6 (interquartile range 1.8–3.8) years.
The longitudinal study schedule comprised annual collection of three consecutive early-morning urine specimens for centralized measurement of the albumin-to-creatinine ratio (ACR) and blood samples for measurements of A1C. Annual assessments also included measurements of height and weight. Parents of the NFS children were also recruited, and they underwent clinical assessment, including height and weight.
Twenty-four-hour ambulatory blood pressure monitoring (ABPM) was performed in a subgroup of offspring and parents from the NFS. Specifically, it was done in 509 children, 311 fathers, and 444 mothers, for a total of 250 complete trios.
Ethics approval was obtained from the regional ethics committee, with written consent from the parents and assent from the children.
24-h ABPM
Each child and parent was fitted with a portable noninvasive oscillometric recorder (Diasys Integra II, Novacor, Rueil-Malmaison, France). The validity of measurements with this monitor was confirmed previously (15). An appropriate cuff size was fitted on the basis of the arm circumference of each subject and applied on the nondominant arm. ABPM was performed during a normal day with typical activities, but subjects were asked to avoid vigorous exercises and to keep their arm relaxed during each daytime inflation. A measurement was automatically repeated if values were outside the following intervals of measurement validity: systolic blood pressure (SBP) <50 mmHg; diastolic blood pressure (DBP) <30 and >150 mmHg; heart rate <35 and >250 bpm; pulse pressure <10 and >150 mmHg at SBP <120 mmHg; and pulse pressure <15 and >150 mmHg at SBP >120 mmHg. Furthermore, all subjects were asked to record in a diary the time they went to bed and the time they awoke, as well as exercise periods and quality of their sleep.
Blood pressure readings were obtained at 30-min intervals during the daytime (0700–2200) and at 60-min intervals during the nighttime (2200–0700). At the end of the ABPM, the monitor was downloaded to a personal computer equipped with DiasySoft software for analysis of the measurements. All readings taken during 24 h were used to calculate mean 24-h SBP and DBP whereas mean daytime and nighttime SBP and DBP were calculated based on the awake and asleep periods, calculated from diary times. To characterize the circadian blood pressure rhythm, the percentage of the nocturnal fall in SBP and DBP was calculated using the formula daytime SBP [or DBP] − nighttime SBP [or DBP]/daytime SBP [or DBP] × 100. Patients were classified as dippers if their daytime SBP and/or DBP decreased by at least 10% during the night; all other subjects were classified as nondippers.
Hypertension in the parents was defined as current use of antihypertensive medication or an elevated ABP, defined as 24-h blood pressure >135/85 mmHg (15). Hypertensive fathers and mothers were excluded from all analyses, apart from those assessing prevalence of hypertension.
Albumin and creatinine
All urine samples were assessed centrally in a single reference laboratory. Samples were stored at −70°C before analysis. Albumin was measured centrally by a double-antibody enzyme-linked immunosorbent assay method. The within- and between-assay coefficients of variation (CV) were 6 and 12%, respectively. Creatinine was initially measured using a modified Jaffe method (Unimate 7; Roche Diagnostic Systems, Basel, Switzerland) on a Cobas Mira (Roche Diagnostic Systems) automated spectrophotometer (CV 2% at 2.2 mmol/l) and, more recently, by stable isotope dilution electrospray mass spectrometry-mass spectrometry (14).
A1C
All samples were analyzed centrally in Cambridge on a TOSOH G7 analyzer (Tosoh Bioscience, Redditch, U.K.), using high-performance liquid chromatography and absorbance change detection and Diabetes Control and Complications Trial–aligned methods. The normal range was 4.9–6.3%, and the CVs were 4.8 and 6.6% at A1C levels of 5.5 and 10.1%, respectively.
