Abstract
Purpose
To developed a genetic mouse model of primary open-angle glaucoma induced by expression of mutated human myocilin in transgenic mice and to test whether expression of mutated human myocilin in the eye angle structures produces more significant damage to the eye than does mutated mouse myocilin.
Methods
Recombineering in Escherichia coli was used to introduce the Tyr437His point mutation into a BAC carrying the full-length human MYOCILIN (MYOC) gene and long flanking regions. This BAC was used to produce transgenic mice. The expression of myocilin in the iridocorneal angle tissues and aqueous humor was studied by immunohistochemistry and Western blot analysis. Intraocular pressure was measured non-invasively with a fiber optic transducer. Retinal ganglion cells were retrograde labeled with fluorescent gold, and counted 5 days after labeling.
Results
BAC transgenic mice expressed elevated levels of myocilin in tissues of the iridocorneal angle. Expression of mutated myocilin induced its intracellular accumulation and prevented secretion of both mutated and wild-type myocilin into the aqueous humor. Transgenic mice demonstrated a moderate elevation of intraocular pressure, which was more pronounced at night than in daytime. In the peripheral retina, transgenic mice lost 20% of the retinal ganglion cells and 55% of large retinal ganglion cells. Axonal degeneration was observed at the periphery of the optic nerve.
Conclusions
Expression of equivalent levels of mutated human or mouse myocilin in the eyes of transgenic mice produce comparable pathologic changes that are similar to those observed in patients with glaucoma.
Glaucoma, a group of neurodegenerative disorders, is one of the leading causes of blindness in the world. It is characterized by the death of retinal ganglion cells, the degeneration of axons in the optic nerve, and a specific deformation of the optic nerve head. In glaucoma, peripheral vision declines first, whereas the loss of central vision occurs much later. Elevated intraocular pressure (IOP) is one of the main risk factors in glaucoma, but it is still not known how elevated IOP kills retinal ganglion cells. Primary open-angle glaucoma is the most common form of glaucoma and will affect more than 60 million people and blind approximately 4.5 million worldwide by 2010.1 Despite the high frequency and severity of glaucoma, little is known about the molecular mechanisms underlying the pathologic effects of glaucoma.
It is now well established that a genetic component may contribute to glaucoma, and several glaucoma-associated genes have been identified. The first identified and the most studied gene is myocilin (MYOC), which is heavily expressed in and secreted by the trabecular meshwork,2–4 one of the key components of the eye’s aqueous humor outflow system. The myocilin protein belongs to a family of glycosylated proteins containing a C-terminal olfactomedin domain.5
Dominant mutations in MYOC are found in 3% to 4% of patients with primary open-angle glaucoma,6 and most of these glaucoma-causing mutations are located in the olfactomedin domain.7–9 Mutant myocilin protein is not secreted and actually blocks the secretion of nonmutated myocilin.10–12 It has been suggested that the intracellular accumulation of myocilin aggregates is deleterious to the trabecular meshwork cells and leads to the deterioration of their function and subsequent elevation of IOP.13,14 Among the identified mutations, the Tyr437His mutation in the olfactomedin domain of myocilin is associated with a severe form of glaucoma, for which the average age at diagnosis is 20 years.15
Animal models of glaucoma represent a useful tool for studying the molecular mechanisms of glaucoma, and several genetic mouse models of glaucoma have been developed. Mice deficient in the glutamate transporters Glast or Eaac1 demonstrate retinal ganglion cell and optic nerve degeneration without elevated IOP.16 DBA/2J mice develop a progressive form of secondary angle-closure glaucoma resembling pigmentary dispersion glaucoma in humans.17 A transgenic mouse strain with a targeted mutation in the gene for the α1 subunit of collagen type I demonstrates a gradual elevation of IOP and progressive optic nerve axon loss.18 Recently, a mouse strain expressing the Tyr423His mouse myocilin point mutation, corresponding to the Tyr437His mutation in the human myocilin, was developed and characterized.19 Expression of mutated myocilin in the iridocorneal angle and sclera induced its accumulation in cell cytoplasm and prevented its secretion into the extracellular space. Transgenic mice demonstrated a moderate elevation of intraocular pressure, the loss of ~20% of the retinal ganglion cells in the peripheral retina, and axonal degeneration in the optic nerve.
