This review covers the epidemiology, pathophysiology, clinical presentation, and diagnosis of cardiac autonomic neuropathy (CAN) in diabetes and discusses current evidence on approaches to prevention and treatment of CAN.
CASE PRESENTATION
A 26-year-old woman with “brittle” type 1 diabetes and severe CAN experienced sudden cardiac death. She had a 16-year history of poor diabetes control presenting with wide blood glucose fluctuations, recurrent episodes of severe hypoglycemia, and hypoglycemia unawareness. Over time she developed persistent orthostatic hypotension with daily falls in systolic blood pressure ranging from 30–60 mmHg. These episodes had significant impact on her daily activities and required intermittent therapy with the α-1 agonist midodrine. Other complications included severe gastroparesis, refractory diarrhea, and painful diabetic peripheral neuropathy. Her last clinical examination revealed resting tachycardia with a fixed rate of 115 bpm, supine blood pressure of 110/78 mmHg, which dropped to 70/48 mmHg while standing, symmetrical absent pinprick and temperature discrimination in stocking distribution, and left Charcot joint. Her last pertinent laboratory findings were A1C 8.7%, creatinine 1.9 mg/dl, microalbumin/creatinine 496 mg/g, and hemoglobin 10.8 g/dl.
CAN represents a significant cause of morbidity and mortality in diabetic patients and is associated with a high risk of cardiac arrhythmias and sudden death, possibly related to silent myocardial ischemia. Therefore, it has important clinical and prognostic relevance. Recent reports of major clinical trials undermine established thinking concerning glycemic control and cardiac risk. Thus, a review of this topic is both timely and important for physicians to better understand how to assess the complexity of conditions present in patients with diabetes in order to establish safe treatment targets.
EPIDEMIOLOGY
Diabetes affects more than 23 million people in the U.S. (www.diabetes.org) and an estimated 250 million worldwide (www.who.int/diabetes). Diabetic neuropathies, including CAN, are a common chronic complication of type 1 and type 2 diabetes and confer high morbidity and mortality to diabetic patients. The reported prevalence of CAN varies greatly depending on the criteria used to identify CAN and the population studied. CAN prevalence ranges from as low as 2.5% of the primary prevention cohort in the Diabetes Control and Complications Trial (DCCT) (1) to as high as 90% of patients with long-standing type 1 diabetes who were potential candidates for a pancreas transplantation (2). In a large cohort of patients with type 1 and type 2 diabetes, Ziegler et al. (3), using predefined heart rate variability (HRV) tests and spectral analysis of the R-R intervals, found that 25.3% of patients with type 1 diabetes and 34.3% of patients with type 2 diabetes had abnormal findings. Age, sex, and other risk factors may also influence CAN development.
PATHOGENESIS
In diabetes, CAN is ultimately the result of complex interactions among degree of glycemic control, disease duration, age-related neuronal attrition, and systolic and diastolic blood pressure (4,5). Hyperglycemia plays the key role in the activation of various biochemical pathways related to the metabolic and/or redox state of the cell, which, in concert with impaired nerve perfusion, contribute to the development and progression of diabetic neuropathies. Experimental data implicate a number of pathogenic pathways that may impact autonomic neuronal function in diabetes including: formation of advanced glycation end products, increased oxidative/nitrosative stress with increased free radical production, activation of the polyol and protein kinase C pathways, activation of polyADP ribosylation, and activation of genes involved in neuronal damage (6–8). A detailed review of these mechanisms and their complex interactions has been covered broadly (6–8) and is beyond the scope of this article.
CAN AND CARDIAC DYSFUNCTION
Autonomic innervation is the primary extrinsic control mechanism regulating HRV and cardiac performance. It has been shown that chronic hyperglycemia promotes progressive autonomic neural dysfunction in a manner that parallels the development of peripheral neuropathy, e.g., beginning distally and progressing proximally. The vagus nerve, the longest autonomic nerve, mediates ∼75% of all parasympathetic activity. Because neuropathy is seen first in the longest fibers, the earliest manifestations of autonomic neuropathy in diabetes tend to be associated with parasympathetic denervation. As such, the initial development of CAN in diabetes is characterized by early augmentation of sympathetic tone (9). Our data (10) and those of others (11) confirm that early in the progression of CAN complicating type 1 diabetes, there is a compensatory increase in the cardiac sympathetic tone in response to subclinical peripheral denervation. Sympathetic denervation follows later beginning at the apex of the ventricles and progressing toward the base (Fig. 1).
Figure 1.
The autonomic innervation of the heart and the effects of diabetes. It has been shown that in diabetes, in a fashion that parallels the development of peripheral neuropathy, which begins at the tip of the toes and can progress proximally, neuropathy affecting the heart begins at the apex of the ventricles and progresses toward the base.
The initial augmentation in cardiac sympathetic activity with subsequent abnormal norepinephrine signaling and metabolism, increased mitochondrial oxidative stress (12), and calcium-dependent apoptosis (13) may contribute to myocardial injury (12,14) and may explain the high risk of cardiac events and sudden death in these patients. The sympathetic imbalance associated with CAN may critically influence myocardial substrate utilization (15) and contribute to mitochondrial uncoupling (16), regional ventricular motion abnormalities, functional deficits, and cardiomyopathy (10).
