Abstract
OBJECTIVE
To examine after gastric bypass the effect of peroral versus gastroduodenal feeding on glucose metabolism.
RESEARCH DESIGN AND METHODS
A type 2 diabetic patient was examined on 2 consecutive days 5 weeks after gastric bypass. A standard liquid meal was given on the first day into the bypassed gastric remnant and on the second day perorally. Plasma glucose, insulin, C-peptide, glucagon, incretin hormones, peptide YY, and free fatty acids were measured.
RESULTS
Peroral feeding reduced 2-h postprandial plasma glucose (7.8 vs. 11.1 mmol/l) and incremental area under the glucose curve (iAUC) (0.33 vs. 0.49 mmol · l−1 · min−1) compared with gastroduodenal feeding. β-Cell function (iAUCCpeptide/Glu) was more than twofold improved during peroral feeding, and the glucagon-like peptide (GLP)-1 response increased nearly fivefold.
CONCLUSIONS
Improvement in postprandial glucose metabolism after gastric bypass is an immediate and direct consequence of the gastrointestinal rearrangement, associated with exaggerated GLP-1 release and independent of changes in insulin sensitivity, weight loss, and caloric restriction.
Resolution of type 2 diabetes after Roux-en-Y gastric bypass (RYGB) has been observed in several studies and involves mechanisms associated with the surgical rearrangement of the gastrointestinal tract in addition to the effect of weight loss (1). The mechanisms have not been established, but changed gut hormone levels after surgery may play a role (2).
Recently, we had the unique opportunity to examine the effects of feeding either perorally (and thereby bypassing the stomach, duodenum, and proximal jejunum) or through a gastric tube inserted into the bypassed gastric remnant on the glucose metabolism in a single patient.
RESEARCH DESIGN AND METHODS
A 51-year-old male patient with type 2 diabetes (BMI 50.2 kg/m2, A1C 8.0%) treated with metformin, sulfonylurea, and insulin underwent a laparoscopic RYGB for morbid obesity.
On the second postoperative day, a leakage from the gastro-jejunostomy was suspected because of fever and abdominal pain. Acute reoperation showed no firm signs of leakage, but nevertheless a percutaneous gastric tube was inserted into the bypassed gastric remnant. The tube served as the only route of nutrition during the following 3 weeks, after which the patient again was allowed peroral feeding through the gastric pouch according to a standard nutrition protocol (1,200 kcal/day). Treatment with insulin and metformin was temporarily required after the reoperation but could be discontinued 3 weeks postoperatively.
We examined the patient 5 weeks postoperatively, at which time the patient was fed perorally but still had the gastric tube. The patient had lost 14 kg (BMI 45.2 kg/m2). Informed consent was obtained prior to examination.
On 2 consecutive days at 8.30 a.m. after an overnight fast (8 h), a standard 200-ml liquid meal (Nutridrink; Nutricia) containing 300 kcal, with 16% protein, 49% carbohydrate, and 36% fat, was given over a period of 10 min, on the first day through the gastric tube and on the second day perorally. Blood samples were drawn from an antecubital vein at 15- to 30-min intervals (Fig. 1).
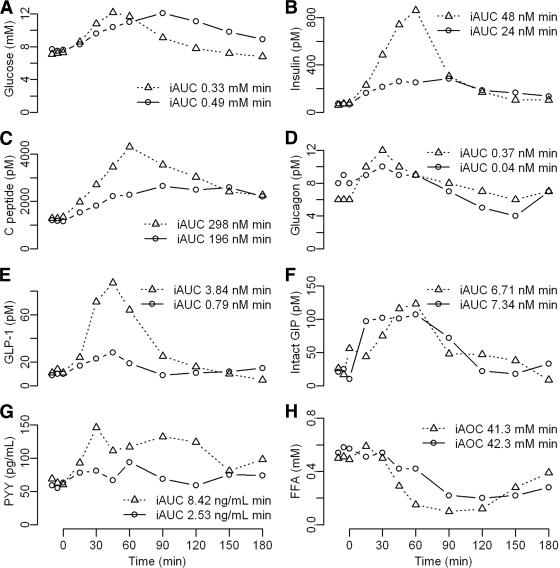
Figure 1.
Plasma concentrations of glucose (A), insulin (B), C-peptide (C), glucagon (D), GLP-1 (E), intact GIP (F), PYY (G), and FFAs (H) after peroral or gastroduodenal feeding in a RYGB-operated patient. Figure includes iAUC estimations. Triangles and dotted lines, peroral feeding; circles and solid lines, gastroduodenal feeding.
Laboratory analyses
Plasma glucose was measured by a glucose oxidase method (ABL800Flex; Radiometer, Br⊘nsh⊘j, Denmark), peptide YY3–36 (PYY) with a radioimmunoassay kit (Linco Research), and free fatty acids (FFAs) by an enzymatic colorimetric method (Wako, Düsseldorf, Germany). Plasma insulin, C-peptide, glucagon, and incretin hormone were quantified as earlier described (3).
Calculations
Incremental area under the curve (iAUC) was calculated using the trapezoidal model. β-Cell function was evaluated by iAUCinsulin, iAUCCpeptide, and insulinogenic index (IGI) and calculated as (insulin30 − insulinfasting)/(Glu30 − Glufasting) and iAUCCpeptide/Glu ratio. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as (insulinfasting × Glufasting)/22.5.
RESULTS
Plasma concentrations and iAUC for glucose, insulin, C-peptide, glucagon, total glucagon-like peptide-1 (GLP-1), intact glucose-dependent insulinotropic polypeptide (GIP), PYY, and FFAs after peroral and gastroduodenal feeding are shown in Fig. 1.
