Abstract
The 1982–1994 National Long-Term Care Surveys indicate an accelerating decline in disability among the U.S. elderly population, suggesting that a 1.5% annual decline in chronic disability for elderly persons is achievable. Furthermore, many risk factors for chronic diseases show improvements, many linked to education, from 1910 to the present. Projections indicate the proportion of persons aged 85–89 with less than 8 years of education will decline from 65% in 1980 to 15% in 2015. Health and socioeconomic status trends are not directly represented in Medicare Trust Fund and Social Security Administration beneficiary projections. Thus, they may have different economic implications from projections directly accounting for health trends. A 1.5% annual disability decline keeps the support ratio (ratio of economically active persons aged 20–64 to the number of chronically disabled persons aged 65+) above its 1994 value, 22:1, when the Hospital Insurance Trust Fund was in fiscal balance, to 2070. With no changes in disability, projections indicate a support ratio in 2070 of 8:1—63% below a cash flow balance.
The changing health status of the U.S. elderly population is likely to, and should, have a substantial impact on the future demand for health services. This, in turn, has major implications for the formulation of both public and private health insurance programs, generally, and the future dynamics of Medicare policy and legislation, in particular. Indeed, the ongoing controversy and uncertainty about the long-term solvency of the Medicare Trust Fund and total Medicare costs can be linked directly to the variation among projections about the future distributions of chronic diseases and disability in a rapidly growing U.S. elderly population.
Current Medicare Trust Fund and Social Security Administration (SSA) projections—despite their broad utilization—do not explicitly incorporate population health trends or, as a consequence, the possibility of cost savings accruing to them. Furthermore, ongoing and likely future biotechnology innovations, together with a wide range of primary prevention and health-promoting programs (many of which are designed using data and statistical information from biomedical and epidemiological research) are playing, and will likely continue to play, a major role in determining the number of disability-free life years experienced by future U.S. elderly populations (1–3). The efficacy of these technologically based curative and preventive health strategies, and of an increasing number of formal and informal behavioral/lifestyle health maintenance programs, is a result of the cumulation of products of a long history of basic biomedical and epidemiological research of chronic health problems in the U.S. (refs. 4–6; K.G.M. and K. C. Land, unpublished results). Nevertheless, these features, which are core ingredients in determining the future demand for health services, and correlatively the fiscal basis and actuarial soundness of public and private health insurance and pension programs lie outside of the forecasting methodology that currently is used to help formulate Medicare legislation and financing.
The purpose of this paper is to present alternative projections to those currently put forth by SSA and the Medicare Trustees—these alternate projections that directly reflect the effects of specific monitored health trends—and to identify the population-level interventions that would be needed to continue those positive health trends in the future. These alternate health-based projections imply a substantial downward revision in estimates of the size of the elderly population with chronic disability and, thus, with high, long-term, cumulating health costs. There necessarily also is implied a corresponding revision in the level and structure of projected health service demand. We also present evidence supporting the roles of a range of primary prevention and curative medical interventions in promoting extant declines in disability among the elderly. We are interested, in particular, in prevention and curative strategies and medical treatments that will transform chronic disease processes requiring chronic care into acute disease events by medically truncating the disease process (e.g., ref. 7). Finally, we will delineate the future longitudinal data sources that will be needed to monitor, and make more informed assessments of, U.S. health status dynamics. These data, and better forecasting models, will make possible more detailed and defensible projections of the complete long-term economic consequences of U.S. health status dynamics.
MATERIALS AND METHODS
We used data from the 1982, 1984, 1989, and 1994 National Long-Term Care Surveys (NLTCS). These are nationally representative longitudinal surveys of the U.S. population (both community and institutional residents) aged 65+ at each date. The survey instruments used in all four waves concentrate on chronic impairments and morbidities. Those sets of questions were unchanged across the four survey waves.