CLINICAL SIGNS
Clinical symptoms of autonomic dysfunction may not appear until long after diabetes onset. However, subclinical CAN, manifested as changes in HRV, may be detected within 1 year of diagnosis in type 2 diabetes and within 2 years of diagnosis in type 1 diabetes (17).
Impaired HRV
The earliest clinical indicator of CAN is a decrease in HRV. Variability in the instantaneous beat-to-beat heart rate intervals is a function of sympathetic and parasympathetic activity that regulates the cardiac functional response to the body's level of metabolic activity. In normal individuals the heart rate has a high degree of beat-to-beat variability and HRV fluctuates with respiration—increasing with inspiration and decreasing with expiration. Initially, clinical relevance of HRV was identified through observations that fetal distress is preceded by alterations in beat-to-beat intervals before any appreciable change occurs in heart rate itself. The serious implications of abnormal HRV became apparent only in the late 1980s, when it was confirmed that HRV was a strong, independent predictor of mortality after acute myocardial infarction (18).
Resting tachycardia
Resting heart rates of ∼100 bpm with occasional increments up to 130 bpm usually occur later in the course of the disease and reflect a relative increase in the sympathetic tone associated with vagal impairment. However, increased resting heart rate may reflect other conditions such as anemia or thyroid dysfunction and is not considered to provide a reliable diagnostic criterion of CAN in the absence of other signs. A fixed heart rate that is unresponsive to moderate exercise, stress, or sleep indicates almost complete cardiac denervation (19) and is indicative of severe CAN.
Exercise intolerance
Autonomic dysfunction may impair exercise tolerance and has been shown to reduce heart rate, blood pressure, and cardiac output responses to exercise (19). It is generally recommended that diabetic patients suspected to have CAN be tested with a cardiac stress test before undertaking an exercise program. Patients with CAN need to rely on their perceived exertion, not heart rate, to avoid hazardous levels of exercise intensity (19).
Abnormal blood pressure regulation
At night, nondiabetic subjects exhibit a predominance of vagal tone and decreased sympathetic tone, associated with reduction in nocturnal blood pressure (20). In diabetic CAN this pattern is altered, resulting in sympathetic predominance during sleep and subsequent nocturnal hypertension (21). These are associated with a higher frequency of left ventricular (LV) hypertrophy and both fatal and severe nonfatal cardiovascular events in diabetic CAN subjects (22,23).
Orthostatic hypotension
In diabetes, orthostatic hypotension occurs largely as a consequence of efferent sympathetic vasomotor denervation, causing reduced vasoconstriction of the splanchnic and other peripheral vascular beds (24). Symptoms associated with orthostatic hypotension include: lightheadness, weakness, faintness, dizziness, visual impairment, and, in most severe cases, syncope on standing. These symptoms can be aggravated by a number of drugs including: vasodilators, diuretics, phenothiazines, insulin (through endothelium-dependent vasodilatation), and tricyclic antidepressants, a class of drugs commonly used for symptomatic relief of pain associated with painful diabetic neuropathy (19). As illustrated in the case presentation, orthostatic hypotension is associated with poor quality of life.
CLINICAL EVALUATION AND DIAGNOSIS CRITERIA
There is no widely accepted single approach to the diagnosis of CAN in diabetes. Assessment of HRV, orthostatic hypotension, and 24-h blood pressure profiles provides indexes of both parasympathetic and sympathetic autonomic function and can be used in clinical settings. Other methods such as cardiac sympathetic imaging, microneurography, occlusion plethysmography, and baroreflex sensitivity are currently used predominantly in research settings but may find a place in the clinical assessment of CAN in the future.
HRV
HRV provides a noninvasive and objective method for assessing cardiovagal function and may be derived from electrocardiogram recordings under paced breathing. Incorporating respiratory signal analysis enables one to independently measure both sympathetic and parasympathetic branches of the autonomic nervous system.
During the 1970s, Ewing et al. (25) devised a number of simple bedside tests of short-term R-R differences to detect CAN in diabetic patients, including: changes in R-R with deep breathing, which measures sinus arrhythmia during quiet respiration and primarily reflects parasympathetic function (26); R-R response to standing, which induces reflex tachycardia followed by bradycardia and is jointly vagal and baroreflex mediated; and Valsalva ratio, which evaluates cardiovagal function in response to a standardized increase in intrathoracic pressure (Valsalva maneuver), primarily parasympathetic mediated (26). These validated tests, described in detail in a statement by the American Diabetes Association (27), are recommended for CAN diagnosis (7,26–28) and can be performed in the practitioner's office. The rapid postural changes that are part of head-up-tilt-table testing, with/without pharmacological provocation, can be used for the investigation of CAN or of predisposition to neurally mediated (vasovagal) syncope due to the wide range of changes in the autonomic input to the heart and in the R-R intervals. This test requires specialized personnel and is not readily available in general practice.
HRV with deep breathing is the most widely used test of cardiovagal function and has about ∼80% specificity (29).
