Abstract
OBJECTIVE
To identify factors associated with declining β-cell compensation for insulin resistance.
RESEARCH DESIGN AND METHODS
In a cohort of Hispanic women with recent gestational diabetes mellitus, oral glucose tolerance tests (OGTTs), intravenous glucose tolerance tests (IVGTTs), and bioelectrical impedance measurements were performed at 15-month intervals for up to 5 years, or until fasting plasma glucose exceeded 140 mg/dl (7.8 mmol/l). Data were analyzed to identify predictors of declining β-cell compensation for insulin resistance (the disposition index [DI]) and to examine the mechanism of weight gain and changes in circulating levels of selected adipokines and inflammatory markers on β-cell compensation decline.
RESULTS
A total of 60 nondiabetic women had a median of four sets of OGTT + IVGTT during a median follow-up of 52 months. Fourteen of the women developed diabetes. None of the baseline characteristics were significantly predictive of a decline in DI. There were significant univariate associations between declining DI and weight gain (specifically fat gain), declining adiponectin and rising C-reactive protein. Multivariate analysis showed that the weight gain was the most significant factor associated with declining DI. The amount of association between weight gain and declining DI was explained 31% by changes in adiponectin and C-reactive protein and 40% by changes in insulin resistance.
CONCLUSIONS
These results identify weight gain as the strongest factor associated with declining β-cell compensation for insulin resistance in Hispanic women at high risk for type 2 diabetes. Such effect may be mediated through at least two effects: alterations in adipokine levels and increasing insulin resistance.
Type 2 diabetes is characterized by inadequate pancreatic β-cell compensation for chronic insulin resistance. Longitudinal studies in Pima Indians (1), Caucasian and African Americans (2,3), and Hispanic Americans (2,4) indicate that β-cell function declines on a background of chronic and often worsening insulin resistance as people progress from normal to impaired glucose tolerance and then to diabetes. Much is known about baseline factors that predict a relatively short time to diabetes—factors such as relatively high glucose levels, insulin resistance, and poor β-cell compensation for insulin resistance. Those factors could be important driving forces for the development of diabetes. They could also simply be markers of individuals closest to diabetes at the initiation of follow-up.
Much less is known about the cause(s) of the progressive deterioration in β-cell function that leads to impaired glucose tolerance and diabetes. Declining β-cell function has been shown to be associated with weight gain in Pima Indians (5) and with increased fat in women with a family history of type 2 diabetes (6). Glucose and lipid toxicity have been suggested as causes of declining β-cell function (7), although data are lacking from humans to support such an effect over the years that it takes to develop diabetes.
We conducted a longitudinal study investigating the pathogenesis of diabetes development in relatively young Hispanic women with recent gestational diabetes mellitus. We observed a progressive decline in β-cell compensation for insulin resistance that was attended by slowly rising glucose levels until β-cell compensation reached very low levels, at which time glucose levels rose to the diabetic range (4). The present analysis examines what baseline characteristics predict the decline in β-cell compensation for insulin resistance and the potential role and mechanism of weight gain and changes in circulating levels of adipokines and inflammatory markers during follow-up on declining β-cell compensation in this high-risk group.
RESEARCH DESIGN AND METHODS
Subjects for the present report were islet cell antibody–negative women who participated in a longitudinal study of the pathogenesis of type 2 diabetes after gestational diabetes mellitus. Selection of the original cohort has been described in detail (8,9). Briefly, all Latino women referred to Los Angeles County Women's Hospital for management of gestational diabetes mellitus between August 1993 and March 1995 were asked to participate if they met all of the following criteria: 1) gestational age between 28 and 34 weeks, 2) no current or prior insulin therapy, 3) all fasting serum glucose concentrations <130 mg/dl (7.2 mmol/l) during pregnancy, 4) otherwise uncomplicated singleton pregnancy, and 5) both parents and at least three of four grandparents from Mexico, Guatemala, or El Salvador. All women had detailed metabolic testing during the third trimester. They were asked to return for a 75-g oral glucose tolerance test (OGTT) 6 months postpartum and then for an OGTT and an intravenous glucose tolerance test (IVGTT) at 15 months postpartum and every 15 months thereafter. Height, weight, and information on contraceptive use and pregnancies were collected at each visit. Bioelectrical impedance (BIA) was measured at each OGTT visit to assess body composition. At the time of diagnosis of impaired glucose tolerance, our diabetic subjects met with a dietitian and received advice on nutrition and daily walking. Subjects remained in follow-up until they withdrew consent, were lost to follow-up, or developed a fasting plasma glucose concentration >140 mg/dl (>7.8 mmol/l), at which time they were referred for pharmacological treatment. Women who were pregnant at the time of a scheduled battery of tests were studied at least 4 months after pregnancy and at least 1 month after completion of breastfeeding. Family history of diabetes was not assessed for this cohort.
