Abstract
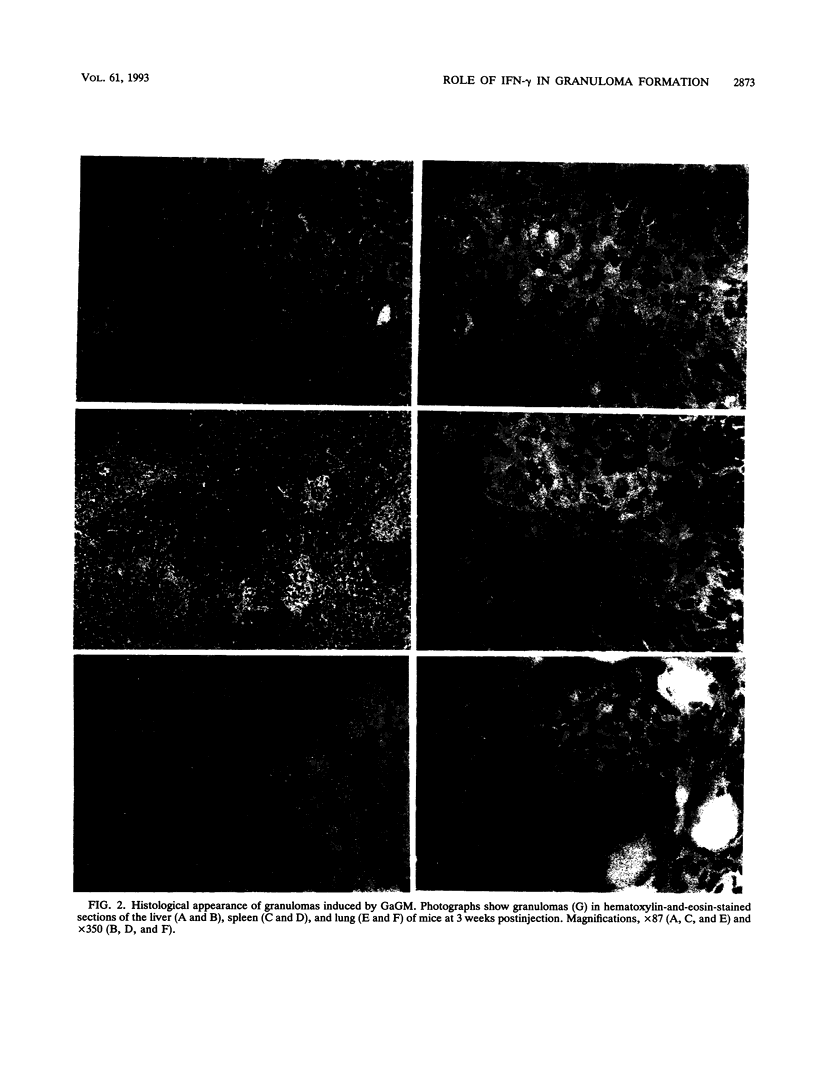
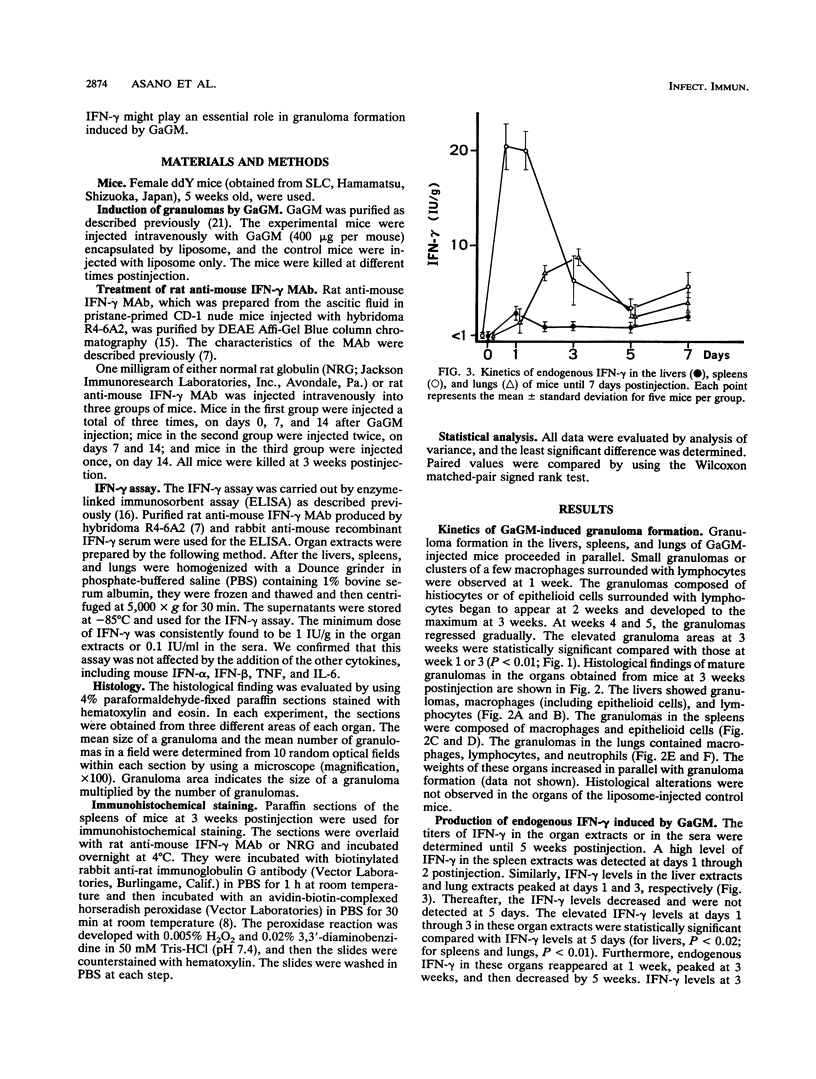
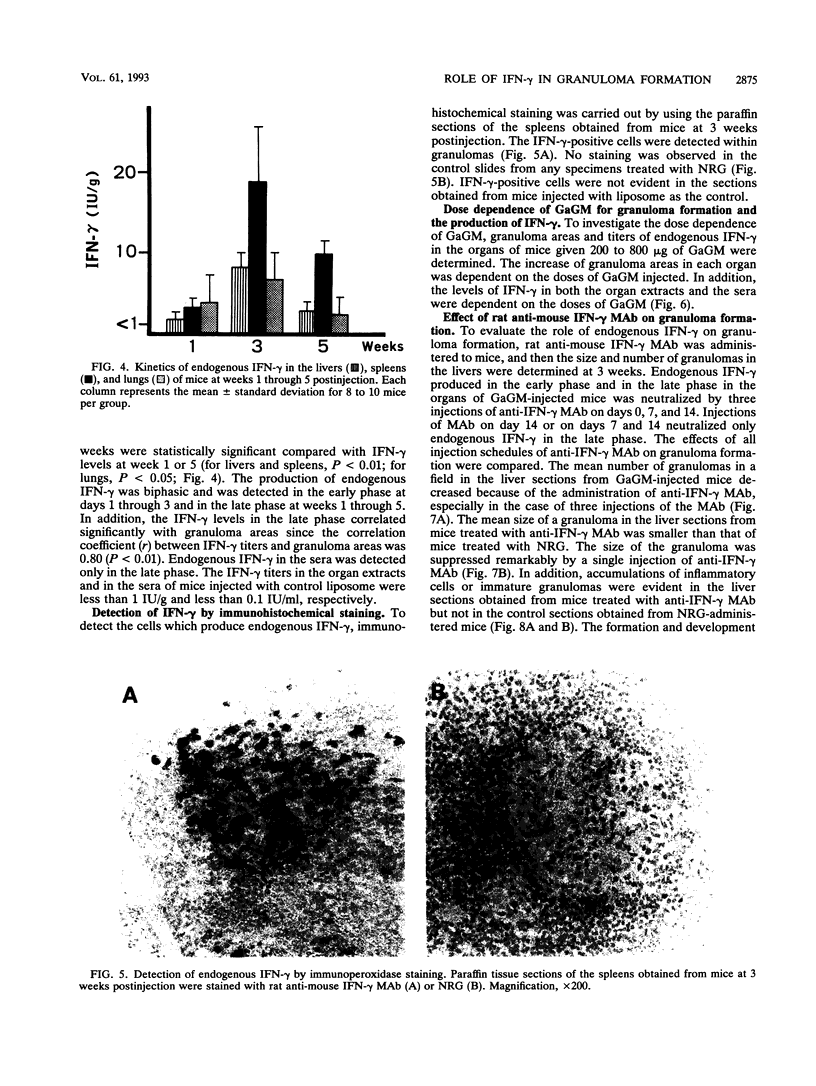
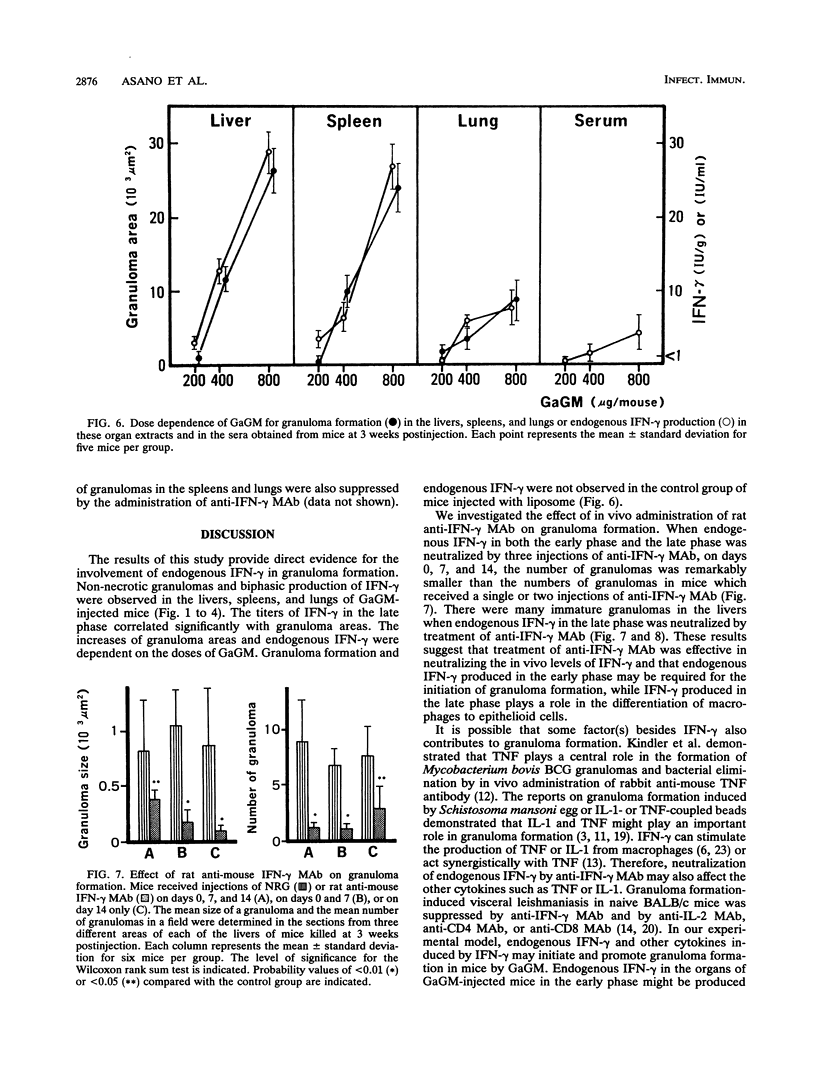
The present study examined the role of endogenous gamma interferon (IFN-gamma) in the formation of granulomas in mice which had been given a single intravenous injection of glycolipid-containing mycolic acid (trehalose 2,3,6'-trimycolate) purified from cell walls of Rhodococcus aurantiacus (Gordona aurantiaca) (GaGM) in the form of liposome. The histological status of granuloma formation in the livers, spleens, and lungs of GaGM-injected mice was studied at weeks 1 through 5, and the titers of endogenous IFN-gamma in all of these organ extracts and in the sera were determined by enzyme-linked immunosorbent assay. The granulomas, composed of epithelioid cells, developed until 3 weeks postinjection, and thereafter the granulomas regressed. The production of endogenous IFN-gamma was biphasic, with an early phase detected at days 1 through 3 and a late phase detected at weeks 1 through 5. The latter peak of endogenous IFN-gamma production proceeded in parallel with granuloma formation. Both the areas of granulomas and titers of IFN-gamma in these organs were dependent on the doses of GaGM used for injection. The cells which produce endogenous IFN-gamma in the spleens appear within the granulomas. To study the role of endogenous IFN-gamma in granuloma formation, the in vivo administration of rat anti-mouse IFN-gamma monoclonal antibody was carried out. Anti-mouse IFN-gamma monoclonal antibody neutralized endogenous IFN-gamma and resulted in the suppression of the number of granulomas and the size of each granuloma. These findings suggest that biphasic production of endogenous IFN-gamma in the local lesions may be crucial to the formation and development of the granulomas.