The R-R variation can be analyzed in a number of different ways, including heart rate range, heart period range, standard deviation (SD), E-to-I ratio (ratio of the shortest R-R during inspiration to the longest R-R during expiration). Calculation of mean circular resultant computed by vector analysis eliminates the effects of trends in heart rate over time, attenuating the effect of basal heart rate and ectopic beats (30). The Valsalva and postural tests are analyzed as the quotient of the largest and shortest R-R intervals recorded during each respective maneuver.
Normative cutpoints had been recommended originally for interpretation of the various HRV indexes (25). Recent studies demonstrate that HRV is affected mainly by age, rate of breathing, and possibly sex (26,30). Therefore, adjustments for these variables are recommended for higher accuracy (19,26,30).
Cardiovagal function can also be evaluated using statistical indexes in the time and frequency domains. Time domain measures of the normal R-R intervals include mean normal-to-normal (NN) interval, mean heart rate, the difference between the longest and shortest NN interval, and the variation during the difference between night and day heart rate (18). Twenty-four–hour R-R recordings allow calculation of more complex statistical time domain measures, such as SD of all normal R-R intervals (SDNN), SD of 5-min average of normal R-R intervals (SDANN), root–mean square of the difference of successive R-R intervals (rMSSD), and the number of instances per hour in which two consecutive R-R intervals differ by >50 ms over 24 h (pNN50). SDNN is thought to represent joint sympathetic and parasympathetic modulation of HRV, and rMSSD and pNN50 are specific for the parasympathetic limb (18). The accuracy of these measures can be affected by various arrhythmias and require normal sinus rhythm and atrioventricular-nodal function.
Spectral analysis of HRV is another tool to evaluate CAN (19). It decomposes the R-R signal into a set of sine and cosine waves and estimates the magnitude of variability as a function of frequency. The main frequency components described are very-low-frequency components (<0.04 Hz) related to fluctuations in vasomotor tone associated with thermoregulation, the low-frequency component (0.04–0.15 Hz) associated with the baroreceptor reflex, and the high-frequency components (0.15–0.4 Hz) related to respiratory activity (19). It is generally thought that the sympathetic system modulates the lower-frequency HRV components, whereas the parasympathetic system controls the high-frequency HRV components. Different mathematical methods have been used to analyze HRV. Fourier transform is the most commonly chosen due to algorithm simplicity and high processing speed (18). This method, limited to stationary signals, is based on the assumption of steady-state conditions discarding any dynamics in the power spectrum and does not allow a precise detection of a sudden change in autonomous tone or a precise localization of a particular event in time when examining nonstationary conditions (31). The continuous wavelet transform equations perform a time-frequency decomposition of the signal yielding a time-dependent version of the typical low- and high-frequency peaks (31,32). Commercially available software programs using these methods are available for assessment of HRV (ANSAR, Philadelphia, PA, and Hokanson, Bellevue, WA).
Orthostatic hypotension
Orthostatic hypotension is documented by a fall >30 mmHg in systolic or >10 mmHg in diastolic blood pressure in response to a postural change from supine to standing (19). There are some controversial aspects related to the cut-off value for the diagnostic fall in systolic blood pressure, i.e., 30 mmHg (7) or 20 mmHg (24,33), despite the definition provided by an ad hoc consensus committee in 1996 (24). Recent evidence suggests that postural hypotension has only moderate concordance with HRV in the diagnosis of CAN (33).
Imaging techniques for CAN
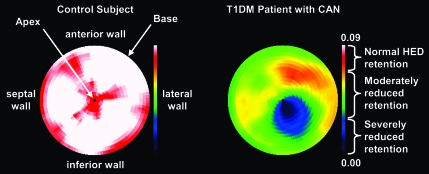
Quantitative scintigraphic assessment of sympathetic innervation of the human heart is possible with positron emission tomography (PET) and either [123I]meta-iodobenzylguanidine (MIBG) or [11C]-meta-hydroxy-ephedrine ([11C]HED) (19,34). Deficits of LV [123I]MIBG and [11C]HED retention have been identified in type 1 and type 2 diabetic subjects (35–37) with (35–37) and without (10) abnormal cardiovascular reflex testing. Metabolically stable [11C]HED undergoes highly specific uptake into sympathetic nerve varicosities via norepinephrine transporters, and quantitative [11C]HED retention may be assessed in 480 independent LV regions (34). The striking consistency of the evolution of the pattern of denervation in type 1 diabetes supports the reliability of [11C]HED to monitor changes in cardiac sympathetic nerve populations and evaluate early anatomical regional deficits of sympathetic denervation (10,34,35). As an example, Fig. 2 shows the polar map analysis of LV [11C]HED retention in subjects with type 1 diabetes expressed as Z score analysis versus control subjects (10).
Figure 2.
Polar maps of [11C]HED retention in normal control subjects (left) and type 1 diabetic patients with CAN (right). The color table is set to a maximum [11C]HED retention index value of 0.09 ml blood · min−1 · ml−1 tissue. To quantify the “extent” of cardiac sympathetic denervation, patients' retention index data are statistically compared with our normal population database using Z score analysis.
Quantitative regional measurements of myocardial β-adrenoreceptor density can also be assessed using PET and the high-affinity β-adrenoreceptor radioligand [11C]CGP-12177 (38). However, postsynaptic β-adrenoreceptor density was never assessed in human diabetes.