For the present report, which is focused on nonpregnant women from 15 to 75 months postpartum (occurred between October 1994 and November 2001), we analyzed data from all subjects who 1) had a baseline OGTT and IVGTT without diabetes in the 15- or 30-month postpartum testing window and 2) returned for at least one additional set of OGTT and IVGTT by the 75-month postpartum test window. All subjects gave written informed consent for participation in the study, which was approved by the Institutional Review Board of the University of Southern California and the Los Angeles County plus University of Southern California Medical Center.
Testing protocols
For a battery of OGTT, IVGTT, and BIA, subjects came to the General Clinical Research Center on 2 separate days, at least 48 h apart, after an 8- to 12-h overnight fast and at least 3 days on an unrestricted diet.
On day 1, BIA was measured immediately before an OGTT between 0700 and 1000. For BIA, subjects lay supine while plastic electrodes were placed on their right hand and foot and a trained technician took dual resistance and reactance readings with a Quantom Impedance Meter (RJL Systems, Michigan). For OGTTs, subjects drank 75 g dextrose. Blood was obtained from an antecubital venous catheter before and 15, 30, 60, 90, and 120 min after the glucose ingestion and placed on ice, and plasma was separated within 20 min and stored at −80°C. Fasting plasma samples from the OGTTs were used later to measure free fatty acids (FFAs), adiponectin, leptin, C-reactive protein (CRP), and interleukin (IL)-6.
On a separate day, an IVGTT was performed starting between 0700 and 1000. Dextrose (300 mg/kg) was injected over 1 min, followed in 20 min by a 5-min infusion of crystalline human insulin (0.03 units/kg). Arterialized venous blood was drawn into iced tubes before (n = 2) and for 240 min after (n = 32) the dextrose injection. Plasma was separated within 20 min and stored at −80°C.
Laboratory analysis
Glucose was measured by a glucose oxidase (Beckman Glucose Analyzer II; Beckman Coulter, Brea, CA). Insulin was measured by a radioimmunoassay (RIA) (Novo Pharmaceuticals, Danbury, CT) that measured insulin and proinsulin with intra- and inter-observer coefficient of variations (CV) of <2.3 and 4.4%. Plasma FFAs were measured by an enzymatic colorimetric method using a kit from WAKO Chemicals (intra- and inter-observer CVs of <0.75 and 0.37%). Plasma adiponectin and leptin levels were measured using RIA kits from LINCO Research (intra- and inter-observer CVs of <8.3 and 9.2%). Plasma CRP and IL-6 were measured using CRP ELISA (intra- and inter-observer CVs of <6.0 and 13.8%) and ultrasensitive IL-6 ELISA kits (intra- and inter-observer CVs of <8.3 and 10.0%) from ALPCO Diagnostics.
Data analysis
BMI was calculated as weight in kilograms divided by the square of height in meters. Diabetes was diagnosed using American Diabetes Association criteria (10). IVGTT results were analyzed using the MINMOD program (11) to obtain measures of fractional glucose disappearance due to an increase in insulin above basal (insulin sensitivity [SI]).
The acute insulin response to intravenous glucose (AIRg) was calculated by the trapezoid rule as the incremental area under the insulin curve during the first 10 min after the glucose injection. The product of SI and AIRg (the disposition index [DI]) was calculated as a measure of acute pancreatic β-cell compensation for insulin resistance (11). Body fat and fat-free mass were calculated by the formula of Kotler et al. (12) using height, weight, and BIA measurements.