Although mouse and human myocilin proteins have many similarities,12,20,21 recent data suggest that only human, not mouse, myocilin, has a peroxisomal targeting sequence at the C terminus and that exposure of this sequence in mutant human myocilin is critical for the interaction with peroxisomal targeting signal type 1 receptor and subsequent elevation of IOP.22 It has been suggested that expression of mutated human myocilin in the eyes of transgenic mice produces more dramatic effects than expression of mutated mouse myocilin and may lead to a valuable mouse model of human primary open-angle glaucoma.22
In this study, we produced transgenic mice carrying the full-length human MYOC gene with a Tyr437His point mutation. These mice showed changes similar to those observed in human primary open-angle glaucoma and provide a new genetic model of this disease. The degree of IOP elevation and retinal damage produced by expression of mutant human myocilin were comparable to that obtained with the mutant mouse myocilin.
Methods
Production of Transgenic Mice
The Tyr437His point mutation (T to C in codon 437) was introduced into BAC DNA RP11-23L17 using oligonucleotide-based recombination in Escherichia coli, as described previously.19,23,24 The presence of missense mutation was confirmed by sequencing. Transgenic mice were produced by injection of mutated BAC DNA into the pronucleus of fertilized mouse FVB/N oocytes. The founders and all subsequent generations were crossed to C57/BL6 mice. All experiments were conducted with F5 and later generations.
IOP Measurement
The mice were managed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were housed in a 12-hour day (6 AM–6 PM) and 12-hour night (6 PM–6 AM) environment. They were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). IOP was measured in anesthetized animals by using a fiber-optic signal (FTI-10) conditioner equipped with a fiber-optic pressure transducer (FISO Technologies, Montreal, Quebec, Canada) as described previously.19,25 Daytime IOP measurements were made from 9 AM to 1 PM and nighttime measurements from 9 PM to 1 AM. IOP from each eye was recorded during a 2-minute session, with four measurements taken at 30-second intervals within each session. The average of these four measurements was used to calculate IOP for each eye. Dacriose sterile eye irrigation solution (Novartis, Basel, Switzerland) was topically applied before and after each IOP measurement. Statistical differences were analyzed with Student’s t-test.
Retrograde Labeling of Retinal Ganglion Cells
For neuronal survival experiments, retinal ganglion cells were retrogradely labeled with 2% fluorescent gold (FluoroGold; Fluorochrome, Englewood, CO). For retrograde labeling, both superior colliculi, the main targets of retinal ganglion cells in the brain, were exposed, and a small piece of sponge (Gelfoam; Pharmacia and Upjohn, Inc., New York, NY) soaked in the gold dye was applied to their surfaces. Five days after application, the time required for labeling the entire retinal ganglion cell population, the animals were scarified and the retinal ganglion somas were counted (n = 10, 18-month-old mice).
Quantification of Retinal Ganglion Cells
Quantification of retinal ganglion cell bodies was performed in duplicate and in a masked fashion. For retinal ganglion cell density counts, mice were perfused intracardially with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer, and both eyes were immediately enucleated. Retinas were dissected and flatmounted on a glass slide with the ganglion cell layer side up. Under fluorescence microscopy, the gold-labeled neurons were counted in four chosen retinal areas located in the periphery of the superior, inferior, nasal and temporal retinal hemispheres. The center of the area is 2142 μm from the optic nerve head and the area is 0.54 × 0.71 μm. Four measurements were averaged and converted to retinal ganglion cell soma density (retinal ganglion cell count per square millimeter). All images were captured by a digital camera (Spot RT; Diagnostic Instruments, Inc., Sterling Heights, MI) attached to a fluorescence microscope (Axioplan 2; Carl Zeiss Meditec, Inc., Thornwood, NY). Quantification of gold fluorescence–labeled retinal ganglion cells were processed by commercial software (Image Pro Plus, ver. 5.1.0 for Windows XP; Media Cybernetics Inc., Silver Spring, MD). Data were analyzed with Student’s t-test.