Baroreflex sensitivity
Baroreflex sensitivity (BRS) is a technique that evaluates the capability to reflexively increase vagal activity and decrease sympathetic activity in response to a sudden increase in blood pressure. It is used in research protocols to assess cardiac vagal and sympathetic baroreflex function and is calculated from the measurement of the heart rate–blood pressure relation after an intravenous bolus of phenylephrine (39). The BRS was a significant independent risk predictor of cardiac mortality in the Autonomic Tone and Reflexes After Myocardial Infarction (ATRAMI) study, a large international multicenter prospective study of 1,284 patients with a recent myocardial infarction (39). It has been shown that the analysis of spontaneous baroreflex sequences gives results equivalent to the pharmacological methods, which lead to development of techniques based on servoplethysmomanometry that measures blood pressure in the finger on a beat-to-beat basis (Finapress) (19).
Microneurography
This technique is based on recording electrical activity emitted by peroneal, tibial, or radial muscle sympathetic nerves and identification of sympathetic bursts. Bursts have a characteristic shape consisting of a gradual rise and fall that is usually constrained by the cardiac cycle and at least twice the amplitude of random fluctuations (40). Recently available fully automated sympathetic neurogram techniques provide a rapid and objective method that is minimally affected by signal quality and preserves beat-by-beat sympathetic neurograms (40).
Assessment of symptoms
Symptoms associated with CAN include exercise intolerance, orthostatic intolerance, and syncope (41). The correlation between symptom scores and deficits is generally weak in mild CAN, as these symptoms usually occur late in the disease process. Low et al. (41), using a validated self-report measure of autonomic symptoms in a population-based study, found that autonomic symptoms were present more commonly in type 1 than in type 2 diabetes. At least one autonomic symptom was reported in 83% of a large cohort of patients with type 2 diabetes (42). The risk of CAN as measured by HRV was positively associated with the number of reported autonomic symptoms (42).
CLINICAL IMPLICATIONS
Mortality risk
CAN is associated with a high risk of cardiac arrhythmias and with sudden death. Longitudinal studies of subjects with CAN have shown 5-year mortality rates 16–50% in type 1 and type 2 diabetes, with a high proportion attributed to sudden cardiac death (25,42,43). In the EURODIAB Prospective Cohort Study of 2,787 type 1 diabetic patients, CAN was the strongest predictor for mortality during a 7-year follow-up, exceeding the effect of traditional cardiovascular risk factors (44). The Hoorn study reported that the presence of diabetic CAN doubled the 9-year mortality risk in an elderly cohort (45). A meta-analysis of 15 studies including 2,900 subjects with diabetes reported a pooled relative risk of mortality of 3.45 (95% CI 2.66–4.47) in patients with CAN, with a progressive increase in the risk with the increase in the number of abnormal CAN function tests (46). The higher predictive value of an increased number of CAN abnormalities was confirmed more recently in two other cohorts of type 1 and type 2 diabetes showing that a combined abnormality in HRV and QT index was a strong predictor of mortality independent of conventional risk factors (47,48).
The increased mortality risk associated with CAN has important implications for diabetes management. A feared consequence of rigorous glycemic control is an increased incidence of hypoglycemia (49,50). Hypoglycemia impairs hormonal and autonomic responses to subsequent hypoglycemia (51). Hypoglycemia unawareness may promote a reduced threshold for malignant arrhythmias and subsequent sudden cardiac death, which was a possible reason for the sudden death experienced by the patient discussed in vignette. Thus, a recent study reported that exposure to hypoglycemia leads to impaired CAN function in healthy volunteers (52).
Silent myocardial ischemia and diabetic cardiomyopathy
In a meta-analysis of 12 published studies, Vinik et al. (7) reported a consistent association between CAN and the presence of silent myocardial ischemia, measured by exercise stress testing, with point estimates for the prevalence rate ratios from 0.85 to 15.53. In the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study of 1,123 patients with type 2 diabetes, CAN was a strong predictor of silent ischemia and subsequent cardiovascular events (53). The association between CAN and silent ischemia has important implications, as reduced appreciation for ischemic pain impairs timely recognition of myocardial ischemia or infarction, thereby delaying appropriate therapy. In patients with diabetes, presence of symptoms such acute onset dyspnea with/without coughing, severe fatigue, and/or acute onset of nausea and vomiting should raise a high index of suspicion for an ischemic event and prompt the appropriate measures (19).
The presence of CAN was also linked to the development of diabetic cardiomyopathy in type 1 diabetes because in these patients LV dysfunction often precedes or occurs in the absence of significant coronary artery disease or hypertension. We have identified diastolic dysfunction early in the course of type 1 diabetes that correlated with abnormal cardiac sympathetic imaging (10). Further studies are needed to clarify the complex interactions between CAN, silent myocardial ischemia, and cardiomyopathy in diabetes.
Intraoperative and perioperative cardiovascular instability
Observations in diabetic patients undergoing general anesthesia reported that individuals with CAN required vasopressor support more often than those without CAN (19). Individuals with CAN may experience a greater decline in heart rate and blood pressure during induction of anesthesia and more severe intraoperative hypothermia resulting in decreased drug metabolism and impaired wound healing (19).