Full text
PDF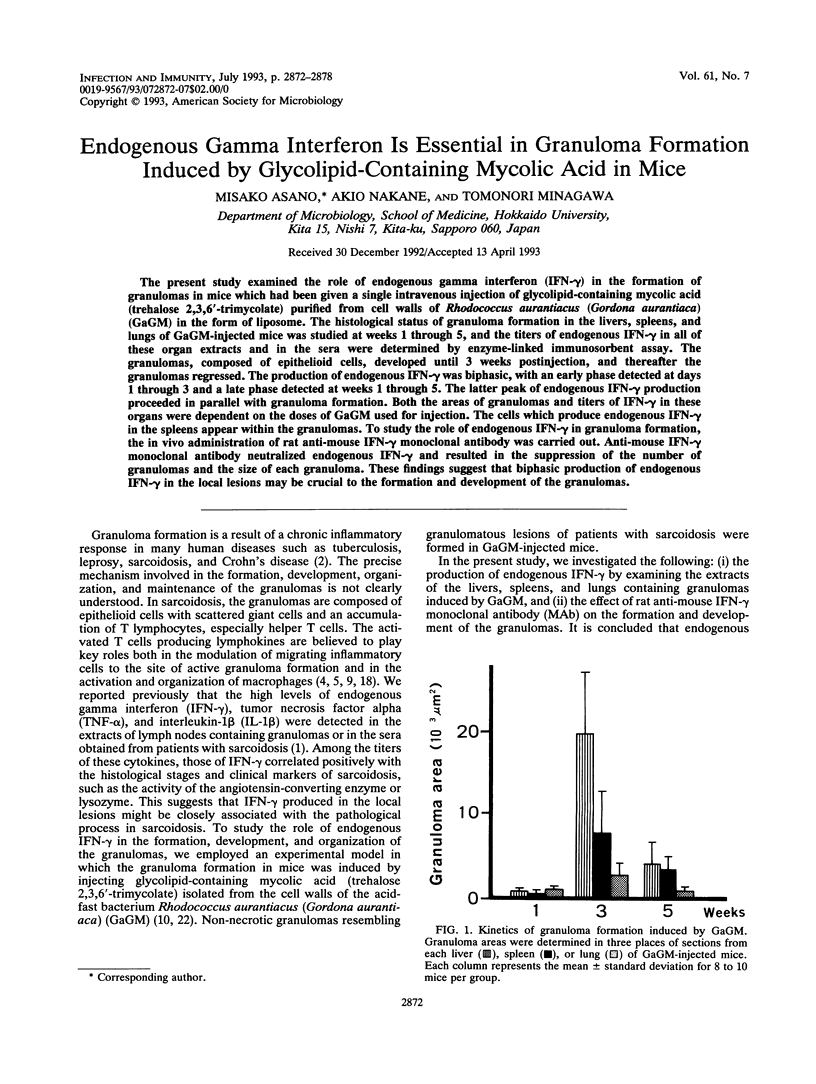


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano M., Minagawa T., Ohmichi M., Hiraga Y. Detection of endogenous cytokines in sera or in lymph nodes obtained from patients with sarcoidosis. Clin Exp Immunol. 1991 Apr;84(1):92–96. doi: 10.1111/j.1365-2249.1991.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Otterness I. G., Higashi G. I., Forsch C. S., Kunkel S. L. Monokine production by hypersensitivity (Schistosoma mansoni egg) and foreign body (Sephadex bead)-type granuloma macrophages. Evidence for sequential production of IL-1 and tumor necrosis factor. J Immunol. 1989 Feb 15;142(4):1281–1286. [PubMed] [Google Scholar]
- Elias J. A., Freundlich B., Kern J. A., Rosenbloom J. Cytokine networks in the regulation of inflammation and fibrosis in the lung. Chest. 1990 Jun;97(6):1439–1445. doi: 10.1378/chest.97.6.1439. [DOI] [PubMed] [Google Scholar]
- Esparza I., Männel D., Ruppel A., Falk W., Krammer P. H. Interferon gamma and lymphotoxin or tumor necrosis factor act synergistically to induce macrophage killing of tumor cells and schistosomula of Schistosoma mansoni. J Exp Med. 1987 Aug 1;166(2):589–594. doi: 10.1084/jem.166.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Purification and further characterization of an anti-murine interferon-gamma monoclonal neutralizing antibody. J Interferon Res. 1986 Oct;6(5):489–497. doi: 10.1089/jir.1986.6.489. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981 May;75(5):734–738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981 Aug 20;305(8):429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- Kaneda K., Sumi Y., Kurano F., Kato Y., Yano I. Granuloma formation and hemopoiesis induced by C36-48-mycolic acid-containing glycolipids from Nocardia rubra. Infect Immun. 1986 Dec;54(3):869–875. doi: 10.1128/iai.54.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K., Kobayashi K., Shikama Y., Yoneya I., Kaga S., Hashimoto M., Odagiri T., Soejima K., Ide H., Takahashi T. The role of monokines in granuloma formation in mice: the ability of interleukin 1 and tumor necrosis factor-alpha to induce lung granulomas. Clin Immunol Immunopathol. 1989 Jun;51(3):419–425. doi: 10.1016/0090-1229(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Oca M. J., Granger A. M., Schreiber R. D. Requirement for T cells and effect of lymphokines in successful chemotherapy for an intracellular infection. Experimental visceral leishmaniasis. J Clin Invest. 1989 Apr;83(4):1253–1257. doi: 10.1172/JCI114009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Squires K. E., Miralles C. D., Stoeckle M. Y., Granger A. M., Granelli-Piperno A., Bogdan C. Acquired resistance and granuloma formation in experimental visceral leishmaniasis. Differential T cell and lymphokine roles in initial versus established immunity. J Immunol. 1992 Mar 15;148(6):1858–1863. [PubMed] [Google Scholar]