Calculations
ACR was summarized as the geometric mean of three consecutive early morning urine samples and log-transformed for analyses. Microalbuminuria was defined as an ACR between 3.5 and 35 mg/mmol in male patients and between 4.0 and 47 mg/mmol in female patients in two of three consecutive early morning urine collections during an annual assessment (corresponding to an overnight albumin excretion rate between 20 and 200 μg/min) (13). The mean of all A1C measurements for the offspring collected from the time of recruitment in the study until the last visit was calculated and included in the analyses.
Statistical analyses
Analyses were performed using SPSS for Windows (version 16.0; SPSS, Chicago, IL). Data are summarized as means ± SD or median (interquartile range) unless otherwise specified. Nonnormally distributed variables, such as ACR, were log-transformed before analysis. Age and sex adjustments for blood pressure were performed by calculating residuals. Comparisons between different groups were performed by unpaired t tests, whereas comparisons across categories were made using χ2 or Fisher exact tests. Multiple regression analysis was applied to test the independent association between parental ABP and offspring ABP and albumin excretion, with adjustment for other confounding variables. For this analysis, data are expressed as the regression coefficient β. P < 0.05 was taken as significant for all analyses.
RESULTS
ABPM was performed in 509 children, 311 fathers, and 444 mothers, for a total of 295 matched father-offspring pairs, 411 matched mother-offspring pairs, and 250 complete trios. All analyses were confined to the 250 families for whom complete trio data were available. Offspring and parental characteristics and ABPM data are reported in supplementary Table 1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-1152/DC1).
Offspring
ABPM was performed in 509 young offspring (278 female and 231 male) with type 1 diabetes. Their mean ± SD age at the ABPM assessment was 15.8 ± 2.3 years, diabetes duration was 6.4 ± 3.9 years, and A1C was 9.3 ± 1.6%.
Fathers
The fathers' age was 46.9 ± 5.9 years (median 46.3 [range 33.0–67.0] years). Paternal ABPM data are reported in supplementary Table 1. Ninety-two fathers (29.6%) were hypertensive, based on ABPM results or current treatment with antihypertensive drugs.
Mothers
The mothers' age was 44.7 ± 5.3 (median 44.2 [range 31.5–60.3] years. Maternal ABPM data are reported in supplementary Table 1. Of the mothers, 65 (14.6%) were hypertensive.
Associations between parental and offspring ABPM parameters
Significant independent associations were found among the patients' ABP and their mothers' and fathers' corresponding ABPM parameters, adjusted for age. Specifically, after adjustment for the offspring's age, sex, A1C, and BMI SD score, all paternal ABPM parameters, except for nighttime SBP, were independently related to the offspring's corresponding ABPM parameters (Table 1). In contrast, only maternal 24-h DBP, daytime DBP, and nighttime SBP were independently related to the offspring's corresponding ABPM parameter (Table 1).
Table 1.
Multiple regression analysis: independent effect of parental ABP on the offspring's ABP
| Fathers |
Mothers |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 1: R2* | Model 2† |
Model 1: R2* | Model 2† |
|||||
| R2 | β | P value | R2 | β | P value | |||
| 24-h SBP | 0.115 | 0.149 | 0.18 | 0.005 | 0.095 | 0.112 | 0.13 | 0.05 |
| 24-h DBP | 0.119 | 0.166 | 0.22 | 0.001 | 0.105 | 0.143 | 0.19 | 0.004 |
| Daytime SBP | 0.073 | 0.134 | 0.25 | <0.001 | 0.070 | 0.098 | 0.06 | 0.2 |
| Daytime DBP | 0.055 | 0.106 | 0.23 | 0.001 | 0.055 | 0.083 | 0.09 | 0.04 |
| Nighttime SBP | 0.114 | 0.115 | 0.04 | 0.5 | 0.083 | 0.139 | 0.24 | 0.001 |
| Nighttime DBP | 0.136 | 0.170 | 0.18 | 0.005 | 0.114 | 0.131 | 0.13 | 0.05 |
Dependent variable: offspring's ABP.
*Model 1: independent variables are child's age, sex, A1C, and BMI SD score.