Stroke
A recent study in 1,458 patients with type 2 diabetes reported that presence of CAN, assessed by standard HRV testing, was one of the strongest predictors of ischemic stroke in this cohort together with age and hypertension (54). Earlier reports showed similar associations (19).
THERAPEUTIC APPROACHES
Glycemic control
The DCCT demonstrated that intensive insulin therapy for type 1 diabetes reduced the incidence of CAN by 53% compared with conventional therapy (1). The Epidemiology of Diabetes Interventions and Complications (EDIC) study, the prospective observational study of the DCCT cohort, has shown persistent beneficial effects of past glucose control on microvascular complications despite the loss of glycemic separation (55). Recently we evaluated CAN in 1,226 well-characterized EDIC participants during the 13th and 14th year of EDIC follow-up. We found that during EDIC CAN progressed substantially in both treatment groups, but the prevalence and incidence of CAN remained significantly lower in the former intensive group than in the former conventional group, despite similar levels of glycemic control in the EDIC study (56). Treatment group differences in the mean A1C level during the DCCT and the EDIC study explained virtually all of the beneficial effects of intensive versus conventional therapy on risk of incident CAN, supporting the concept that intensive treatment of type 1 diabetes should be initiated as early as is safely possible (56).
In type 2 diabetes, the effects of glycemic control are less conclusive. The VA Cooperative Study demonstrated no difference in the prevalence of autonomic neuropathy in type 2 diabetic patients after 2 years of tight glycemic control compared with those without tight control (57). On the other hand, the Steno-2 Trial reported that a targeted, intensive intervention involving glucose control and multiple cardiovascular risk factors reduced the prevalence of CAN among patients with type 2 diabetes and microalbuminuria (58).
Other therapies
Data regarding the impact of lifestyle interventions in preventing progression of CAN are emerging. Strictly supervised endurance training combined with dietary changes was associated with weight loss and improved HRV in patients with minimal abnormalities (19). In the Diabetes Prevention Program, indexes of CAN improved most in the lifestyle modification arm compared with the metformin or placebo arm.
ACE inhibitors, angiotensin receptor blockers, or aldose reductase inhibitors appear promising but are yet to be validated (19).
Orthostatic hypotension
The treatment of orthostatic hypotension is challenging. Nonpharmacological treatments include avoidance of sudden changes in body posture to the head-up position; avoiding medications that aggravate hypotension, such as tricyclic antidepressants and phenothiazines; eating small, frequent meals to avoid postprandial hypotension; and avoiding activities that involve straining, since increased intra-abdominal and intra-thoracic pressure decrease venous return (19). Several physical counter maneuvers, such as leg crossing, squatting, and muscle pumping can help maintain blood pressure during daily activities by inducing increased cardiac filling pressures and stroke volume.
PHARMACOLOGICAL TREATMENTS
Midodrine
Midodrine, a peripheral-selective α1-adrenoreceptor agonist is the only Food and Drug Administration–approved agent for the treatment of orthostatic hypotension in doses of 2.5–10 mg three times/day. Several double-blind, placebo-controlled studies have documented its efficacy in the treatment of orthostatic hypotension (7). It does not cross the blood-brain barrier, resulting in fewer central side effects. The main adverse effects are piloerection, pruritis, paresthesias, urinary retention, and supine hypertension.
Fludrocortisone acetate
Fludrocortisone acetate, a synthetic mineralocorticoid with a long duration of action, induces plasma expansion and may enhance the sensitivity of blood vessels to circulating catecholamines (59). The effects usually occur over a 1- to 2-week period. Supine hypertension, hypokalemia, and hypomagnesemia may occur. Caution must be used, particularly in patients with congestive heart failure, to avoid fluid overload. Treatment with fludrocortisone should begin with 0.05 mg at bedtime and may be titrated gradually to a maximum of 0.2 mg/day. Doses up to 0.3–0.4 mg used in refractory cases are associated with high risk for hypokalemia, excessive fluid retention, hypertension, and congestive heart failure.
Erythropoietin
Erythropoietin may improve orthostatic hypotension, but the mechanism of action for this pressor effect is still unresolved. Possibilities include the increase in red cell mass and central blood volume, correction of the normochromic normocytic anemia that frequently accompanies severe CAN, and direct or indirect neurohumoral effects on the vascular wall and vascular tone regulation mediated by the interaction between hemoglobin and the vasodilator nitric oxide (59). Erythropoietin is administered subcutaneously or intravenously at doses of 25–75 units/kg three times a week until the hematocrit level approaches normal followed by lower maintenance doses (∼25 units/kg three times/week) (59).
Nonselective β-blockers
Nonselective β-blockers, particularly those with intrinsic sympathomimetic activity, may have a limited role in the treatment of orthostatic hypotension (59). The suggested mechanism of action of these agents is the blockade of vasodilating β-2 receptors allowing unopposed α-adrenoreceptor–mediated vasoconstriction. To date there is no clear efficacy evidence in diabetic CAN.
Clonidine
Clonidine, an α-2 antagonist, produces a central sympatholytic effect and a consequent decrease in blood pressure. Patients with severe CAN have little central sympathetic efferent activity, and the use of clonidine (0.1–0.6 mg/day) could result in an increase in venous return without a significant increase in peripheral vascular resistance. Its use is limited by the inconsistent hypertensive effect and serious side effects.