To be defined as chronically disabled when initially selected for a detailed interview, a sample person had to have at least one disability in an activity of daily living (ADL; ref. 8) or instrumental activity of daily living (IADL; ref. 9), and such disability had to have lasted, or was expected to last, at least 90 days. Chronic disability was grouped into five categories, i.e., those with one or more IADLs impaired (but no ADL impaired), those with 1–2, 3–4, or 5–6 ADLs impaired (with or without IADL impairments), or persons residing in chronic care institutions who report being chronically disabled. All other persons were defined as not being chronically disabled.
Response rates were uniformly high (95%) in all four surveys. Mortality follow-up is virtually 100% because reporting of deaths is required by the Health Care Financing Administration (HCFA) and is computerized in Medicare data systems. Samples of approximately 20,000 persons were drawn in each survey year from Medicare enrollment lists of persons aged 65+. Overall, in the four surveys, there were 35,848 distinct individuals. Roughly 17,000 deaths occurred from 1982 to 1996. To track both positive and negative changes in disability, in each year persons who were chronically disabled or institutionalized in prior survey years were automatically selected for detailed community or institutional interviews. In addition, roughly 14,000 persons in each survey year were screened for incident disability. Longitudinal samples were supplemented to ensure representation of all persons in the U.S. aged 65 and older by drawing new Medicare list samples of roughly 5,000 beneficiaries who passed age 65 between any two interview dates.
Estimates of the range of potential effects of early interventions on risk factor processes (10) on disability were based on multivariate analyses of longitudinal risk factor, morbidity, and mortality data from the Framingham Heart Study (11). Initiated in 1950, with 2,336 males and 2,873 females aged 29–62, the Framingham Study included biennial assessments of the following risk factors for chronic diseases, which we analyzed by using a multivariate, stochastic process model of state variable dynamics: diastolic blood pressure, pulse pressure, serum cholesterol, vital capacity index, hemoglobin, hematocrit, smoking, body mass index, blood sugar, ventricular (heart) rate, and left ventricular hypertrophy. The fundamental relation of risk factors to chronic morbidity, disability, and mortality was estimated from the 34-year follow-up data from Framingham by using a stochastic process model of risk factor dynamics coupled to a hazard function for morbidity/mortality that was an age-dependent quadratic form in the risk factor variables. For details about the parametric structure of this model and details of its estimation, see ref. 12. Although risk factor mean levels and their variances and covariances will vary across populations, it is assumed that the basic model parameters estimated from the longitudinally followed Framingham study population represent physiological mechanisms intrinsic to the temporally evolving relation of risk factors to chronic disease risks in humans.
Age-specific disability rates for persons in 5-year categories from ages 65 to 69, 90 to 94, and 95+ were calculated from the 1982 and 1994 NLTCS. The two sets of age-specific disability rates were direct-age-standardized to U.S. population projections for 1994–2070 (13). This differs from the usual demographic application of the age standardization of populations in which two or more sets of age-specific rates are compared using one select population as a standard. Here we have only two sets of age-specific rates but 76 different standard populations (i.e., for each year 1994–2070) to which we apply those rates. The years for which the detailed calculations are reported were 1982, 1994, and 2010 [before the World War II (WWII) baby boom cohorts pass age 65], 2030 (after the last WWII baby boom cohort passes age 65), and 2060 and 2070 (two dates near the end of the projection to see which effects the later non-WWII baby boom cohorts have on Medicare expenditures). Furthermore, the 1994 disability rates were reduced by 1.5% for each year since 1994—consistent with the most recent (1989–1994) annual disability rates of decline (1). The 1982 disability rates were held constant. Thus, both sets of age-standardized chronic disability rates changed because of projected changes in the age distribution of the elderly population.
