Abstract
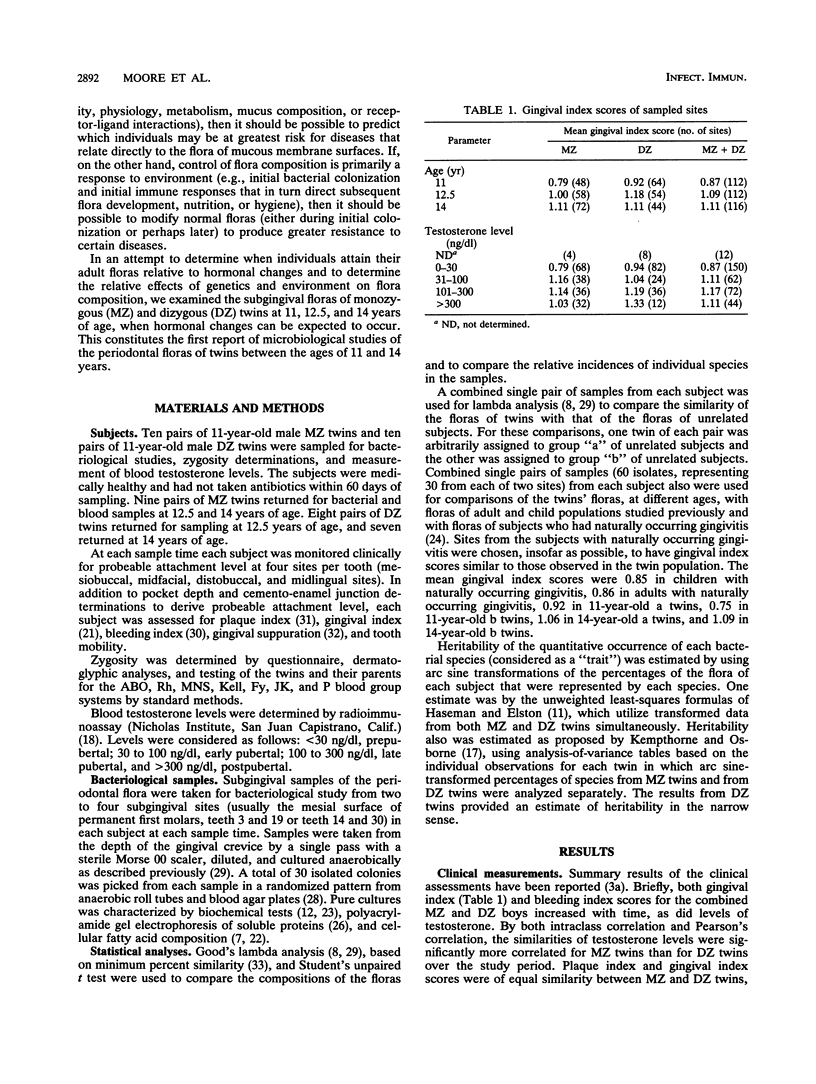
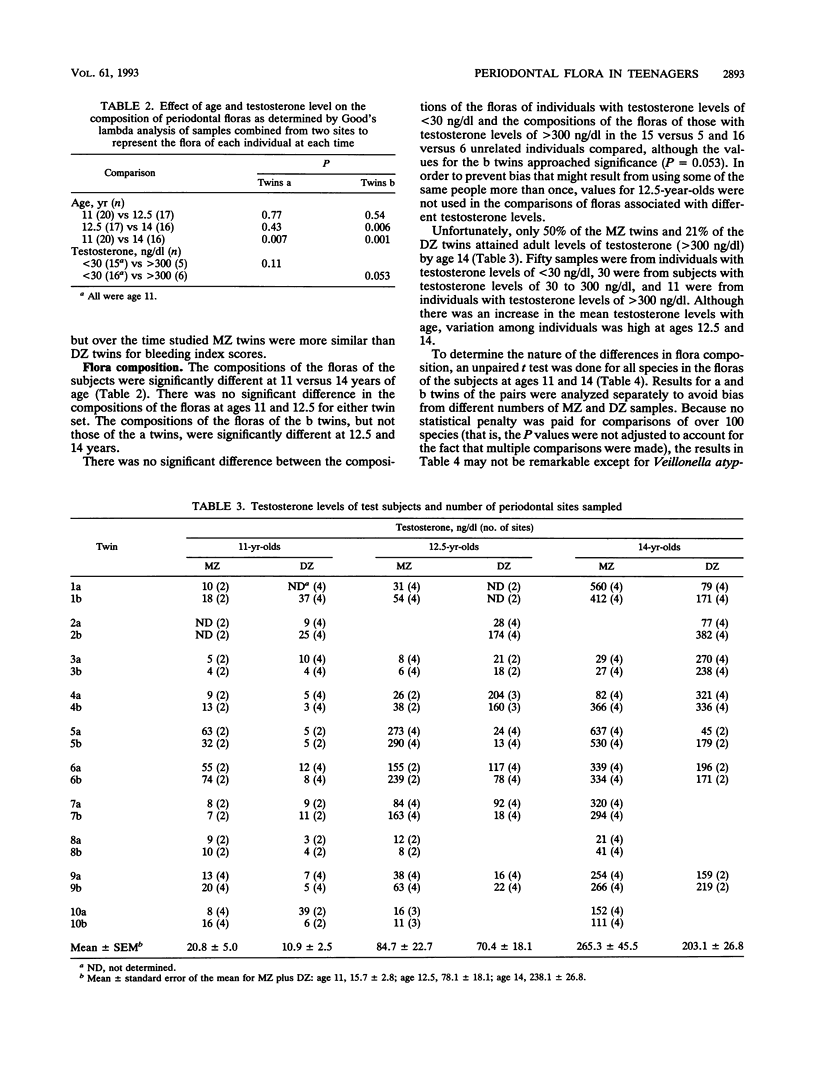
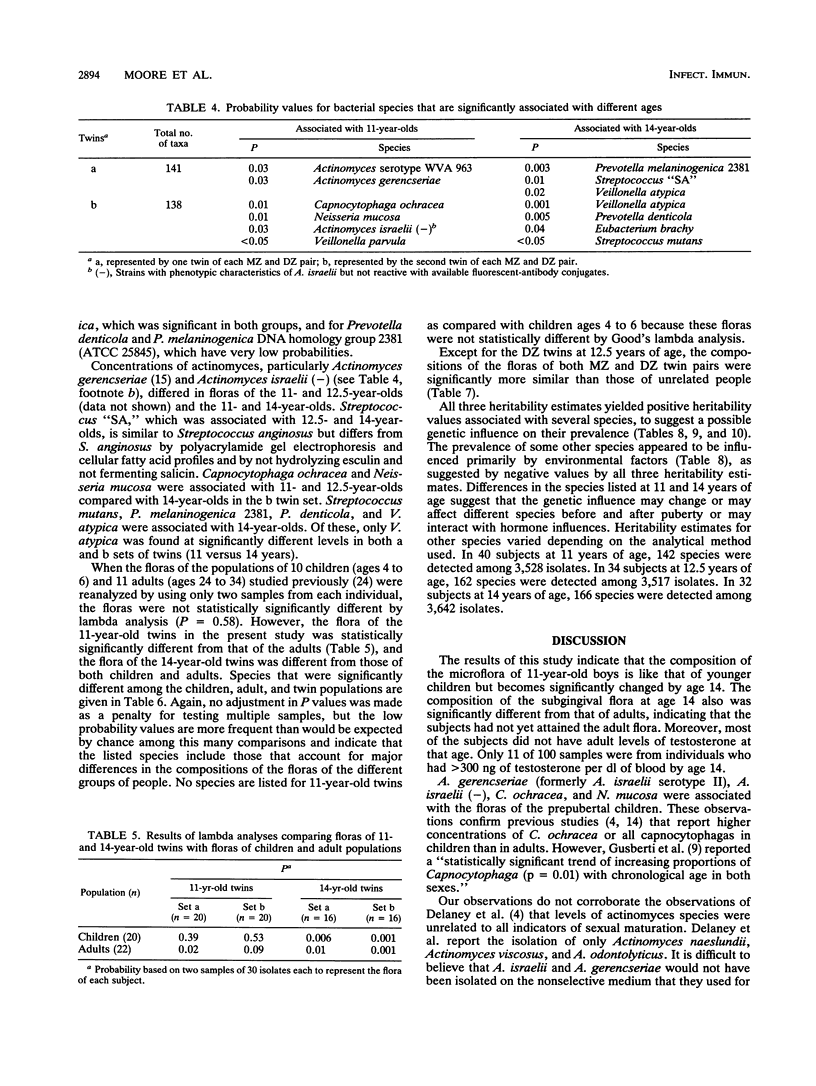
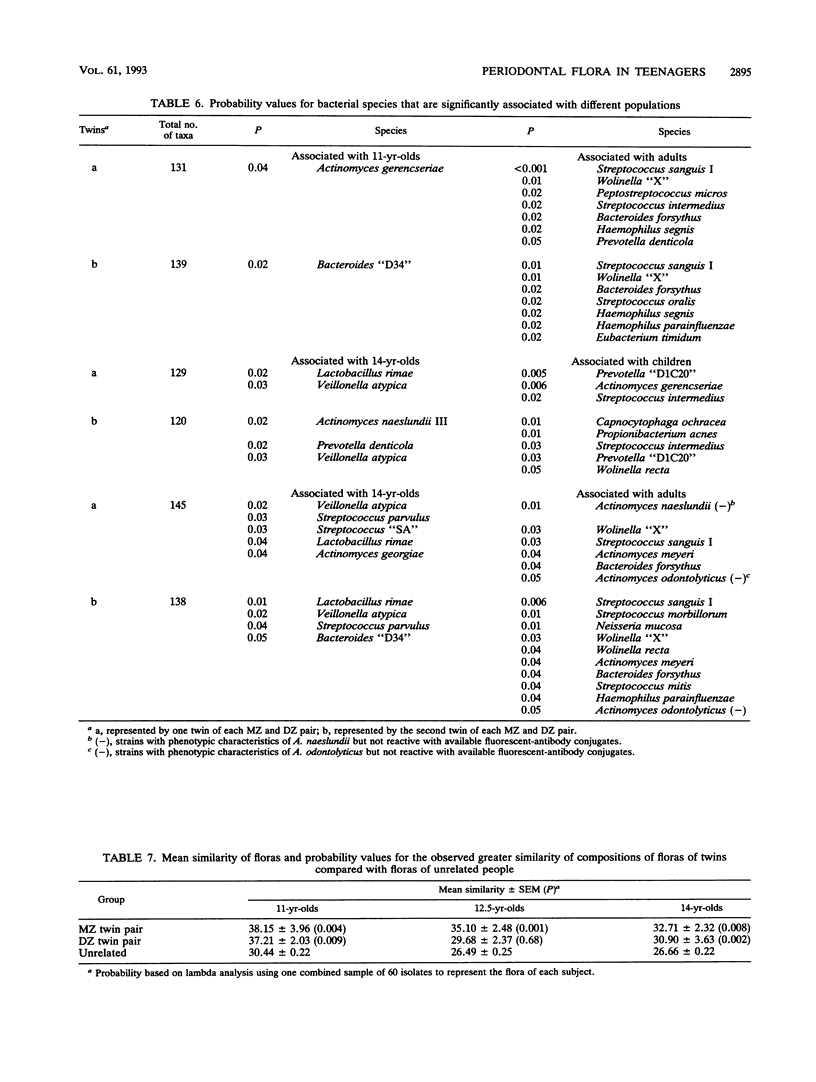
The classical twin model was utilized in this study in an attempt to determine the importance of host genetics to the composition of the subgingival flora. Simultaneously, the effect of puberty on the flora composition was assessed. The compositions of the floras were significantly different at ages 11 and 14 in the same people, indicating that transition to an adult flora composition may be initiated during puberty. However, the numbers of subjects who had prepubertal and postpubertal testosterone levels in this study were too small to demonstrate significant differences based solely on testosterone level (P = 0.053 and 0.11 for tests of unrelated members, i.e., all twins "a," the first twin of each pair, and all twins "b," the second twin of each pair). Sixteen unrelated 11-year-old subjects had prepubertal levels of less than 30 ng of testosterone per dl of serum, and only six of these unrelated subjects had levels above 300 ng/dl by age 14. Of their twin siblings, who formed the second group of unrelated individuals, 15 had prepubertal levels and only 5 reached postpubertal levels. Unpaired t tests indicated that Veillonella atypica, Prevotella denticola, and Prevotella melaninogenica were among the species that contributed most to changes in flora composition during puberty. The compositions of subgingival floras of 11-year-old monozygous and dizygous male twins were significantly more similar than those of unrelated subjects in the study (P = 0.004 and 0.009, respectively). At 12.5 years of age, the floras of monozygous twins remained more similar than those of unrelated subjects (P = 0.001), but the dizygous-twin floras were not significantly more similar than those of unrelated people. This