Baseline characteristics are presented as mean (SD) and ranges (Table 1). For data analysis, log-transformation was applied for CRP, fasting and 2-h insulin, 30-min incremental insulin, SI, AIRg, and DI. Thus, geometric means are presented for these variables where appropriate. Rates of change for variables that were measured at each follow-up visit (weight, body composition, AIRg, SI, and DI) were estimated by regressing follow-up data in each subject against follow-up time for that subject. Fasting FFA, adiponectin, CRP, leptin, and IL-6 were measured only at baseline and last follow-up visit, so their rates of change were estimated as [last baseline]/[follow-up time] for each subject. Fasting total, HDL, and LDL cholesterol and triglycerides were not collected during follow-up. Relationship between follow-up changes in glucose and DI decline has been evaluated previously (4) and was not the focus in this report; thus, follow-up changes on OGTT glucose and insulin were not included in this report. Random coefficient mixed-effect models were used to test for significant changes over time for DI, SI, AIRg, weight, and body composition. Mixed-effect models were also used to test for significant associations between changes in the primary outcome variable, DI, and 1) baseline variables (Table 1) and 2) changes during follow-up in pregnancy status, use of hormonal contraception, body weight, fat and fat-free mass, circulating levels of FFA, adiponectin, CRP, leptin and IL-6. In evaluating the prediction of baseline SI, AIRg, and DI on the primary outcome rate of change in DI, we applied a bias correction to adjust for possible artificial negative correlation due to measurement error, sometimes referred to as “regression to the mean.” Measurement error accounted for 15, 12, and 15% of the total variation for SI, AIRg, and DI, respectively (estimated from 109 Hispanic women with similar characteristics who participated in a separate study where IVGTTs were repeated after 3 months without any treatment [13]). The bias corrected point estimates were obtained using an empirical formula developed through simulation for longitudinal repeated data (14). Standard errors of the estimates were obtained by the bootstrap method with 100 replications. In each bootstrap sample, 60 subjects were randomly selected with replacement from the original sample; mixed-effect models were then fitted and bias corrected estimates were obtained.
Table 1.
Baseline and follow-up characteristics
| Mean ± SD* | Range (minimum, maximum) | |
|---|---|---|
| Baseline | ||
| Age (years) | 32.6 ± 5.6 | 22.0, 42.6 |
| BMI (kg/m2) | 30.6 ± 4.9 | 18.2, 45.4 |
| Body percent fat† | 44.3 ± 5.8 | 28, 57 |
| Fasting total cholesterol (mg/dl) | 173.8 ± 30.5 | 114, 251 |
| Fasting HDL cholesterol (mg/dl) | 39.5 ± 10.5 | 20, 74 |
| Fasting LDL cholesterol (mg/dl) | 105.9 ± 30.7 | 50.2, 166.2 |
| Fasting triglycerides (mg/dl) | 136.1 ± 71.2 | 43, 374 |
| Fasting FFA (μmol/l) | 500 ± 205 | 265, 1,372 |
| Adiponectin (ng/ml) | 6,326 ± 2,179 | 1,099, 12,002 |
| CRP (ng/ml) | 28.6 ± 30.8 | 30, 233 |
| Leptin (ng/ml) | 11.8 ± 4.5 | 5, 33 |
| IL-6 (pg/ml) | 2.4 ± 1.2 | 0.6, 7.7 |
| Fasting glucose (mg/dl)‡ | 96.8 ± 8.5 | 82, 116 |
| 2-h glucose (mg/dl)‡ | 142.6 ± 30.4 | 64, 194 |
| Fasting insulin (μU/ml)‡ | 17.0 ± 9.1 | 3, 56 |
| 2-h insulin (μU/ml)‡ | 105.5 ± 72.3 | 16, 446 |
| Δ Insulin at 30 min (μU/ml)§ | 82.2 ± 43.1 | 14, 321 |
| Insulin sensitivity (SI, min−1 per μU/ml × 10−4)‖ | 1.44 ± 0.46 | 0.5, 5.5 |
| Acute insulin response (AIRg, μU/ml × min)¶ | 514 ± 257 | 47, 4,803 |
| DI (SI × AIRg)# | 726 ± 249 | 50, 2,249 |
| Rates of change during follow-up | ||
| Weight (kg/year) | 0.75 ± 1.18‡‡ | −2.4, 4.5 |
| Body fat (kg/year)† | 0.56 ± 1.01‡‡ | −2.4, 3.7 |
| Fasting FFA (μmol/l per year) | 96 ± 138‡‡ | −236, 445 |
| Adiponectin (ng/ml per year) | −211 ± 532†† | −2,327, 771 |
| CRP (log ng/ml per year) | −0.02 ± 0.32 | −1.07, 1.49 |
| Leptin (ng/ml per year) | 1.34 ± 2.18‡‡ | −3.48, 8.07 |
| IL-6 (pg/ml per year) | −0.07 ± 0.38 | −0.94, 1.25 |
| SI (log unit per year) | −0.02 ± 0.14 | −0.48, 0.51 |
| AIRg (log unit per year) | −0.08 ± 0.14‡‡ | −0.60, 0.20 |
| DI (log unit per year) | −0.06 ± 0.08‡‡ | −0.21, 0.15 |
n = 60.