Projections of the number of chronically disabled elderly persons were produced by applying the age-specific disability or institutionalized rates observed for 1982 and 1994—assuming a 1.5% per annum decline after 1994—to three alternate population projections produced in 1996 by the U.S. SSA (14) and an approximation of the “low-cost” projection developed in 1996 for the Federal Hospital Insurance (Medicare Part A) Trust Fund (17). Life tables (18) were, in a separate set of calculations, combined with age-specific disability rate estimates to show the changes in the period-specific age relation of disability and mortality in 1994 and as it is projected for 2070.
An alternative life expectancy scenario was based on population projections for the years 2010, 2030, 2060, and 2070 constrained by estimates based on alternative risk factor distributions (means and variances) and altered risk factor dynamics (both deterministic and stochastic process components) from the Framingham Heart Study. To assess the upper range of life expectancy that was biologically feasible, it was assumed that, beginning in 2010, major risk factor interventions were possible. We assumed that risk factor means could be set to “optimal” levels (12) and that risk factor variances were reduced by 75% initially, with the variance reduction increasing to 100% 20 years after initiation of the interventions. Not surprisingly, this produced much higher life expectancy projections than the official series of projections put forth by the SSA and HCFA. Details of the analytical and statistical strategy used to generate the risk factor-related mortality changes and life expectancy forecasts are reported in ref. 4.
RESULTS
To understand what the changes in the chronically disabled population aged 65+ meant in terms of the payroll tax burden on the U.S. population aged 20–64, we calculated a health-adjusted support ratio—i.e., the ratio of the number of persons in the U.S. population aged 20–64 to the number of chronically disabled persons aged 65+. This differs from standard economic “support ratio” calculations made for SSA by being adjusted for changes in the prevalence of chronic disability in the elderly population—an important determinant of health costs. A non-health-adjusted support ratio may be more appropriate for Social Security Trust Fund calculations in which all survivors aged 65+, regardless of health, receive a pension.
The health-adjusted support ratio assumes that the average health care costs for persons aged 65+ are a function of the population prevalence of chronic disability. Chronic disability is a good predictor of health care costs because chronically disabled persons generally have much higher medical expenses than nondisabled persons and have them for more prolonged periods of time (17).
The prevalence of chronic disability also can be viewed as a “leading” indicator of health changes in the elderly population. This is supported by the fact that the observed declines in the proportion of the U.S. elderly population chronically disabled over time make it plausible that the proportion of the population aged 65+ with chronic morbidity, before the onset of disability, also has been declining (as discussed in ref. 5 and K.G.M. and K. C. Land, unpublished results). Thus, the proportion of the elderly population chronically disabled is a good index of the population ill-health burden. It foreshadows most shifts in the total distribution of acute and chronic health care costs among the U.S. elderly population. It is of interest that the differentials in certain major health cost components (e.g., home health care use) because of disability have increased significantly from 1982 to 1994.
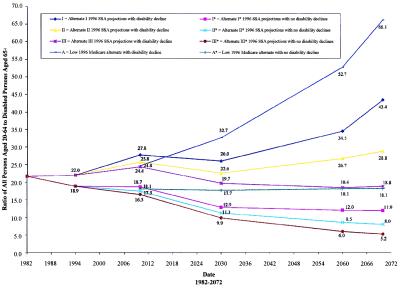
Disability-adjusted support ratios, calculated for each of eight population growth scenarios, are shown in Fig. 1. Six scenarios are based on population forecasts (I*, II*, and III*) made by SSA in 1996. I* assumes high mortality, fertility, and immigration; III* assumes low mortality, fertility, and immigration; and II* assumes intermediate levels on all three components (14). These projections provide the demographic basis for the Hospital Insurance (HI) Trust Fund projections. Two more alternatives, A and A*, are an interpretation of the “low-cost” projections used in the HI Trust Fund report where life expectancy is assumed to peak at the values (73.0 years for males; 79.3 years for females) projected by SSA (12) for their alternate projection I for the year 2000.
Figure 1.