difference corresponded with moderate and varied testosterone levels within dizygous-twin pairs at age 12.5. By age 14 both monozygous and dizygous twins again had floras with compositions more similar than those of unrelated people (P = 0.008 and 0.002, respectively). Estimates of the genetic contributions to the increased similarity of the floras of twins as compared with floras of unrelated people indicated that the concentrations of several species in the flora may be influenced by host genetic factors. The prevalence of certain other species appeared to be controlled primarily by environment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asikainen S., Alaluusua S., Kari K., Kleemola-Kujala E. Subgingival microflora and periodontal conditions in healthy teenagers. J Periodontol. 1986 Aug;57(8):505–509. doi: 10.1902/jop.1986.57.8.505. [DOI] [PubMed] [Google Scholar]
- BAILIT H. L., BALDWIN D. C., HUNT E. E., Jr THE INCREASING PREVALENCE OF GINGIVAL BACTEROIDES MELANINOGENICUS WITH AGE IN CHILDREN. Arch Oral Biol. 1964 Jul-Aug;9:435–438. doi: 10.1016/0003-9969(64)90028-7. [DOI] [PubMed] [Google Scholar]
- Bornside G. H., Cohn I., Jr Stability of normal human fecal flora during a chemically defined, low residue liquid diet. Ann Surg. 1975 Jan;181(1):58–60. doi: 10.1097/00000658-197501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney J. E., Ratzan S. K., Kornman K. S. Subgingival microbiota associated with puberty: studies of pre-, circum-, and postpubertal human females. Pediatr Dent. 1986 Dec;8(4):268–275. [PubMed] [Google Scholar]
- Drasar B. S., Jenkins D. J., Cummings J. H. The influence of a diet rich in wheat fibre on the human faecal flora. J Med Microbiol. 1976 Nov;9(4):423–431. doi: 10.1099/00222615-9-4-423. [DOI] [PubMed] [Google Scholar]