†Model 2: as for model 1 plus parental ABP parameter.
Relationship between parental ABP and offspring's ACR
Potential associations between parental ABP and the offspring's albumin excretion were assessed. All maternal ABP parameters were significantly associated with logACR in the offspring (Table 2, model 1). These associations remained significant even after adjustment of ACR for age, sex, and A1C (model 2). In contrast, after adjustment for the corresponding ABP parameter in the offspring (model 3), 24-h, daytime, and nighttime SBPs were no longer significantly associated with logACR, whereas diastolic ABP parameters were still independently associated with ACR. In contrast, there was no significant association between any paternal ABPM parameter and the offspring ACR (Table 2).
Table 2.
Associations between parental ABP and offspring's ACR
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| β | P value | β | P value | β | P value | |
| Mothers | ||||||
| 24-h SBP | 0.15 | 0.01 | 0.15 | 0.03 | 0.13 | 0.05 |
| 24-h DBP | 0.17 | 0.01 | 0.17 | 0.01 | 0.16 | 0.02 |
| Daytime SBP | 0.15 | 0.02 | 0.14 | 0.03 | 0.12 | 0.05 |
| Daytime DBP | 0.16 | 0.01 | 0.16 | 0.01 | 0.16 | 0.02 |
| Nighttime SBP | 0.16 | 0.02 | 0.14 | 0.04 | 0.11 | 0.1 |
| Nighttime DBP | 0.17 | 0.01 | 0.16 | 0.02 | 0.15 | 0.03 |
| Fathers | ||||||
| 24-h SBP | 0.13 | 0.06 | 0.01 | 0.08 | 0.08 | 0.2 |
| 24-h DBP | 0.11 | 0.1 | 0.10 | 0.1 | 0.07 | 0.3 |
| Daytime SBP | 0.13 | 0.05 | 0.12 | 0.07 | 0.08 | 0.3 |
| Daytime DBP | 0.08 | 0.2 | 0.08 | 0.2 | 0.05 | 0.4 |
| Nighttime SBP | 0.13 | 0.06 | 0.11 | 0.1 | 0.10 | 0.1 |
| Nighttime DBP | 0.13 | 0.06 | 0.13 | 0.1 | 0.11 | 0.1 |
Data are regression coefficients β and P values. Regression models with logACR as the dependent variable. Three models were generated: model 1: maternal (or paternal) ABP parameter as an independent variable; model 2: model 1 plus offspring's age, sex, and mean A1C; and model 3: model 2 plus offspring's corresponding ABP parameter.
Parents' ABP and offspring's microalbuminuria status
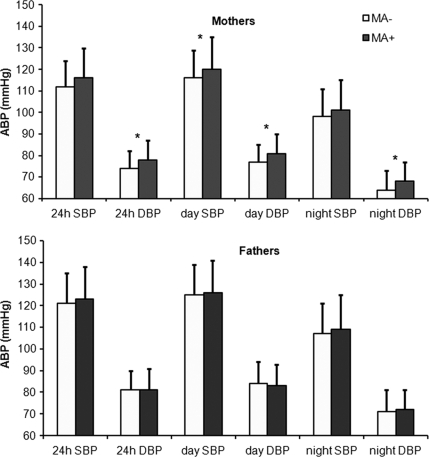
The association between parental blood pressure and ACR in the offspring was further investigated by comparing parents of offspring who developed microalbuminuria (both persistent and transient cases of microalbuminuria were considered) during the study period with parents of offspring who remained normoalbuminuric. Specifically, 38 fathers of patients with microalbuminuria (MA+) were compared with the remaining 212 fathers of patients with normoalbuminuria (MA−). Fathers of MA+ patients were similar in age, height, weight, and BMI compared with fathers of MA− patients (supplementary Table 2). There was no significant difference in any ABPM parameter between the two groups (Fig. 1). Similarly, the nocturnal fall in blood pressure (SBP fall [MA+ vs. MA− group] 15.2 ± 7.6 vs. 14.3 ± 7.3%, P = 0.4; DBP fall 15.2 ± 8.2 vs. 16.3 ± 8.4%, P = 0.4) and the percentage of hypertensive fathers was not different between the two groups (MA+ vs. MA− group 41.7 vs. 30.9%, P = 0.4).