Immunohistochemistry
The mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) and perfused transcardially with 4% paraformaldehyde in 0.1 M PB (pH 7.4), and the eyes were postfixed at 4°C for 2 hours before processing for cryosection embedding or whole-retina preparation. Radial cross-sections (10 μm) of iridocorneal angle were prepared and then incubated with affinity-purified antibodies against mouse Myoc at 4°C overnight.19,26 The sections were then incubated with fluorophore-conjugated goat anti-mouse IgG (4 μg/mL; Alexa 594; Invitrogen-Molecular Probes, Eugene, OR) at room temperature for 1 hour, washed in PBS, and mounted using an antifade reagent (Slow-Fade; Invitrogen-Molecular Probes). For whole-retina immunostaining, tissue was permeabilized in PBS containing 2% Triton X-100 and 0.5% DMSO at 4°C for 3 days and incubated in blocking solution (10% normal goat serum in 2% Triton X-100 and 0.5% DMSO) for 1 hour at room temperature. The retinas were incubated with monoclonal neurofilament (NF) RT-97 antibody, which recognizes phosphorylated NF-H (10 μg/mL; Chemicon International, Temecula, CA) or monoclonal neurofilament (SMI-32) antibody against nonphosphorylated NF-H (1:100, Sternberger-Covance, Lutherville, MD), followed by incubation with Alexa 594 goat anti-mouse IgG secondary antibody (4 μg/mL). Fluorescent staining was examined by fluorescence microscopy, as described earlier.
Western Blot Analysis
For aqueous humor collection, the mouse eyes were rinsed with normal saline and dried with nonabrasive wipes (Kimwipes; Kimberly-Clark, Neenah, WI). The center of the cornea was punctured with a 32-gauge needle syringe, and aqueous humor was withdrawn from the anterior chamber of the eye, approximately 4 μL from each eye. Aqueous humor proteins were separated by SDS-PAGE (10% Tris/glycine gels) and then transferred to a nitrocellulose membrane. Another gel loaded with the same amount of aqueous humor was stained with Coomassie blue for normalization. The iridocorneal angle tissues included the ciliary body, trabecular meshwork, and base of the iris and cornea. Retinal pigment epithelium, choroid, and sclera were mixed together in one pool. Twenty micrograms of each extract were used per lane in Western blot experiments. Nonspecific binding was blocked by incubating blots in 10 mM Tris (pH 8.0), 150 mM NaCl, 0.1% Tween 20 (TBST), and 5% lyophilized skim milk at room temperature for 1 hour. Membranes were incubated with an affinity-purified rabbit polyclonal antibody against mouse myocilin at a 1:1000 dilution. The blots were washed in TBST and then incubated with horse anti-rabbit secondary antibodies conjugated to horseradish peroxidase (Vector Laboratories, Burlingame, CA). Protein signals were detected with a chemiluminescence reagent (ECL plus kit; GE Healthcare, Biosciences, Piscataway, NJ), followed by exposure of blots to X-OMAT (Eastman-Kodak, Rochester, NY) imaging film. For β-actin normalization, blots were reprobed with CY3 conjugated monocolonal anti-actin antibody and scanned (Typhoon 9410 Variable Mode Image; GE Healthcare) and analyzed (Image Pro Plus 5.1; Media Cybernetics).
Semithin Section and Light Microscopy
For morphologic studies, mice were perfused transcardically with 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M PB (pH 7.4). The eye globe and optic nerve fragment (n = 8, 18-month-old mice) 0.5 mm posterior to the eye globe were dissected and placed in 1% osmium tetroxide. After dehydration and infiltration with propylene oxide alone and with propylene oxide mixed with epoxy, the pieces were embedded in Araldite 502/Embed-812 embedding medium (Electron Microscopy Science, Hatfield, PA). Semithin (1 μm) sections were cut on an ultramicrotome with a glass knife. Semithin sections were stained with a mixture of toluidine blue, methylene blue, and azure II, 0.25% each in 1.0% sodium borate, or with paraphenylene diamine (1% in 1:1 isopropanol: methanol mixture).
Results
Generation of BAC Transgenic Mice Expressing Mutated Human MYOC
BAC DNA RP11-23L17 containing the full-length human MYOC gene, as well as approximately 89 kb and 51 kb of the 5′-flanking and 3′-flanking sequences, respectively was used for transgenic mouse production. Since available data suggest that pathogenesis of MYOC-related glaucoma is dependent on the expression of abnormal mutant protein, the Tyr437His point mutation was introduced into MYOC BAC DNA in vitro, as described in the Materials and Methods section. Four lines of mice, carrying mutated mouse MYOC BAC DNA, were developed. One line was selected for further analysis.