- Frisken K. W., Tagg J. R., Laws A. J., Orr M. B. Suspected periodontopathic microorganisms and their oral habitats in young children. Oral Microbiol Immunol. 1987 Jun;2(2):60–64. doi: 10.1111/j.1399-302x.1987.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Ghanem F. M., Ridpath A. C., Moore W. E., Moore L. V. Identification of Clostridium botulinum, Clostridium argentinense, and related organisms by cellular fatty acid analysis. J Clin Microbiol. 1991 Jun;29(6):1114–1124. doi: 10.1128/jcm.29.6.1114-1124.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusberti F. A., Mombelli A., Lang N. P., Minder C. E. Changes in subgingival microbiota during puberty. A 4-year longitudinal study. J Clin Periodontol. 1990 Nov;17(10):685–692. doi: 10.1111/j.1600-051x.1990.tb01054.x. [DOI] [PubMed] [Google Scholar]
- Gusberti F. A., Syed S. A., Bacon G., Grossman N., Loesche W. J. Puberty gingivitis in insulin-dependent diabetic children. I. Cross-sectional observations. J Periodontol. 1983 Dec;54(12):714–720. doi: 10.1902/jop.1983.54.12.714. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Elston R. C. The estimation of genetic variance from twin data. Behav Genet. 1970 Feb;1(1):11–19. doi: 10.1007/BF01067367. [DOI] [PubMed] [Google Scholar]
- Holdeman L. V., Good I. J., Moore W. E. Human fecal flora: variation in bacterial composition within individuals and a possible effect of emotional stress. Appl Environ Microbiol. 1976 Mar;31(3):359–375. doi: 10.1128/aem.31.3.359-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdeman L. V., Moore W. E., Cato E. P., Burmeister J. A., Palcanis K. G., Ranney R. R. Distribution of capnocytophaga in periodontal microfloras. J Periodontal Res. 1985 Sep;20(5):475–483. doi: 10.1111/j.1600-0765.1985.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Moore L. V., Kaneko B., Moore W. E. Actinomyces georgiae sp. nov., Actinomyces gerencseriae sp. nov., designation of two genospecies of Actinomyces naeslundii, and inclusion of A. naeslundii serotypes II and III and Actinomyces viscosus serotype II in A. naeslundii genospecies 2. Int J Syst Bacteriol. 1990 Jul;40(3):273–286. doi: 10.1099/00207713-40-3-273. [DOI] [PubMed] [Google Scholar]