*Geometric means ± SDs were presented for baseline CRP, fasting and 2-h insulin, Δinsulin at 30 min, insulin sensitivity, acute insulin response, and DI.
†Estimated by bioelectrical impedance.
‡During 75-g OGTTs.
§30 min minus baseline value for plasma insulin.
‖Calculated by minimal model analysis of IVGTT insulin and glucose data.
¶Incremental insulin area during first 10 min of IVGTT.
#A measure of β-cell compensation for insulin resistance. Significant changes:
††P < 0.01,
‡‡P < 0.001. To convert the glucose in units of mg/dl to the SI unit of mmol/l, multiply the number in the table by 0.0555.
All statistical tests were two-sided, and statistical significance was defined as P ≤ 0.05.
RESULTS
Baseline characteristics
Sixty women met the inclusion criteria for this report; 28 had normal glucose tolerance and 32 had impaired glucose tolerance. At baseline (Table 1), the cohort had an average age of 32 years and an average BMI and body fat consistent with obesity. Mean glucose levels were consistent with impaired glucose tolerance, and mean insulin sensitivity was very low. However, there was a wide range for all baseline values in the cohort.
Changes during follow-up
The median duration of follow-up was 52 months. There were 25 women who had all five possible visits, 19 had four, 12 had three, and 4 had two. During follow-up, 14 of the women developed diabetes, 15% of the women used estrogen-progestin combination oral contraceptives, and 15% used progestin-only contraceptives; two women (3%) used both at different times. A total of 25% of the women experienced one or more additional pregnancies during the follow-up period.
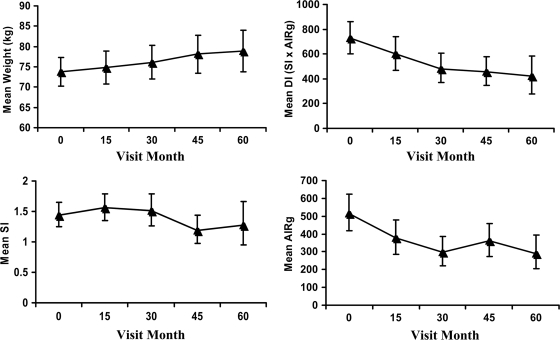
During follow-up (Table 1), mean weight increased significantly (0.75 ± 1.18 kg/year; P < 0.0001), although some women gained and some lost weight. Body fat also increased significantly (0.56 ± 1.01 kg/year; P < 0.0001), and changes in body fat accounted for 75% of the changes in body weight. Fasting FFA and leptin increased significantly (P < 0.001) and adiponectin decreased significantly (P < 0.01). There were no significant changes across the cohort in CRP, IL-6, or SI. AIRg and DI both fell significantly (P < 0.001). Figure 1 depicts mean values for weight, DI, SI, and AIRg at each follow-up time.
Figure 1.
Plot of mean weight, DI, SI, and first-phase insulin secretion (AIRg) by visit month. Geometric means are shown for DI, SI, and AIRg; arithmetic mean is shown for weight. Vertical lines are the associated 95% CIs for the means.
Factors associated with the decline in β-cell compensation
Univariate analysis with random coefficients mixed-effect modeling revealed that none of the baseline characteristics listed in Table 1 except baseline AIRg and DI were significantly associated with rate of change in DI during follow-up (P > 0.15 for each). Baseline AIRg and DI were significantly negatively associated with rate of change in DI (regression coefficient ± SE = −0.031 ± 0.0107 log DI unit per year/log AIRg unit, P = 0.004, for baseline AIRg; −0.0337 ± 0.0103 log DI unit per year/log DI unit for baseline DI, P = 0.0014, respectively). After correcting for potential bias due to regression to the mean, baseline AIRg and DI were no longer significantly associated with rate of change in DI (regression coefficients were reduced to −0.0056 ± 0.0123 log DI unit per year/log AIRg unit, P = 0.65, for baseline AIRg; −0.0009 ± 0.0096 log DI unit per year/log DI unit for baseline DI, P = 0.92, respectively).