1996 SSA projections with and without disability decline.
Three of the four projections with the 1.5% per annum disability decline keep the support ratio above its 1994 value (i.e., 22 economically active persons aged 25–64 for each chronically disabled person aged 65+) in all years for which the ratio is calculated. In contrast, when no changes in disability are assumed, the support ratio rapidly drops below the 22:1 ratio observed in 1994, when the HI Trust Fund was in approximate cash flow balance. The support ratio of 8:1 projected for the intermediate SSA assumptions with no changes in disability is, in 2070, 63% below the observed ratio for 1994. The calculations assume that the unemployment- and disability-dependent proportions of the population aged 20–64 remain, on average, at their 1994 levels, when program income and costs were roughly in balance.
In addition to these scenarios it is important to consider that there is more uncertainty about future projections of U.S. population size than SSA recognizes (15, 16). Projections implying longer life expectancy than in SSA scenarios could lead to problems with HI Trust Fund balances even with the continuation of 1.5% annual disability declines. Achieving a balance between program income and costs then might require a somewhat higher rate of disability decline and/or longer active working life for the aged 65+ population.
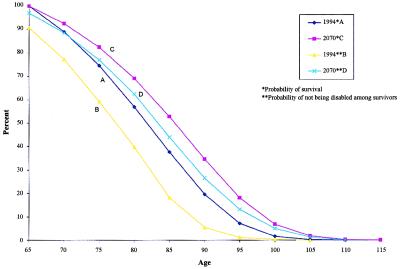
To indicate what plausibly might be accomplished by early-in-life major interventions on multiple risk factors, Fig. 2 displays the probabilities of not being disabled and survival probabilities for specific ages under different forecasting scenarios.
Figure 2.
Comparisons of overall survival and disability-free survival for persons aged 65+ in 1994 and 2070.
Curve A represents the age-specific survival probabilities in 1994. As an alternative, curve C represents age-specific survival probabilities in 2070, projected by using the stochastic process model of risk factor dynamics (12) calibrated on Framingham data and assuming major interventions in multiple risk factors as of 2010. The total area under this curve is the risk factor-adjusted projected life expectancy in 2070, i.e., 98+ years, far exceeding any of the current SSA or HCFA life expectancy projections. Curve B represents the age-specific probabilities of being free of chronic disability in 1994. Curve D represents the age curves corresponding to the probabilities of not being disabled in 2070, assuming a 1.5% per annum decline after 1994. The area between curves A and B (compare with the relation of C and D) represents the expected number of years spent chronically disabled under the respective scenarios. The key point is that the area between C and D in 2070 indicates that about 3 years is expected to be lived in a chronically impaired state. This is less than the difference between A and B, which suggests about 6 years of chronically impaired life expectancy. That is, even with the much greater overall life expectancy forecast for 2070, there are fewer years expected to be lived by individuals, absolutely and relatively, in chronically disabled states.
Since health costs are a function of both the duration and intensity of medical problems, this suggests that the projected declines in chronic disability could have a large effect on future health costs. Although disability is assumed to decline to ages 95+, the largest absolute number of persons with disability prevented by 2070 would be aged 65–90.
Additional calculations done for the above scenarios indicate that the differences in 2070 in the number of persons chronically disabled who are older than 65, assuming a 1.5% per annum reduction in disability, vs. an assumption of no disability change is about 20 million persons. Furthermore, 16.2 million (82.7%) of the total 19.6 million cases of chronic disability projected to be avoided in 2070 if disability rates declined 1.5% per annum are in the age range of 65–89. Thus, most of the projected disability decline would occur at relatively young old ages for which we already have significant clinical experience in reducing disability (20, 21) or in treating many chronic medical problems (22, 23).