- NIH conference. Pulmonary sarcoidosis: a disease characterized and perpetuated by activated lung T-lymphocytes. Ann Intern Med. 1981 Jan;94(1):73–94. doi: 10.7326/0003-4819-94-1-73. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kohanawa M., Chen Y., Sato H., Moriyama M., Tsuruoka N. Interactions between endogenous gamma interferon and tumor necrosis factor in host resistance against primary and secondary Listeria monocytogenes infections. Infect Immun. 1989 Nov;57(11):3331–3337. doi: 10.1128/iai.57.11.3331-3337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Numata A., Asano M., Kohanawa M., Chen Y., Minagawa T. Evidence that endogenous gamma interferon is produced early in Listeria monocytogenes infection. Infect Immun. 1990 Jul;58(7):2386–2388. doi: 10.1128/iai.58.7.2386-2388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Numata A., Chen Y., Minagawa T. Endogenous gamma interferon-independent host resistance against Listeria monocytogenes infection in CD4+ T cell- and asialo GM1+ cell-depleted mice. Infect Immun. 1991 Oct;59(10):3439–3445. doi: 10.1128/iai.59.10.3439-3445.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi P. K., Goldstein R. A. Immunopathogenesis, immunology and assessment of activity of sarcoidosis. Ann Allergy. 1984 May;52(5):316–328. [PubMed] [Google Scholar]
- Shikama Y., Kobayashi K., Kasahara K., Kaga S., Hashimoto M., Yoneya I., Hosoda S., Soejima K., Ide H., Takahashi T. Granuloma formation by artificial microparticles in vitro. Macrophages and monokines play a critical role in granuloma formation. Am J Pathol. 1989 Jun;134(6):1189–1199. [PMC free article] [PubMed] [Google Scholar]
- Squires K. E., Schreiber R. D., McElrath M. J., Rubin B. Y., Anderson S. L., Murray H. W. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. J Immunol. 1989 Dec 15;143(12):4244–4249. [PubMed] [Google Scholar]
- Tomiyasu I., Yano I. Separation and analysis of novel polyunsaturated mycolic acids from a psychrophilic, acid-fast bacterium, Gordona aurantiaca. Eur J Biochem. 1984 Feb 15;139(1):173–180. doi: 10.1111/j.1432-1033.1984.tb07991.x. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Kobayashi M., Rosen M., Loudon R., Murphy M., Perussia B. Tumor necrosis factor and lymphotoxin induce differentiation of human myeloid cell lines in synergy with immune interferon. J Exp Med. 1986 Oct 1;164(4):1206–1225. doi: 10.1084/jem.164.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]