Figure 1.
ABP in parents of MA+ and MA− offspring. Bars show means ± SD. *P < 0.01 for the comparison between mothers of MA+ vs. MA− offspring.
Mothers of MA+ patients were similar in age, height, weight, and BMI compared with mothers of MA− patients (supplementary Table 2). When we compared ABPM parameters of mothers of MA+ patients with those of mothers of MA− patients, we found that 24-h DBP, daytime SBP, daytime DBP, and nighttime DBP were significantly higher in mothers of MA+ than in mothers of MA− patients (Fig. 1). In addition, the percentage of hypertensive mothers was higher in the MA+ than in the MA− group (34.3 vs. 11.2%, P = 0.004). There was no difference in the nocturnal fall in blood pressure between the two groups (SBP fall [MA+ vs. MA− group] 14.0 ± 7.1 vs. 14.8 ± 7.0%, P = 0.5; DBP fall 13.2 ± 6.7 vs. 14.1 ± 8.1%, P = 0.5).
CONCLUSIONS
In the present study we found a significant association of both paternal and maternal ABP with the same trait in young offspring with type 1 diabetes. In addition, we found that maternal ABP was independently related to the offspring's albumin excretion.
Several lines of evidence have shown that blood pressure is a genetically determined trait and an heritability of ∼30–40% has been reported, even though, so far, no consistent genetic determinants have been identified (16). In line with previous studies (17), we found that parental blood pressure significantly influenced the same trait in the offspring, therefore supporting a potential genetic influence on blood pressure in young people with diabetes.
A predominant effect of paternal blood pressure in influencing the same trait in the offspring emerged from the present study. This result is similar to previous data showing that parental blood pressure was a significant determinant of blood pressure in young offspring with type 1 diabetes, with a stronger effect of paternal SBP on the offspring's SBP, whereas the contribution of maternal and paternal DBP on the offspring's DBP was similar (17).
Interestingly, we found significant associations between maternal ABP and ACR and the development of microalbuminuria in the offspring, whereas no relationship was found between paternal ABP and ACR in the offspring. Few previous studies (2,18–20) have assessed maternal and paternal blood pressure effects separately, mainly because the majority of them have been underpowered for this kind of analysis. A stronger association of a maternal history of hypertension with diabetic nephropathy, as in our study, was previously found in adult offspring with type 1 diabetes (2), even though the risk was significantly higher when both parents were hypertensive. In a cohort of children and adolescents with type 1 diabetes, Lévy-Marchal et al. (20), found an independent effect of maternal hypertension on the risk of microalbuminuria in the offspring. The stronger association we found between maternal ABP and ACR in the offspring might be related to a dominant influence of maternal genes. Mitochondria-specific genes could be potential candidates (16), but imprinted genes, in which only the maternal allele is expressed, might be also implicated in the predisposition to microalbuminuria in the offspring.
Whereas familial predisposition to hypertension may indicate a genetic effect, the potential contribution of environmental and behavioral components also needs to be considered (21,22). The effects of the intrauterine environment might contribute to the stronger influence of maternal blood pressure on albumin excretion (21). However, a greater postnatal sharing of environmental factors between mothers and offspring than between fathers and offspring might also explain the stronger maternal influence (22).