DISCUSSION
Our calculations assumed that chronic disability could, with the appropriate public health programs and sufficient biomedical research investments, decline by 1.5% per annum for each year in the 75-year period, 1995–2070. Achievement of declines of this magnitude would require large-scale adoption of preventive strategies, broad dissemination of the existing “best” and new medical technologies to the U.S. elderly population, and significant new investment in biomedical research to continue, and increase, the current rate of biotechnology innovations. Several lines of evidence suggest that this rate of improvement (i.e., 1.5% per annum) with the appropriate interventions is a plausible average rate of decline to maintain for a prolonged period of time with a well developed multidimensional national strategic health program.
The 1982–1994 NLTCS already shows that a 1.5% rate of decline in chronic disability above age 65 is achievable, and other data even suggest that the rate of disability decline may be further accelerating. Furthermore, other evidence suggests that sizable declines in chronic disease prevalence and related disabilities have been occurring in the U.S. for more than 75 years without the benefit of major health research programs. Fogel (23) found that chronic disease prevalence in males declined 6% per decade from 1910 to 1985—roughly a 0.62% per annum decline in chronic disease risks for the 75-year period. These declines were achieved largely because of unplanned interventions, with relatively primitive medical and public health technologies, when relatively little was known about the mechanisms of chronic disease processes occurring at relatively advanced ages. For example, one major source of the decline was a reduction in congestive heart failure apparently due to declines in rheumatic heart disease at younger ages over time.
New data suggest that nutritional factors affecting maternal health and fetal development help explain why chronic disease risks declined from 1910 to 1985 (2). Maternal nutritional deficiencies (e.g., for the 1910 elderly Civil War veteran cohorts, who were born in 1825–1844, whose mothers would have such nutritional deficiencies up to 30 years earlier) could have affected maternal pelvic development and increased subsequent late-age risks for cerebrovascular disease in the child (24). Maternal nutritional deficiency during pregnancy may impact the development of the pancreas and liver in the fetus, which may alter risks of diabetes and heart disease in the child as he or she ages (24).
In addition, environmental factors almost certainly altered chronic disease risks between 1910 and 1985. For example, in the 1920s and 1930s, changes in food preservation, thermal preparation, and storage that affected microbial food contaminants, salt intake, and changes in water quality (e.g., reducing the prevalence of Helicobacter pylori infections) could have affected a wide range of chronic diseases including stroke, hypertension, gastric and other cancer, and gastric ulcers (25, 26). Thus, it is of further interest that Perutz (27) recently found a similar temporal pattern implied by the time rate of growth of the British centenarian population. Up until the 1940s it grew 1% per year. After the 1940s (i.e., centenarians born after the 1840s) the growth rate was nearer 6%. Thus, in Britain, as well as the U.S., there appeared to be major socioeconomic and nutritional changes affecting the health and survival of the post-1840 birth cohorts.
If unplanned nutritional and environmental changes with primitive medical technologies produced a 0.62% per annum decrease in chronic morbidity from 1910 to 1985, it seems that the 1.5% per annum decrease in chronic disability observed in recent years might be maintained in a cost-effective fashion by appropriate investment in biomedical and epidemiological research, rapid promulgation of new biotechnologies, more aggressive efforts at public health intervention (e.g., smoking cessation, promotion of physical activity, behavioral changes in dietary consumption), and, in the more complete population, dissemination of the highest-quality health care.
There are currently many diseases for which known interventions have been proved cost-effective but for which, for a variety of reasons, the full-population health benefit of the intervention has not been experienced. For example, coronary revascularization procedures in U.S. African Americans appear underutilized. In patients for whom the procedure was most likely to be effective (i.e., for whom the extension of life was expected to be greater than 1 year), there were significantly fewer Blacks (42%) than Whites (61%) being treated (28). It has been estimated that adjunctive drug therapy after acute myocardial infarction may reduce mortality by 5–30%. Such therapies, if fully utilized, thus could prevent large numbers of premature deaths (29).