- KEMPTHORNE O., OSBORNE R. H. The interpretation of twin data. Am J Hum Genet. 1961 Sep;13:320–339. [PMC free article] [PubMed] [Google Scholar]
- Kelstrup J. The incidence of bacteroides melaniogenicus in human gingival sulci, and its prevalence in the oral cavity at different ages. Periodontics. 1966 Jan-Feb;4(1):14–18. [PubMed] [Google Scholar]
- Kinouchi T., Pages L., Horton R. A specific radioimmunossay for testosterone in peripheral plasma. J Lab Clin Med. 1973 Aug;82(2):309–316. [PubMed] [Google Scholar]
- Kornman K. S., Loesche W. J. Effects of estradiol and progesterone on Bacteroides melaninogenicus and Bacteroides gingivalis. Infect Immun. 1982 Jan;35(1):256–263. doi: 10.1128/iai.35.1.256-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornman K. S., Loesche W. J. The subgingival microbial flora during pregnancy. J Periodontal Res. 1980 Mar;15(2):111–122. doi: 10.1111/j.1600-0765.1980.tb00265.x. [DOI] [PubMed] [Google Scholar]
- LOE H., SILNESS J. PERIODONTAL DISEASE IN PREGNANCY. I. PREVALENCE AND SEVERITY. Acta Odontol Scand. 1963 Dec;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Moore L. V., Moore W. E., Cato E. P., Smibert R. M., Burmeister J. A., Best A. M., Ranney R. R. Bacteriology of human gingivitis. J Dent Res. 1987 May;66(5):989–995. doi: 10.1177/00220345870660052401. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Hash D. E., Holdeman L. V., Cato E. P. Polyacrylamide slab gel electrophoresis of soluble proteins for studies of bacterial floras. Appl Environ Microbiol. 1980 Apr;39(4):900–907. doi: 10.1128/aem.39.4.900-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Cato E. P., Good I. J., Smith E. P., Ranney R. R., Palcanis K. G. Variation in periodontal floras. Infect Immun. 