Parental blood pressure could contribute to the risk of diabetic nephropathy in offspring through an effect on the offspring's blood pressure and/or through other mechanisms (4). However, previous studies have not always included adjustments for offspring blood pressure. Where these adjustments were made, as in the EURODIAB study (4), the association between parental hypertension and albuminuria in the offspring was attenuated and lost statistical significance, suggesting that most of the influence of parental blood pressure is mediated through an effect on the offspring's blood pressure. In the present study, we also found that after adding the offspring's blood pressure into the regression models, there was an attenuation of the effect of some maternal blood pressure parameters on albumin excretion, but the majority of them and in particular diastolic parameters were still significantly associated with the outcome. In contrast to the EURODIAB study (4), in which only a family history of hypertension was considered, we performed a direct assessment of blood pressure, which was included as a continuous variable in our regression models, and this could explain, at least in part, our different finding.
One of the strengths of the present study was that a direct assessment of 24-h blood pressure was performed in the parents, instead of a collection of a self-reported history of hypertension, which was used in the majority of previous studies (2,4,7,11). A recent study assessing the heritability of blood pressure has shown that ABP is more accurate and sensitive than office blood pressure in capturing the heritable part of this trait (23). Office blood pressure is based only on a single assessment, which is affected by environmental factors and does not reflect the physiological daily variability in blood pressure (23).
In the present study we considered albumin excretion, adjusted as a quantitative trait, as the main outcome variable, and the presence of microalbuminuria as a secondary outcome, to avoid potential misclassifications due to cases of transient microalbuminuria, which can be very common, particularly in younger populations (13). The use of albumin excretion as a continuous variable is supported by recent evidence that progressive increases in albumin excretion, even within the normal range, are detectable soon after diagnosis and represent a strong predictor for the development of microalbuminuria (24). In addition, we have recently reported that increases in blood pressure parallel rises in albumin excretion, even before the onset of microalbuminuria in young people with type 1 diabetes (14). Furthermore, ACR adjusted for other factors known to influence it, such as age, sex, duration of diabetes, and A1C (25), provides a more robust estimate of the independent effects of familial blood pressure on this quantitative trait. However, a weakness of our study is that we do not have long-term outcomes related to the establishment of advanced stages of diabetic nephropathy.
The present study is one of the few reports on the role of parental blood pressure on albumin excretion in a cohort of young offspring with type 1 diabetes. Previously, the effect of familial factors on the risk of developing diabetes complications in the offspring has been assessed mainly in adults, with only a few investigations including young people with diabetes. Rudberg et al. (11) found that a family history of hypertension and CVD was associated with an increased prevalence of microalbuminuria in a cohort of 300 offspring with type 1 diabetes. In an early report from the Oxford Regional Prospective Study, there was no effect of parental absolute ABP values or of parental hypertension on albumin excretion in young offspring with type 1 diabetes (12). The different findings of the latter study compared with those of the present study might be related to differences in the ages of the populations studied or differences in the ACR range between the two studies.
In our study, as in previous studies (4), the association between parental blood pressure and albumin excretion in the offspring was weak, suggesting that, even though there is an effect of parental blood pressure, this is likely to be just one of several factors implicated in the complex pathogenesis of diabetic nephropathy. This assumption is supported by the observation that the presence of a clustering of several CVD risk factors in the parents has a stronger effect on the risk for diabetic nephropathy in the offspring than the heritability of a single factor, such as hypertension alone (2).
A stronger effect of parental blood pressure emerged from studies in which the offspring had a more advanced stage of diabetic nephropathy, characterized by clinical proteinuria (7). Therefore, it could be argued that the effect of parental blood pressure might become more evident with further follow-up of our cohort and when more individuals with persistent microalbuminuria or macroalbuminuria are identified.
In summary, in the present study we found that in a cohort of young people with childhood-onset type 1 diabetes, parental ABP significantly influenced the offspring's blood pressure, therefore confirming a familial influence on this trait. Of particular interest was the finding that maternal, but not paternal, ABP was closely related to albumin excretion and the presence of microalbuminuria in the offspring, suggesting a dominant effect of maternal genes or an effect of the intrauterine environment on the offspring's risk of developing diabetic nephropathy.