For follow-up characteristics, additional pregnancies, use of hormonal contraception, changes in FFAs, leptin, and IL-6 were not significantly associated with rate of change in DI (P > 0.30). Rate of change in DI was significantly associated with rates of change in weight (−0.026 ± 0.007 log DI unit/kg, P = 0.0003), adiponectin (0.055 + 0.018 log DI unit per ng/ml × 10−3, P = 0.002), and CRP (−0.110 ± 0.035 log DI unit per log ng/ml, P = 0.002) univariately. The association with CRP was noted despite the fact that there was on average no significant change in CRP in this cohort (Table 1). Results of the multivariate analysis with which we assessed the potential contributions of changing adiponectin and CRP to the association between change in DI and weight gain appear in Table 2. The univariate association between weight gain and declining DI was reduced by 19% after adjustment for changes in either adiponectin or CRP and by 31% after adjustment for both (Table 2). Nonetheless, change in weight remained significantly associated with change in DI after these adjustments (Table 2, adjusted P ≤ 0.02). Thus, weight gain was the strongest factor that associated with declining DI, and that association was reduced but remained significant after adjustment for changes in adiponectin and CRP.
Table 2.
Impact of changes in adiponectin and CRP on the relationship between changes in weight and DI during follow-up
| Variables adjusted | Regression coefficient ± SE (log DI unit/kg)* | P | % Reduction compared with no adjustment† |
|---|---|---|---|
| None | −0.026 ± 0.007 | 0.0003 | — |
| Change in adiponectin | −0.021 ± 0.007 | 0.006 | 19% |
| Change in CRP | −0.021 ± 0.007 | 0.005 | 19% |
| Changes in adiponectin and CRP | −0.018 ± 0.008 | 0.02 | 31% |
*Estimated from random-coefficient mixed-effect model regression analysis.
†Calculated as 100 × (unadjusted regression coefficient − adjusted regression coefficient)/unadjusted regression coefficient.
We have previously shown that insulin resistance can contribute to declining β-cell function in Hispanic women (13,15). To determine whether the pattern of association we observed between DI, weight gain, and changing CRP and adiponectin was simply due to insulin resistance, we performed analysis with follow-up SI as a covariate. Adjustment for changes in SI alone reduced the association between weight gain and declining DI by 40%, but the association remained statistically significant (P = 0.037). Adding adjustment for changes in SI to the previous 31% reduction caused by adjustment for adiponectin and CRP resulted in a total of 70% reduction of the relationship between weight gain and declining DI. Indeed, the association between weight gain and declining DI was no longer statistically significant (P = 0.29). The adjustment for changes in SI had little impact on the relationship between failing DI and changes in adiponectin and CRP after weight gain was included. Thus, changes in insulin resistance, adiponectin, and CRP all appeared to contribute to the relationship between weight gain and declining DI.
Multivariate analysis including the two major components of weight (body fat and fat-free mass) indicated that the decline in DI was significantly associated with the gain in fat mass (P = 0.01), but not the fat-free mass (P = 0.16).
CONCLUSIONS
In relatively young and nondiabetic Hispanic women with prior gestational diabetes mellitus, we were unable to identify baseline characteristics that were predictive of declining β-cell compensation for insulin resistance over the next 5 years. By contrast, we identified several changes that were associated with declining DI. The strongest was weight gain. Additional factors were declining levels of adiponectin and rising CRP. In multivariate analysis, approximately one-third of the association between weight gain and declining β-cell compensation was accounted for statistically by these two variables. These associations were not simply due to increasing insulin resistance; adjustment for change in insulin sensitivity accounted for an additional ∼40% of the association between weight gain and declining β-cell function. On average, 75% of weight gain was body fat, and it was gain in fat, not lean mass, that was associated with declining DI. Taken together, our findings indicate that weight gain was the strongest factor that contributed to the decline in β-cell compensation for insulin resistance in Hispanic women at high risk for type 2 diabetes. Such an effect was mediated through at least two effects: increasing insulin resistance and alterations in adipokine levels.