Characterization of Myocilin Expression in the Eyes of Transgenic Mice and Morphologic Analysis of the Iridocorneal Angle
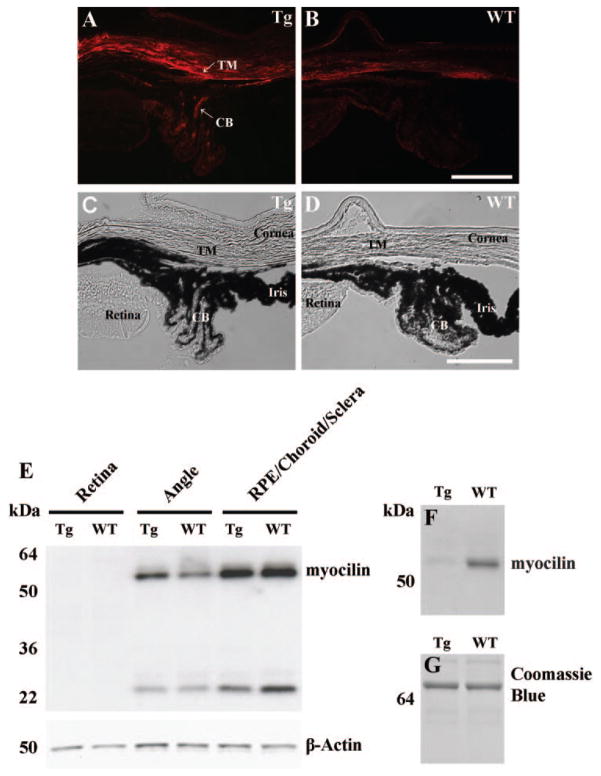
Distribution of myocilin in the eyes of wild-type (WT) and transgenic mice was studied by immunostaining and by Western blot analysis using affinity-purified antibodies against mouse myocilin. Previous experiments showed that these antibodies recognize both mouse and human myocilin proteins.12 Immunostaining of the eye cross-sections demonstrated that myocilin was highly expressed in the trabecular meshwork, ciliary body, and sclera of transgenic mice, indicating that the expression pattern of the human MYOC transgene in the eyes reproduces the expression pattern of the endogenous mouse Myoc gene (Fig. 1A). When compared with WT littermates, transgenic mice showed reproducibly higher levels of immunostaining in the trabecular meshwork and ciliary body (four pairs were analyzed; compare Figs. 1A and 1B).
Figure 1.
Myocilin expression in the eyes of 18-month-old WT and transgenic mice. (A–B) Myocilin immunostaining showing higher levels of immunostaining in the trabecular meshwork–sclera region and iris of transgenic mice (A) as compared with WT control animals (B). (C, D) The same images as in (A) and (B), but in bright field, to show morphological structures. Red, myocilin. (E, F) Western blot analysis of the myocilin content of eye tissues (E) and aqueous humor (F) of WT and transgenic mice. Twenty micrograms of protein per well for eye tissues and 3 μg of protein per well for aqueous humor were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and stained with myocilin antibodies (1:1000 dilution). Staining with actin antibodies was used as a normalization control for the eye tissue extracts. Staining with Coomassie blue was used for the control of equal protein loading of aqueous humor (G). The band with apparent molecular mass at ~24 kDa in (E) represents a product of specific endoproteolitic cleavage of myocilin. CB, ciliary body; TM, trabecular meshwork.
Elevated levels of myocilin in the angle tissues of transgenic mice were confirmed by Western blot analyses (Fig. 1E). Digital chemiluminescence quantification of myocilin on Western blots showed that myocilin contents in the eye angle tissues of 18-month-old transgenic mice were two to threefold higher, compared with their WT littermates. When young (4- to 6-month-old) transgenic mice were analyzed, the magnitude of total myocilin increase in the angle tissues was significantly smaller (not shown). The levels of myocilin proteins were comparable in the combined retinal pigmented epithelium, choroid, and sclera of WT and transgenic animals (Fig. 1E).
Our previous experiments demonstrated that human and mouse myocilin proteins are able to form heterodimers and that the presence of mutated human myocilin prevents secretion of WT mouse myocilin in cell culture.12 To check whether expression of mutated human myocilin blocks secretion of WT mouse myocilin in the eye in vivo, we collected aqueous humor from the anterior chambers of transgenic or WT mouse eyes and used Western blot analysis to estimate the levels of myocilin proteins. A specific myocilin band was detected in the aqueous humor of WT but not in that of transgenic animals (Fig. 1F). These data demonstrate that the observed levels of the mutated human myocilin expression in the trabecular meshwork and iris are sufficient to block secretion of WT mouse myocilin.