Concerning prevention per se, there is a substantial and growing body of literature documenting the protective effects of regular, vigorous physical exercise (30, 31), appropriate dietary supplementation of vitamins B6, B12, E, and folate (32), and reductions in fat intake. For example, supplementation with B vitamins could reduce circulatory disease deaths by more than 50,000 per year (32). It has been estimated (6) that a nationwide reduction in fat intake could defer approximately 2% of deaths in U.S. adults each year and increase total U.S. life expectancy by 3–4 months. A recent study (33) showed that persons with lower health risks (defined in terms of smoking, body-mass index, and exercise) have less incident disability at older ages and lower levels of cumulative disability and disability at any given age than do persons with higher health risks from the above set. They showed that, while life expectancy increased because of health interventions, the years lived in an active state also increased. A recent longitudinal study (34) showed that better risk-factor profiles were associated with reduced Medicare Part A spending.
Adoption of protective behavioral and lifestyle practices often depends on a person’s level of education and consequent compliance. Changes in the educational level of the elderly population could help sustain a decline of 1.5% per annum in chronic disability for at least another 35 years. Preston (3) projected that the proportion of persons aged 85–89 with less than 8 years of education would decline from about 65% in 1980 to about 15% in 2015. A projection assuming all persons aged 65+ had 8 or more years of education in 1990 suggests that disability could decline to 2025 (when the 1990 cohorts aged 65+ reach ages 100+) by 2.1–2.2% per annum (35, 36)—nearly 40% faster than the rates assumed in the projections presented above.
More informed assessments of health status dynamics and defensible projections of their economic consequences would be greatly facilitated by relatively modest amendments to the design and conduct of future NLTCS. It would be important to enroll younger cohorts with lifestyle, behavioral, and physiologic risk-factor assessments to allow for prospective evaluation of the population adoption of preventive interventions and later-life incident disability in individuals. More extensive assessment of the range of daily physical and social activities of the elderly would allow for documentation of leisure activities and expenditures for them. We view this latter addendum to current data collection efforts to be particularly important to accurately assess national health trends at the individual level, for developing a more comprehensive economic analysis of the impact of high-technology preventive and curative interventions (e.g., which chronic diseases are cured or truncated to acute events; ref. 38), and to assess the long-term economic returns to basic biomedical research investment.
Much of the extant health economics literature (38, 39) views the future availability of such high-technology interventions as directly linked to an increase in demand for them and, hence, substantial increases in health insurance costs. One of the elements neglected in such analyses is the simultaneous consideration of the positive economic benefit of a vastly expanded leisure/sports/vacation/health-fitness industry consequential to significantly increased disability-free life years (40). Another factor possibly not given sufficient weight is the effect of “postinnovation innovation” (41), or the maturation of a therapy or technology after its introduction (e.g., CAT scanners), where efficacy and indication for use can greatly expand. Furthermore, with the extant fertility declines leading to relatively smaller future cohorts of working-age people—an additional 5 years of productive disability-free working life for persons aged 65+ could have a very positive benefit in terms of SSA and Medicare Trust Fund balances. The 1.5% per annum reduction in chronic disability would provide 3 of those 5 years without any changes in U.S. life expectancy. To the extent that such disability reduction extends active life expectancy the benefit could be far more than 5 years. A comprehensive economic analysis incorporating these various economic, health, and technological dynamics lies in the future.
Acknowledgments
K.G.M.’s efforts in this research were supported by grants from the National Institute on Aging. B.H.S.’s contribution was supported by National Institutes of Health Grant P30 HD32030 to Princeton University.