The significant association between declining β-cell compensation and weight gain confirmed observations made previously by Weyer et al. in Pima Indians (5) and by Kriketos et al. (6) in women with a family history of type 2 diabetes. In Weyer's study, 209 nondiabetic Pima Indians (151 with normal glucose tolerance and 58 with impaired glucose tolerance) were evaluated an average of 2.6 years after baseline. Weight gain was significantly correlated with declining DI in both normal glucose tolerance and impaired glucose tolerance; the correlation was stronger in the subgroup with impaired glucose tolerance (5). In Kriketos's study, 20 women with a family history of type 2 diabetes (risk group) and 15 women without a family history of type 2 diabetes (control group) were tested at baseline and ∼6 years later. Insulin resistance and secretion were assessed by homeostasis model assessment (HOMA)-R and HOMA-β. Baseline levels and changes over time in total and central abdominal fat mass were inversely related to HOMA-β (6). Our study provides novel information about the potential mediation of declining β-cell function associated with weight gain. Some may be due to increasing insulin resistance, which is not surprising given the beneficial effects of chronic use of insulin-sensitizing medications on β-cell function in this population (13,15). Additional contributors may be endocrine and/or inflammatory products of adipose tissue, a novel finding to our knowledge.
Epidemiological studies have revealed direct associations between diabetes risk and markers of inflammation (16) and inverse associations between diabetes risk and adiponectin levels (17,18). In vitro studies reveal evidence for detrimental effects of cytokines (19) and beneficial effects of adiponectin (20) on β-cells in the short term. The present study demonstrated longitudinal association between adipocyte-derived hormones and declining β-cell compensation for insulin resistance in humans. The results raise the possibility that circulating products of adipose tissue such as adiponectin and inflammatory cytokines are harmful to β-cells.
We did not find evidence for an effect of leptin, IL-6, FFA, baseline level of glucose, cholesterol, triglyceride, insulin resistance, insulin secretion, and β-cell compensation on changes in β-cell compensation in this cohort. The lack of impact of initial glucose levels is consistent with other studies we have conducted in this high-risk group (13) and speaks against glucotoxicity as a major cause of declining β-cell function in pre-diabetic individuals. The lack of an effect of lipid levels, including FFAs, speaks against lipotoxicity, although circulating lipid levels obtained in the fasting state may not be a good indicator of lipid delivery to or metabolism by β-cells. The lack of an effect of initial insulin resistance, secretion, and β-cell compensation after correcting for the bias due to measurement error did not support the hypothesis that high insulin resistance, high insulin secretion, and β-cell compensation lead to faster declining of β-cell compensation in this high-risk, very insulin-resistant group. We recognized that the relative small sample size in this report limited our ability to identify small effects of any of these factors on β-cell compensation.
Our observation is consistent with results of several type 2 diabetes prevention trials in which the best evidence for slowing or arrest of declining β-cell function comes from interventions that either reduce body weight (21) or change the biology of fat (13,15,22). The role of inflammation and adipokines in diabetes prevention in these studies remains to be determined. However, studies using aspirin and salcylate (23,24) and IL-1 receptor antagonist anakinra (25) indicate that treatment of inflammation per se may have beneficial effects on glucose regulation in pre-diabetic and diabetic humans.
In summary, we found a significant association between weight gain and declining β-cell compensation for insulin resistance in Hispanic women at high risk for type 2 diabetes. The effect was explained in part by changes in insulin resistance and in part by changes in circulating adiponectin and CRP. The results raise the possibility that increased body fat contributes to declining β-cell function not only through insulin resistance and increased demands on β-cells (13,15), but also through direct effects of circulating adipose tissue peptides on β-cell replication and/or survival. Our findings highlight the importance of reducing body fat and/or its detrimental metabolic effects to preserve pancreatic β-cell function and prevent or arrest the progression to/of diabetes in high-risk individuals.