Somatostatin analogs
Somatostatin analogs (25–200 μg/day) may attenuate orthostatic hypotension in patients with CAN by inhibiting the release of vasoactive gastrointestinal peptides, enhancing cardiac output, and increasing forearm and splanchnic vascular resistance. However, severe cases of hypertension were reported with their use in patients with diabetic CAN (60).
Pyridostigmine bromide
Pyridostigmine bromide, a cholinesterase inhibitor, was recently shown to ameliorate orthostatic hypotension by enhancing ganglionic transmission without worsening supine hypertension (61).
CONCLUSIONS
CAN is a serious chronic complication of diabetes and an independent predictor of cardiovascular disease mortality. As illustrated by the case vignette and by the evidence presented in this review, CAN is associated with a poor prognosis and poor quality of life. Conclusive clinical evidence from randomized prospective trials supports a central role for hyperglycemia in the pathogenesis of CAN, although other metabolic and vascular factors contribute to the disease state. The clinical presentation of CAN comprises a broad constellation of symptoms and deficits. Assessment of HRV is an easily available tool to document the presence of CAN. Cardiac scintigraphic imaging with sympathetic analogs offers more sensitive diagnostic alternatives for research use. The treatment of CAN is challenging. Recent clinical evidence continues to prove the benefits of glycemic control, while the benefits of lifestyle interventions are emerging.
Acknowledgments
R.P.B. is supported by American Diabetes Association Grant 1-08-CR-48, Juvenile Diabetes Research Foundation (JDRF) Grant 1-2008-1025, and JDRF for the Study of Complications of Diabetes Grant 4-200-421.
No potential conflicts of interest relevant to this article were reported.
R.P.B. acknowledges the work of Dr. David Raffel, Department of Radiology, University of Michigan, for assistance in generating the polar map of [11C]HED retention.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia 1998; 41: 416– 423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kennedy WR, Navarro X, Sutherland DE. Neuropathy profile of diabetic patients in a pancreas transplantation program. Neurology 1995; 45: 773– 780 [DOI] [PubMed] [Google Scholar]
- 3. Ziegler D, Dannehl K, Mühlen H, Spüler M, Gries FA. Prevalence of cardiovascular autonomic dysfunction assessed by spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses at various stages of diabetic neuropathy. Diabet Med 1992; 9: 806– 814 [DOI] [PubMed] [Google Scholar]
- 4. Stella P, Ellis D, Maser RE, Orchard TJ. Cardiovascular autonomic neuropathy (expiration and inspiration ratio) in type 1 diabetes: incidence and predictors. J Diabetes Complications 2000; 14: 1– 6 [DOI] [PubMed] [Google Scholar]
- 5. Witte DR, Tesfaye S, Chaturvedi N, Eaton SE, Kempler P, Fuller JH. EURODIAB Prospective Complications Study Group. Risk factors for cardiac autonomic neuropathy in type 1 diabetes mellitus. Diabetologia 2005; 48: 164– 171 [DOI] [PubMed] [Google Scholar]
- 6. Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabó E, Szabó C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes 2002; 51: 514– 521 [DOI] [PubMed] [Google Scholar]
- 7. Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care 2003; 26: 1553– 1579 [DOI] [PubMed] [Google Scholar]
- 8. Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacol Ther 2008; 120: 1– 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Axelrod S, Lishner M, Oz O, Bernheim J, Ravid M. Spectral analysis of fluctuations in heart rate: an objective evaluation of autonomic nervous control in chronic renal failure. Nephron 1987; 45: 202– 206 [DOI] [PubMed] [Google Scholar]
- 10. Pop-Busui R, Kirkwood I, Schmid H, Marinescu V, Schroeder J, Larkin D, Yamada E, Raffel DM, Stevens MJ. Sympathetic dysfunction in type 1 diabetes: association with impaired myocardial blood flow reserve and diastolic dysfunction. J Am Coll Cardiol 2004; 44: 2368– 2374 [DOI] [PubMed] [Google Scholar]
- 11. Taskiran M, Rasmussen V, Rasmussen B, Fritz-Hansen T, Larsson HB, Jensen GB, Hilsted J. Left ventricular dysfunction in normotensive Type 1 diabetic patients: the impact of autonomic neuropathy. Diabet Med 2004; 21: 524– 530 [DOI] [PubMed] [Google Scholar]
- 12. Givertz MM, Sawyer DB, Colucci WS. Antioxidants and myocardial contractility: illuminating the “Dark Side” of beta-adrenergic receptor activation? Circulation 2001; 103: 782– 783 [DOI] [PubMed] [Google Scholar]
- 13. Iwai-Kanai E, Hasegawa K, Araki M, Kakita T, Morimoto T, Sasayama S. alpha- and beta-adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation 1999; 100: 305– 311 [DOI] [PubMed] [Google Scholar]
- 14. Paulson DJ, Light KE. Elevation of serum and ventricular norepinephrine content in the diabetic rat. Res Commun Chem Pathol Pharmacol 1981; 33: 559– 562 [PubMed] [Google Scholar]