ABBREVIATIONS
- SSA
Social Security Administration
- HI
Hospital Insurance
- NLTCS
National Long-Term Care Survey
References
- 1.Manton K G, Corder L, Stallard E. Proc Natl Acad Sci USA. 1997;94:2593–2598. doi: 10.1073/pnas.94.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith G D. Lancet. 1996;348:1478–1480. doi: 10.1016/S0140-6736(96)03482-4. [DOI] [PubMed] [Google Scholar]
- 3.Preston S H. In: Forecasting the Health of Elderly Populations. Manton K G, Singer B H, Suzman R M, editors. New York: Springer; 1993. pp. 51–77. [Google Scholar]
- 4.Manton K G, Stallard E, Woodbury M A, Dowd J E. J Gerontol Biol Sci. 1994;49:B169–B190. doi: 10.1093/geronj/49.4.b169. [DOI] [PubMed] [Google Scholar]
- 5.Manton K G, Stallard E, Corder L S. J Gerontol Biol Sci. 1998;53:B59–B70. doi: 10.1093/gerona/53a.1.b59. [DOI] [PubMed] [Google Scholar]
- 6.Tosteson A N A, Weinstein M C, Hunink G M, Mittleman M A, Williams L W, Goldman P A, Goldman L. Circulation. 1997;95:24–30. doi: 10.1161/01.cir.95.1.24. [DOI] [PubMed] [Google Scholar]
- 7.Fagan S C, Morganstern L B, Petitta A, Ward R E, Tilley B C, Marler J R, Levine S R, Broderick J P, Kwiatkowski T G, Frankel M, et al. Neurology. 1998;50:883–890. doi: 10.1212/wnl.50.4.883. [DOI] [PubMed] [Google Scholar]
- 8.Katz S, Branch L G, Branson M H, Papsidero J A, Beck J C, Greer D S. N Engl J Med. 1983;309:1218–1223. doi: 10.1056/NEJM198311173092005. [DOI] [PubMed] [Google Scholar]
- 9.Lawton M P, Brody E M. Gerontology. 1969;9:179–186. [PubMed] [Google Scholar]
- 10.Manton K G, Dowd J E, Woodbury M A. In: Forecasting the Health of Elderly Populations. Manton K G, Singer B H, Suzman R M, editors. New York: Springer; 1993. pp. 137–158. [Google Scholar]
- 11.Dawber T R. The Framingham Study: The Epidemiology of Arteriosclerotic Disease. Cambridge, MA: Harvard Univ. Press; 1980. [Google Scholar]
- 12.Manton K G, Stallard E, Singer B H. Int J Health Forecast. 1992;8:433–458. doi: 10.1016/0169-2070(92)90057-g. [DOI] [PubMed] [Google Scholar]
- 13.Day J C. Population Projections of the United States, by Age, Sex, Race, and Hispanic Origin: 1993 to 2050. Washington, DC: U.S. Government Printing Office; 1993. , Series P25–11004. [Google Scholar]
- 14.Social Security Administration. Social Security Area Population Projections: 1996. Baltimore: Social Security Administration; 1996. , Actuarial Study No. 110. [Google Scholar]
- 15.Ahlburg D, Vaupel J. Demography. 1990;27:639–652. [PubMed] [Google Scholar]
- 16.Lee R D, Tuljapurkar S. J Am Stat Assoc. 1994;89:1175–1189. [PubMed] [Google Scholar]
- 17.Board of Trustees, Federal Hospital Insurance Trust Fund. 1996 Annual Report of the Board of Trustees of the Federal Hospital Insurance. Washington, DC: U.S. Government Printing Office; 1996. , the 1996 Annual Report of the Board, pursuant to section 1817(b) of the Social Security Act, as amended. [Google Scholar]
- 18.Social Security Administration. Life Tables for the United States Social Security Area 1900–2080. Baltimore: Social Security Administration; 1992. , Actuarial Study 107, SSA Pub. No. 11–11536. [Google Scholar]