These findings underline the importance of considering familial factors, in particular blood pressure, when one is estimating the renal risk in young offspring with diabetes. The identification of familial risk factors predisposing to nephropathy could help in identifying subjects at higher risk for diabetes complications, who might require more intensive treatments, including antihypertensive drugs, to prevent diabetic micro- and macrovascular complications.
Supplementary Material
Acknowledgments
The NFS is funded by the Juvenile Diabetes Research Foundation (JDRF), Wellcome Trust, and Diabetes U.K. M.L.M. is the recipient of a European Society for Pediatric Endocrinology (ESPE) research fellowship (sponsored by Novo Nordisk A/S) and an ESPE Visiting Scholarship.
No other potential conflicts of interest relevant to this article were reported.
We acknowledge the study field workers, pediatricians, physicians, and diabetes nurse specialists involved in the NFS, JDRF, Wellcome Trust, the National Institute for Health Research Cambridge Comprehensive Biomedical Research Centre and Novacor U.K. We thank John Todd and Jason Cooper from the JDRF/Wellcome Trust Diabetes and Inflammation Laboratory, Cambridge Institute for Medical Research, University of Cambridge, Cambridge, U.K.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Rossing P, Hougaard P, Borch-Johnsen K, Parving HH. Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ 1996; 313: 779– 784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorn LM, Forsblom C, Fagerudd J, Pettersson-Fernholm K, Kilpikari R, Groop PHFinnDiane Study Group. Clustering of risk factors in parents of patients with type 1 diabetes and nephropathy. Diabetes Care 2007; 30: 1162– 1167 [DOI] [PubMed] [Google Scholar]
- 3.Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med 1989; 320: 1161– 1165 [DOI] [PubMed] [Google Scholar]
- 4.Roglic G, Colhoun HM, Stevens LK, Lemkes HH, Manes C, Fuller JH. Parental history of hypertension and parental history of diabetes and microvascular complications in insulin-dependent diabetes mellitus: the EURODIAB IDDM Complications Study. Diabet Med 1998; 15: 418– 426 [DOI] [PubMed] [Google Scholar]
- 5.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1: 1430– 1432 [DOI] [PubMed] [Google Scholar]
- 6.Barzilay J, Warram JH, Bak M, Laffel LM, Canessa M, Krolewski AS. Predisposition to hypertension: risk factor for nephropathy and hypertension in IDDM. Kidney Int 1992; 41: 723– 730 [DOI] [PubMed] [Google Scholar]
- 7.Krolewski AS, Canessa M, Warram JH, Laffel LM, Christlieb AR, Knowler WC, Rand LI. Predisposition to hypertension and susceptibility to renal disease in insulin-dependent diabetes mellitus. N Engl J Med 1988; 318: 140– 145 [DOI] [PubMed] [Google Scholar]
- 8.Fagerudd JA, Tarnow L, Jacobsen P, Stenman S, Nielsen FS, Pettersson-Fernholm KJ, Grönhagen-Riska C, Parving HH, Groop PH. Predisposition to essential hypertension and development of diabetic nephropathy in IDDM patients. Diabetes 1998; 47: 439– 444 [DOI] [PubMed] [Google Scholar]
- 9.Hannedouche TP, Marques LP, Natov S, Delgado AG, Boitard C, Lacour B, Grünfeld JP. Renal abnormalities in normotensive insulin-dependent diabetic offspring of hypertensive parents. Hypertension 1992; 19: 378– 384 [DOI] [PubMed] [Google Scholar]
- 10.Jensen JS, Mathiesen ER, Nørgaard K, Hommel E, Borch-Johnsen K, Funder J, Brahm J, Parving HH, Deckert T. Increased blood pressure and erythrocyte sodium/lithium countertransport activity are not inherited in diabetic nephropathy. Diabetologia 1990; 33: 619– 624 [DOI] [PubMed] [Google Scholar]