- 15. Drake-Holland AJ, Van d V, Roemen TH, Hynd JW, Mansaray M, Wright ZM, Noble MI. Chronic catecholamine depletion switches myocardium from carbohydrate to lipid utilisation. CardiovascDrugs Ther 2001; 15: 111– 117 [DOI] [PubMed] [Google Scholar]
- 16. Schrauwen P, Hoeks J, Hesselink MK. Putative function and physiological relevance of the mitochondrial uncoupling protein-3: involvement in fatty acid metabolism? Prog Lipid Res 2006; 45: 17– 41 [DOI] [PubMed] [Google Scholar]
- 17. Pfeifer MA, Weinberg CR, Cook DL, Reenan A, Halter JB, Ensinck JW, Porte D., Jr Autonomic neural dysfunction in recently diagnosed diabetic subjects Diabetes Care 1984; 7: 447– 453 [DOI] [PubMed] [Google Scholar]
- 18. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996; 93: 1043– 1065 [PubMed] [Google Scholar]
- 19. Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation 2007; 115: 387– 397 [DOI] [PubMed] [Google Scholar]
- 20. Furlan R, Guzzetti S, Crivellaro W, Dassi S, Tinelli M, Baselli G, Cerutti S, Lombardi F, Pagani M, Malliani A. Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and RR variabilities in ambulant subjects. Circulation 1990; 81: 537– 547 [DOI] [PubMed] [Google Scholar]
- 21. Spallone V, Bernardi L, Ricordi L, Soldà P, Maiello MR, Calciati A, Gambardella S, Fratino P, Menzinger G. Relationship between the circadian rhythms of blood pressure and sympathovagal balance in diabetic autonomic neuropathy. Diabetes 1993; 42: 1745– 1752 [DOI] [PubMed] [Google Scholar]
- 22. Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 2002; 347: 797– 805 [DOI] [PubMed] [Google Scholar]
- 23. Schwartz PJ, La Rovere MT, Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation 1992; 85: I77– I91 [PubMed] [Google Scholar]
- 24. Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology 1996; 46: 1470. [DOI] [PubMed] [Google Scholar]
- 25. Ewing DJ, Campbell IW, Clarke BF. Assessment of cardiovascular effects in diabetic autonomic neuropathy and prognostic implications. Ann Intern Med 1980; 92: 308– 311 [DOI] [PubMed] [Google Scholar]
- 26. Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O'Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve 1997; 20: 1561– 1568 [DOI] [PubMed] [Google Scholar]
- 27. Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005; 28: 956– 962 [DOI] [PubMed] [Google Scholar]
- 28. Assessment: clinical autonomic testing report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 1996; 46: 873– 880 [PubMed] [Google Scholar]
- 29. England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, Asbury AK, Szigeti K, Lupski JR, Latov N, Lewis RA, Low PA, Fisher MA, Herrmann D, Howard JF, Lauria G, Miller RG, Polydefkis M, Sumner AJ. American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, American Academy of Physical Medicine and Rehabilitation. Evaluation of distal symmetric polyneuropathy: the role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Muscle Nerve 2009; 39: 106– 115 [DOI] [PubMed] [Google Scholar]
- 30. Gelber DA, Pfeifer M, Dawson B, Schumer M. Cardiovascular autonomic nervous system tests: determination of normative values and effect of confounding variables. J Auton Nerv Syst 1997; 62: 40– 44 [DOI] [PubMed] [Google Scholar]
- 31. Pichot V, Gaspoz JM, Molliex S, Antoniadis A, Busso T, Roche F, Costes F, Quintin L, Lacour JR, Barthélémy JC. Wavelet transform to quantify heart rate variability and to assess its instantaneous changes. J Appl Physiol 1999; 86: 1081– 1091 [DOI] [PubMed] [Google Scholar]
- 32. Toledo E, Gurevitz O, Hod H, Eldar M, Akselrod S. Wavelet analysis of instantaneous heart rate: a study of autonomic control during thrombolysis. Am J Physiol Regul Integr Comp Physiol 2003; 284: R1079– 91 [DOI] [PubMed] [Google Scholar]
- 33. Spallone V, Morganti R, Fedele T, D'Amato C, Maiello MR. Reappraisal of the diagnostic role of orthostatic hypotension in diabetes. Clin Auton Res 2009; 19: 58– 64 [DOI] [PubMed] [Google Scholar]
- 34. Raffel DM, Wieland DM. Assessment of cardiac sympathetic nerve integrity with positron emission tomography. Nucl Med Biol 2001; 28: 541– 559 [DOI] [PubMed] [Google Scholar]
- 35. Stevens MJ, Raffel DM, Allman KC, Dayanikli F, Ficaro E, Sandford T, Wieland DM, Pfeifer MA, Schwaiger M. Cardiac sympathetic dysinnervation in diabetes: implications for enhanced cardiovascular risk. Circulation 1998; 98: 961– 968 [DOI] [PubMed] [Google Scholar]
- 36. Stevens MJ, Raffel DM, Allman KC, Schwaiger M, Wieland DM. Regression and progression of cardiac sympathetic dysinnervation complicating diabetes: an assessment by C-11 hydroxyephedrine and positron emission tomography. Metabolism 1999; 48: 92– 101 [DOI] [PubMed] [Google Scholar]