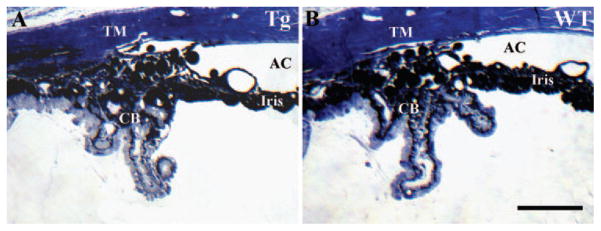
To determine whether the accumulation of WT and mutated human myocilin in the trabecular meshwork and iris of transgenic mice affects the aqueous human drainage structures, we used histology of the iridocorneal angle tissues. Semithin sections through the eyes of 18-month-old transgenic mice (n = 6) showed normal iridocorneal angle structures indistinguishable from those of WT eyes (n = 6; Fig. 2).
Figure 2.
Morphopathologic analysis of the iridocorneal angle. Semithin sections of the eye angle tissues of 18-month-old transgenic (A) and WT (B) mice. Scale bar, 100 μm.
IOP Measurements
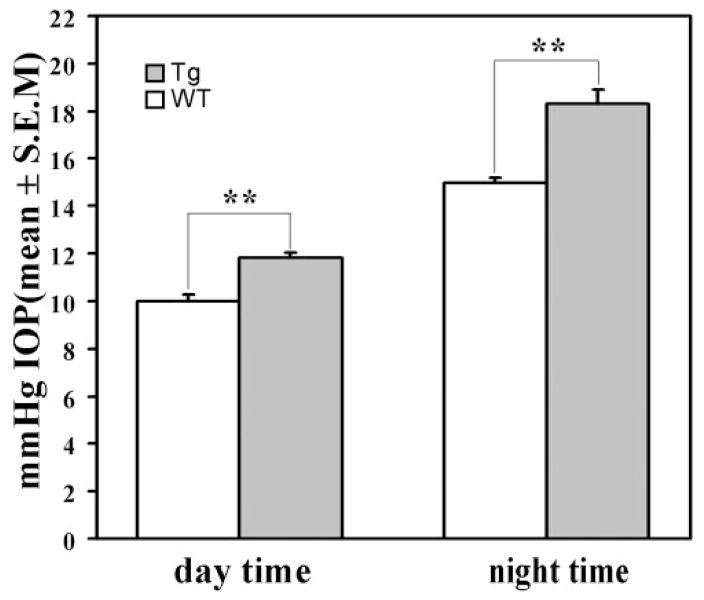
To check whether expression of mutated human myocilin may affect the aqueous humor balance, we measured IOP in transgenic mice and their WT littermates by using a noninvasive applanation tonometer as described in the Materials and Methods section.19,25 IOP was first measured during the day from 9 AM to 1 PM. We did not find any difference in IOP between 6-month-old transgenic and WT mice (not shown). IOP was approximately 2 mm Hg higher in the eyes of 18-month-old transgenic animals than that in the eyes of their WT littermates (11.8 ± 1.2 vs. 10.0 ± 0.8 mm Hg, n = 26, P < 0.001; Fig. 3).
Figure 3.
IOP elevation in the eyes of transgenic mice. Results of IOP measurements in 18-month-old transgenic and WT mice.
It is now well established that there is a diurnal variation of IOP in humans, with the highest IOPs detectable in the morning. Moreover, the magnitude of the variation is higher in glaucoma than in normal individuals.27 Mice are nocturnal animals, and it has been shown that IOP is increased in the dark period in some mouse strains, including the C57/BL6 strain.28,29 Therefore, we measured IOP during the night from 9 PM to 1 AM (Fig. 3). IOP was approximately 3.4 mm Hg higher in the eyes of the 18-month-old transgenic animals than that in the eyes of the WT littermates (18.3 ± 2.2 vs. 14.9 ± 0.9 mm Hg, n = 18, P < 0.001).