- 19.Manton K G, Singer B H, Suzman R M, editors. Forecasting the Health of Elderly Populations. New York: Springer; 1993. [Google Scholar]
- 20.Fiatarone M A, O’Neill E F, Doyle N, Clements K M, Roberts S, Kehayias J, Lipsitz I, Evans D. J Am Geriatr Soc. 1993;41:333–337. doi: 10.1111/j.1532-5415.1993.tb06714.x. [DOI] [PubMed] [Google Scholar]
- 21.Hosking S W, Ling T K W, Chung S C S, Yung M, Cheng A, Sung J, Li A. Lancet. 1994;343:508–510. doi: 10.1016/s0140-6736(94)91460-5. [DOI] [PubMed] [Google Scholar]
- 22.Elayda M A, Hall R J, Reul R M, Alonzo D, Gillette N, Reul G, Cooley D. Circulation. 1993;88:11–16. [PubMed] [Google Scholar]
- 23.Fogel R W. Am Econ Rev. 1994;84:369–395. [Google Scholar]
- 24.Martyn C N, Barker D J P, Osmond C. Lancet. 1996;348:1264–1268. doi: 10.1016/s0140-6736(96)04257-2. [DOI] [PubMed] [Google Scholar]
- 25.Joossens J V, Hill M J, Elliott P, Stamler R, Lesaffre E, Dyer A, Nichols R, Kesteloot H. Int J Epidemiol. 1996;25:494–504. doi: 10.1093/ije/25.3.494. [DOI] [PubMed] [Google Scholar]
- 26.Parsonnet J. N Engl J Med. 1996;335:278–280. doi: 10.1056/NEJM199607253350411. [DOI] [PubMed] [Google Scholar]
- 27.Perutz, M. (1998) Economist Feb. 7, 82–83.
- 28.Peterson E D, Shaw L K, DeLong E R, Pryor D B, Califf R M, Mark D B. N Engl J Med. 1997;336:480–486. doi: 10.1056/NEJM199702133360706. [DOI] [PubMed] [Google Scholar]
- 29.Hennekens C H, Albert C M, Godfried S L, Guziano J M, Buring J L. N Engl J Med. 1997;335:1660–1667. doi: 10.1056/NEJM199611283352207. [DOI] [PubMed] [Google Scholar]
- 30.Paffenbarger R S, Hyde R T, Wing A L, Lee I M, Jung D L, Kampert J B. N Engl J Med. 1993;328:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 31.Blair S N, Kohl H W, Paffenbarger R S, Clark D G, Cooper K H, Gibbons L W. J Am Med Assoc. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 32.Boushey C J, Beresford S A A, Omenn G S, Motulsky A G. J Am Med Assoc. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 33.Vita A J, Terry R B, Hubert H B, Fries J F. N Engl J Med. 1998;338:1035–1041. doi: 10.1056/NEJM199804093381506. [DOI] [PubMed] [Google Scholar]
- 34.Daviglus M, Liu K, Greenland P, Dyer A, Garside D, Manheim L, Lowe L, Rodin M, Lubitz J, Stamler J. N Engl J Med. 1998;339:1122–1129. doi: 10.1056/NEJM199810153391606. [DOI] [PubMed] [Google Scholar]
- 35.Manton K G, Lowrimore G L, Yashin A I, Tolley H D. Computers Math Appl. 1997;25:89–107. [Google Scholar]
- 36.Manton K G, Corder L S. In: Aging, Social Security and Affordability. Marmor T R, DeJong P R, editors. London: Avebury; 1998. pp. 327–348. [Google Scholar]
- 37.Weisbrod B A. J Econ Literature. 1991;29:523–552. [Google Scholar]
- 38.Garber A M, Phelps C E. J Health Econ. 1997;16:1–31. doi: 10.1016/s0167-6296(96)00506-1. [DOI] [PubMed] [Google Scholar]
- 39.Meltzer D. J Health Econ. 1997;16:33–64. doi: 10.1016/s0167-6296(96)00507-3. [DOI] [PubMed] [Google Scholar]
- 40.Fogel R W, Costa D L. Demography. 1997;34:49–66. [PubMed] [Google Scholar]
- 41.Gelijins A C, Rosenberg N, Moskowitz A J. N Engl J Med. 1998;339:693–698. doi: 10.1056/NEJM199809033391010. [DOI] [PubMed] [Google Scholar]