Plasma glucose concentration peaked earlier and returned more rapidly to fasting values after peroral than gastroduodenal feeding, as illustrated by a markedly reduced 2-h plasma glucose concentration (7.8 vs. 11.1 mmol/l). iAUCGlu was noticeably lower after peroral feeding. The peak values of plasma insulin and C-peptide were higher after peroral than gastroduodenal feeding (fourfold and twofold, respectively) and iAUCinsulin and iAUCCpeptide were also clearly elevated. IGI was improved after peroral feeding (115 vs. 72 pmol/mmol), and the iAUCCpeptide/Glu ratio was more than twofold increased (0.90 vs. 0.40 nmol/mmol). HOMA-IR remained unchanged on the 2 examination days (3.3 vs. 3.5).
GLP-1 plasma concentration peaked simultaneously after peroral and gastroduodenal feeding, but the peak value was more than threefold increased (87 vs. 28 pmol/l), and iAUCGLP-1 was nearly fivefold increased after peroral feeding. Insulin and GLP-1 correlated strongly after peroral (r = 0.92, P < 0.001) but not gastroduodenal (r = 0.55, P = 0.08) feeding. Plasma concentrations of glucagon and intact GIP were similar on both days. Responses of PYY and FFAs are depicted in the figure.
CONCLUSIONS
Rapid improvement in glucose tolerance after RYGB surgery is a clinical reality (2). Here, we report important differences in β-cell function and glucose metabolism after peroral compared with gastroduodenal feeding in a patient with RYGB and a gastrostomy, where differences in insulin sensitivity, weight loss, and caloric restriction can be ruled out as explanations for the improved glucose tolerance.
Our results show marked improvement in glucose tolerance with near normalization of 2-h postprandial plasma glucose value and a 33% reduction in iAUCGlu after peroral feeding compared with gastroduodenal feeding. In contrast, during gastroduodenal feeding glucose tolerance was diabetic with a 2-h postprandial plasma glucose value ∼11 mmol/l. The improvement was accompanied by a twofold increase in β-cell secretory response (AUCCpeptide/Glu), which was associated with a fivefold increase in iAUCGLP-1. Insulin and GLP-1 concentrations during peroral feeding were strongly correlated, which is suggestive of a causal relationship. Interestingly, the insulin and C-peptide response curves found after gastroduodenal feeding resemble the responses found in type 2 diabetic patients, whereas the response curves after peroral feeding are similar to those found in healthy control subjects (4). The emptying time is likely to be slower after feeding into the bypassed gastric remnant, which could explain the slower peak in plasma glucose observed after gastroduodenal feeding but would also, per se, be expected to result in decreased postprandial glucose excursions.
The observed improvements in glucose tolerance and GLP-1 secretion are in concordance with earlier findings from patients examined before and after RYGB surgery (5–13). Regarding GIP, some studies have demonstrated increased (7,10) and others decreased (9,13) responses after RYGB. In our patient, GIP responses were similar on the 2 days, suggesting that changes in GIP were not responsible for the differences in insulin secretion and glucose tolerance. Also glucagon responses were similar.
In conclusion, our results suggest that RYGB has a direct beneficial effect on postprandial glucose metabolism, most likely due to an increased insulin secretion caused by the massive increase in GLP-1 that is probably due to the rapid exposure of l-cells in the distal small intestine to nutrients (14). It has been suggested that duodenal exclusion inherent in the RYGB somehow might be responsible for the improvement in glucose tolerance (15). In this respect, it is of interest that the secretion of the upper jejunal hormone, GIP, was similar during peroral or gastroduodenal feeding.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292: 1724– 1737 [DOI] [PubMed] [Google Scholar]
- 2.Bose M, Oliván B, Teixeira J, Pi-Sunyer FX, Laferrère B. Do incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: what are the evidence? Obes Surg 2009; 19: 217– 229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Højberg PV, Vilsbøll T, Rabøl R, Knop FK, Bache M, Krarup T, Holst JJ, Madsbad S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia 2009; 52: 199– 207 [DOI] [PubMed] [Google Scholar]
- 4.Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29: 46– 52 [DOI] [PubMed] [Google Scholar]
- 5.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg 2006; 93: 210– 215 [DOI] [PubMed] [Google Scholar]
- 6.de Carvalho CP, Marin DM, de Souza AL, Pareja JC, Chaim EA, de Barros Mazon S, da Silva CA, Geloneze B, Muscelli E, Alegre SM. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg 2009; 19: 313– 320 [DOI] [PubMed] [Google Scholar]
- 7.Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, Schneider BE, Holst JJ, Patti ME. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab 2007; 92: 4678– 4685 [DOI] [PubMed] [Google Scholar]
- 8.Holdstock C, Zethelius B, Sundbom M, Karlsson FA, Edén Engström B. Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes (Lond) 2008; 32: 1640– 1646 [DOI] [PubMed] [Google Scholar]
- 9.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 2007; 3: 597– 601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008; 93: 2479– 2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 2006; 243: 108– 114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morínigo R, Moizé V, Musri M, Lacy AM, Navarro S, Marín JL, Delgado S, Casamitjana R, Vidal J. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 2006; 91: 1735– 1740 [DOI] [PubMed] [Google Scholar]
- 13.Rodieux F, Giusti V, D'Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008; 16: 298– 305 [DOI] [PubMed] [Google Scholar]
- 14.Holst JJ, Sørensen TI, Andersen AN, Stadil F, Andersen B, Lauritsen KB, Klein HC. Plasma enteroglucagon after jejunoileal bypass with 3:1 or 1:3 jejunoileal ratio. Scand J Gastroenterol 1979; 14: 205– 207 [DOI] [PubMed] [Google Scholar]
- 15.Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006; 244: 741– 749 [DOI] [PMC free article] [PubMed] [Google Scholar]