- 11.Rudberg S, Stattin EL, Dahlquist G. Familial and perinatal risk factors for micro- and macroalbuminuria in young IDDM patients. Diabetes 1998; 47: 1121– 1126 [DOI] [PubMed] [Google Scholar]
- 12.Schultz CJ, Dalton RN, Selwood M, Dunger DB, Neil HAOxford Regional Prospective Study Group. Paternal phenotype is associated with microalbuminuria in young adults with type 1 diabetes mellitus of short duration. Diabet Med 2004; 21: 246– 251 [DOI] [PubMed] [Google Scholar]
- 13.Amin R, Widmer B, Prevost AT, Schwarze P, Cooper J, Edge J, Marcovecchio L, Neil A, Dalton RN, Dunger DB. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ 2008; 336: 697– 701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcovecchio ML, Dalton RN, Schwarze CP, Prevost AT, Neil HA, Acerini CL, Barrett T, Cooper JD, Edge J, Shield J, Widmer B, Todd JA, Dunger DB. Ambulatory blood pressure measurements are related to albumin excretion and are predictive for risk of microalbuminuria in young people with type 1 diabetes. Diabetologia 2009; 52: 1173– 1181 [DOI] [PubMed] [Google Scholar]
- 15.O'Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Staessen J, Stergiou G, Verdecchia PEuropean Society of Hypertension Working Group on Blood Pressure Monitoring. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens 2005; 23: 697– 701 [DOI] [PubMed] [Google Scholar]
- 16.Binder A. A review of the genetics of essential hypertension. Curr Opin Cardiol 2007; 22: 176– 184 [DOI] [PubMed] [Google Scholar]
- 17.Tarn AC, Thomas JM, Drury PL. Correlates of blood pressure in young insulin-dependent diabetics and their families. J Hypertens 1990; 8: 795– 803 [DOI] [PubMed] [Google Scholar]
- 18.Hadjadj S, Duengler F, Torremocha F, Faure-Gerard G, Bridoux F, Boissonnot M, Mauco G, Guilhot J, Maréchaud R. Maternal history of type 2 diabetes is associated with diabetic nephropathy in type 1 diabetic patients. Diabetes Metab 2007; 33: 37– 43 [DOI] [PubMed] [Google Scholar]
- 19.Wada K, Tamakoshi K, Yatsuya H, Otsuka R, Murata C, Zhang H, Takefuji S, Matsushita K, Sugiura K, Toyoshima H. Association between parental histories of hypertension, diabetes and dyslipidemia and the clustering of these disorders in offspring. Prev Med 2006; 42: 358– 363 [DOI] [PubMed] [Google Scholar]
- 20.Lévy-Marchal C, Sahler C, Cahané M, Czernichow PGECER Study Group. Risk factors for microalbuminuria in children and adolescents with type 1 diabetes. J Pediatr Endocrinol Metab 2000; 13: 613– 620 [DOI] [PubMed] [Google Scholar]
- 21.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008; 359: 61– 73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveria SA, Ellison RC, Moore LL, Gillman MW, Garrahie EJ, Singer MR. Parent-child relationships in nutrient intake: the Framingham Children's Study. Am J Clin Nutr 1992; 56: 593– 598 [DOI] [PubMed] [Google Scholar]
- 23.Fava C, Burri P, Almgren P, Groop L, Hulthén UL, Melander O. Heritability of ambulatory and office blood pressure phenotypes in Swedish families. J Hypertens 2004; 22: 1717– 1721 [DOI] [PubMed] [Google Scholar]
- 24.Schultz CJ, Neil HA, Dalton RN, Dunger DBOxforn Regional Prospective Study Group. Risk of nephropathy can be detected before the onset of microalbuminuria during the early years after diagnosis of type 1 diabetes. Diabetes Care 2000; 23: 1811– 1815 [DOI] [PubMed] [Google Scholar]
- 25.Dunger DB, Schwarze CP, Cooper JD, Widmer B, Neil HA, Shield J, Edge JA, Jones TW, Daneman D, Dalton RN. Can we identify adolescents at high risk for nephropathy before the development of microalbuminuria? Diabet Med 2007; 24: 131– 136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.