1984 Dec;46(3):720–726. doi: 10.1128/iai.46.3.720-726.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Cato E. P., Burmeister J. A., Palcanis K. G., Ranney R. R. Bacteriology of experimental gingivitis in children. Infect Immun. 1984 Oct;46(1):1–6. doi: 10.1128/iai.46.1.1-6.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Good I. J., Burmeister J. A., Palcanis K. G., Ranney R. R. Bacteriology of experimental gingivitis in young adult humans. Infect Immun. 1982 Nov;38(2):651–667. doi: 10.1128/iai.38.2.651-667.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlemann H. R., Son S. Gingival sulcus bleeding--a leading symptom in initial gingivitis. Helv Odontol Acta. 1971 Oct;15(2):107–113. [PubMed] [Google Scholar]
- SILNESS J., LOE H. PERIODONTAL DISEASE IN PREGNANCY. II. CORRELATION BETWEEN ORAL HYGIENE AND PERIODONTAL CONDTION. Acta Odontol Scand. 1964 Feb;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- Speck R. S., Calloway D. H., Hadley W. K. Human fecal flora under controlled diet intake. Am J Clin Nutr. 1970 Nov;23(11):1488–1494. doi: 10.1093/ajcn/23.11.1488. [DOI] [PubMed] [Google Scholar]
- Wojcicki C. J., Harper D. S., Robinson P. J. Differences in periodontal disease-associated microorganisms of subgingival plaque in prepubertal, pubertal and postpubertal children. J Periodontol. 1987 Apr;58(4):219–223. doi: 10.1902/jop.1987.58.4.219. [DOI] [PubMed] [Google Scholar]
- Yanover L., Ellen R. P. A clinical and microbiologic examination of gingival disease in parapubescent females. J Periodontol. 1986 Sep;57(9):562–567. doi: 10.1902/jop.1986.57.9.562. [DOI] [PubMed] [Google Scholar]
- Zambon J. J., Reynolds H. S., Slots J. Black-pigmented Bacteroides spp. in the human oral cavity. Infect Immun. 1981 Apr;32(1):198–203. doi: 10.1128/iai.32.1.198-203.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