Acknowledgments
This work was supported by grant R01-DK-46374 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH); grant M01-RR-43 from the Division of Clinical Research, National Center for Research Resources, NIH; and a Distinguished Clinical Scientist Award from the American Diabetes Association.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
The authors thank Susie Nakao, Carmen Martinez, and the staff of the General Clinical Research Center for assistance with metabolic studies; Lilit Zeberians, Mike Salce, Jay Sisson, and Martha Hernandez for performance of insulin and glucose assays; and Jerry Palmer for performance of islet cell antibody assays.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care 2001; 24: 89– 94 [DOI] [PubMed] [Google Scholar]
- 2.Festa A, Williams K, D'Agostino R, Jr, Wagenknecht LE, Haffner SM. The natural course of β-cell function in nondiabetic and diabetic individuals. Diabetes 2006; 55: 1114– 1120 [DOI] [PubMed] [Google Scholar]
- 3.Cnop M, Vidal J, Hull RL, Utzschneider KM, Carr DB, Schraw T, Scherer PE, Boyko EJ, Fujimoto WY, Kahn SE. Progressive loss of β-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care 2008; 30: 677– 682 [DOI] [PubMed] [Google Scholar]
- 4.Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic β-cell function in Latino women at high risk for type 2 diabetes. Diabetes 2006; 55: 1074– 1079 [DOI] [PubMed] [Google Scholar]
- 5.Weyer C, Hanson K, Bogardus C, Pratley RE. Long-term changes in insulin action and insulin secretion associated with gain, loss, regain and maintenance of body weight. Diabetologia 2000; 43: 36– 46 [DOI] [PubMed] [Google Scholar]
- 6.Kriketos AD, Carey DG, Jenkins AB, Chisholm DJ, Furler SM, Campbell LV. Central fat predicts deterioration of insulin secretion index and fasting glycemia: 6-year follow-up of subjects at varying risk of type 2 diabetes mellitus. Diabet Med 2003; 20: 294– 300 [DOI] [PubMed] [Google Scholar]
- 7.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008; 29: 351– 366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiang AH, Peters RK, Trigo E, Kjos SL, Lee WP, Buchanan TA. Multiple metabolic defects during late pregnancy in women at high risk for type 2 diabetes mellitus. Diabetes 1999; 48: 848– 854 [DOI] [PubMed] [Google Scholar]
- 9.Buchanan TA, Xiang A, Kjos SL, Lee WP, Trigo E, Nader I, Bergner EA, Palmer JP, Peters RK. Gestational diabetes mellitus: antepartum metabolic characteristics that predict postpartum glucose intolerance and type 2 diabetes in Latino women. Diabetes 1998; 47: 1302– 1310 [DOI] [PubMed] [Google Scholar]
- 10.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003; 26 ( Suppl. 1): S5– S20 [DOI] [PubMed] [Google Scholar]
- 11.Bergman RN. Lilly lecture 1989: Toward physiological understanding of glucose tolerance: minimal model approach. Diabetes 1989; 38: 1512– 1527 [DOI] [PubMed] [Google Scholar]
- 12.Kotler DP, Burastero S, Wang J, Pierson JRN. Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: effect of race, sex and disease. Am J Clin Nutr 1996; 64 ( Suppl.): 489S– 497S [DOI] [PubMed] [Google Scholar]
- 13.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic B-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002; 51: 2769– 2803 [DOI] [PubMed] [Google Scholar]
- 14.Xiao J. Effect of Measurement Error on the Association Between Baseline and Longitudinal Change PhD thesis, University of Southern California, 2007 [Google Scholar]
- 15.Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Kawakubo M, Buchanan TA. Effect of pioglitazone on pancreatic β-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes 2006; 55: 517– 522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6 and risk of developing type 2 diabetes mellitus. JAMA 2001; 286: 327– 334 [DOI] [PubMed] [Google Scholar]
- 17.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, Knowler WC, Krakoff J. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 2002; 360: 57– 58 [DOI] [PubMed] [Google Scholar]
- 18.Spranger J, Kroke A, Möhlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003; 361: 226– 228 [DOI] [PubMed] [Google Scholar]
- 19.Donath MY, Størling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T. Cytokines and beta-cell biology: from concept to clinical translation. Endocr Rev 2008; 29: 334– 350 [DOI] [PubMed] [Google Scholar]
- 20.Rakatzi I, Mueller H, Ritzeler O, Tennagels N, Eckel J. Adiponectin counteracts cytokine- and fatty acid-induced apoptosis in the pancreatic beta-cell line INS-1. Diabetologia 2004; 47: 249– 258 [DOI] [PubMed] [Google Scholar]
- 21.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DMDiabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: randomised controlled trial. Lancet 2006; 368: 1096– 1105 [DOI] [PubMed] [Google Scholar]
- 23.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care 2008; 31: 289– 294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest 2002; 109: 1321– 1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antogonist in type 2 diabetes mellitus. N Engl J Med 2007; 356: 1517– 1526 [DOI] [PubMed] [Google Scholar]