- 37. Schnell O, Muhr D, Weiss M, Dresel S, Haslbeck M, Standl E. Reduced myocardial 123I-metaiodobenzylguanidine uptake in newly diagnosed IDDM patients. Diabetes 1996; 45: 801– 805 [DOI] [PubMed] [Google Scholar]
- 38. Caldwell JH, Link JM, Levy WC, Poole JE, Stratton JR. Evidence for Pre- to Postsynaptic Mismatch of the Cardiac Sympathetic Nervous System in Ischemic Congestive Heart Failure. J Nucl Med 2008; 49: 234– 241 [DOI] [PubMed] [Google Scholar]
- 39. La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 1998; 351: 478– 484 [DOI] [PubMed] [Google Scholar]
- 40. Hamner JW, Taylor JA. Automated quantification of sympathetic beat-by-beat activity, independent of signal quality. J Appl Physiol 2001; 91: 1199– 206 [DOI] [PubMed] [Google Scholar]
- 41. Low PA, Benrud-Larson LM, Sletten DM, Opfer-Gehrking TL, Weigand SD, O'Brien PC, Suarez GA, Dyck PJ. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care 2004; 27: 2942– 2947 [DOI] [PubMed] [Google Scholar]
- 42. Navarro X, Kennedy WR, Sutherland DE. Autonomic neuropathy and survival in diabetes mellitus: effects of pancreas transplantation. Diabetologia 1991; 34( Suppl. 1): S108– S112 [DOI] [PubMed] [Google Scholar]
- 43. O'Brien IA, McFadden JP, Corrall RJ. The influence of autonomic neuropathy on mortality in insulin-dependent diabetes. Q J Med 1991; 79: 495– 502 [PubMed] [Google Scholar]
- 44. Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH. EURODIAB Prospective Complications Study Group. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care 2008; 31: 1360– 1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, Heethaar RM, Stehouwer CD. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care 2001; 24: 1793– 1798 [DOI] [PubMed] [Google Scholar]
- 46. Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care 2003; 26: 1895– 901 [DOI] [PubMed] [Google Scholar]
- 47. Lykke JA, Tarnow L, Parving HH, Hilsted J. A combined abnormality in heart rate variation and QT corrected interval is a strong predictor of cardiovascular death in type 1 diabetes. Scand J Clin Lab Invest 2008; 12: 1– 6 [DOI] [PubMed] [Google Scholar]
- 48. Ziegler D, Zentai CP, Perz S, Rathmann W, Haastert B, Döring A, Meisinger C. KORA Study Group. Prediction of mortality using measures of cardiac autonomic dysfunction in the diabetic and nondiabetic population: the MONICA/KORA Augsburg Cohort Study. Diabetes Care 2008; 31: 556– 561 [DOI] [PubMed] [Google Scholar]
- 49. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus: the Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329: 977– 986 [DOI] [PubMed] [Google Scholar]
- 50. UKPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837– 853 [PubMed] [Google Scholar]
- 51. Cryer PE. Hypoglycemia-associated autonomic failure in diabetes. Am J Physiol Endocrinol Metab 2001; 281: E1115– E1121 [DOI] [PubMed] [Google Scholar]
- 52. Adler GK, Bonyhay I, Failing H, Waring E, Dotson S, Freeman R. Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes 2009; 58: 360– 366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Heller GV, Iskandrian AE, Wittlin SD, Filipchuk N, Ratner RE, Inzucchi SE. DIAD Investigators. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301: 1547– 1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ko SH, Song KH, Park SA, Kim SR, Cha BY, Son HY, Moon KW, Yoo KD, Park YM, Cho JH, Yoon KH, Ahn YB. Cardiovascular autonomic dysfunction predicts acute ischaemic stroke in patients with Type 2 diabetes mellitus: a 7-year follow-up study. Diabet Med 2008; 25: 1171– 1177 [DOI] [PubMed] [Google Scholar]
- 55. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003; 290: 2159– 2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, Sommer C, Cleary PA, Lachin JM, Herman WH. DCCT/EDIC Research Group. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation 2009; 119: 2886– 2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Azad N, Emanuele NV, Abraira C, Henderson WG, Colwell J, Levin SR, Nuttall FQ, Comstock JP, Sawin CT, Silbert C, Rubino FA. The effects of intensive glycemic control on neuropathy in the VA cooperative study on type II diabetes mellitus (VA CSDM). J Diabetes Complications 1999; 13: 307– 313 [DOI] [PubMed] [Google Scholar]
- 58. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383– 393 [DOI] [PubMed] [Google Scholar]
- 59. Freeman R. Treatment of orthostatic hypotension. Semin Neurol 2003; 23: 435– 442 [DOI] [PubMed] [Google Scholar]
- 60. Pop-Busui R, Chey W, Stevens MJ. Severe hypertension induced by the long-acting somatostatin analogue sandostatin LAR in a patient with diabetic autonomic neuropathy [In Process Citation]. J Clin Endocrinol Metab 2000; 85: 943– 946 [DOI] [PubMed] [Google Scholar]
- 61. Singer W, Sandroni P, Opfer-Gehrking TL, Suarez GA, Klein CM, Hines S, O'Brien PC, Slezak J, Low PA. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol 2006; 63: 513– 518 [DOI] [PubMed] [Google Scholar]