Analysis of Retinal Ganglion Cell Degeneration in Wholemount Retinas of Transgenic Mice
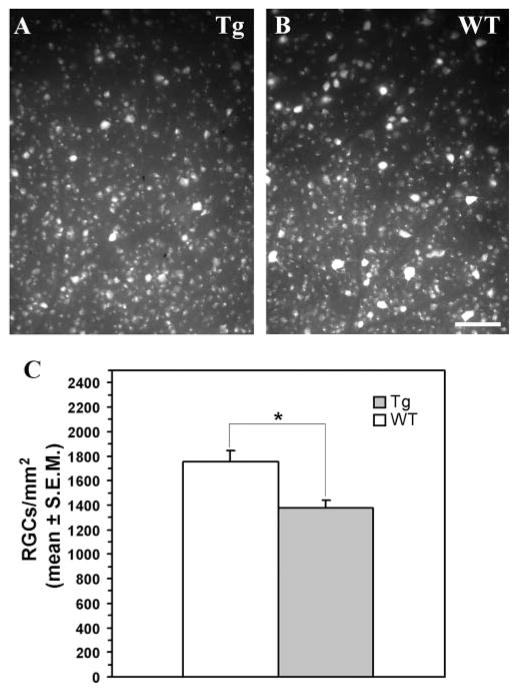
To find out whether the expression of the mutated human MYOC gene causes damage to retinal ganglion cells, we visualized these cells in wholemounts by retrograde gold fluorescent labeling (FluoroGold; Fluorochrome). There were no statistically significant differences in the density of retinal ganglion cells in the central retina between transgenic and WT mice (not shown). However, loss of approximately 20% of retinal ganglion cells was detected in the peripheral retinas of the 18-month-old transgenic mice compared with the WT littermates (1379 ± 59 vs. 1753 ± 89 cells/mm2, n = 10, P < 0.01; Fig. 4).
Figure 4.
Retinal ganglion cell degeneration in the peripheral retina of 18-month-old transgenic mice. Fluorescent gold–labeled retinal ganglion cells in transgenic (A) and WT (B) retinas. (C) Quantitative analysis of retinal ganglion cell density in WT and transgenic retinas.
There are at least 14 different morphologic types of retinal ganglion cells in mice.30,31 Large ganglion cells were define as cells having a large soma and a large, sparsely branched dendritic trees.30–33 To determine whether large retinal ganglion cells are more vulnerable to damage in transgenic animals, we stained wholemount retinas from WT and transgenic mice with monoclonal antibody SMI-32 against a nonphosphorylated epitope neurofilament H. This antibody preferentially labels soma and dendrites of large retinal ganglion cells in both WT and genetically modified mice.33 There were 55% fewer SMI-32-marked large retinal ganglion cells in the peripheral retinas of the transgenic mice (Fig. 5A) in relation to the corresponding areas of WT littermates (Fig. 5B; 87 ± 18 vs. 39 ± 23 cells/mm2, n = 6, P < 0.05, 18-month-old mice).
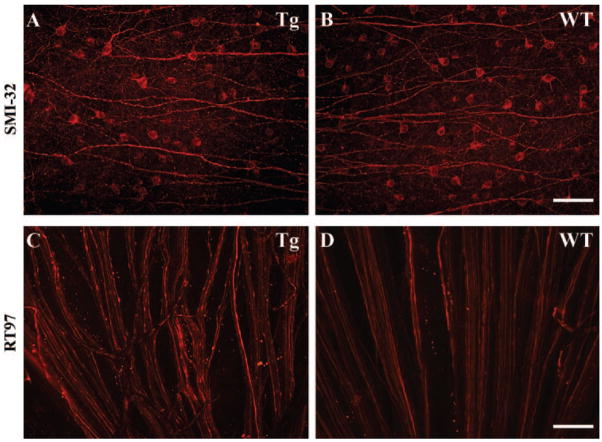
Figure 5.
Immunofluorescence analysis of wholemounted retinas of 18-month-old transgenic mice. Representative images of the retinal ganglion cell layer of transgenic (A) and WT (B) mice stained with the SMI-32 antibody. The estimates showed that there were 55% less SMI-labeled cells in transgenic versus WT retinas. Representative images of retinal ganglion cell axons in the nerve fiber layer of transgenic (C) and WT (D) mice stained with the RT-97 antibody.
Analysis of Retinal Axonal Degeneration
Glaucoma is characterized by the degeneration of retinal ganglion cell axons in both the retinal nerve fiber layer and the optic nerve. Hence, we investigated the effect of mutant human myocilin on retinal ganglion cell axons. Figures 5C and 5D show unmyelinated axons of the retinal nerve fiber layer visualized by staining of wholemount retinas with RT-97 antibody, which recognizes the phosphorylated 200-kDa neurofilament H subunit. In WT retinas, immunoreactive axons formed well-ordered bundles near the optic nerve head (Fig. 5D). In transgenic retinas, the overall structure of the axon bundles was distorted, and many fibers had a beaded appearance characteristic of degeneration33 (Fig. 5C).
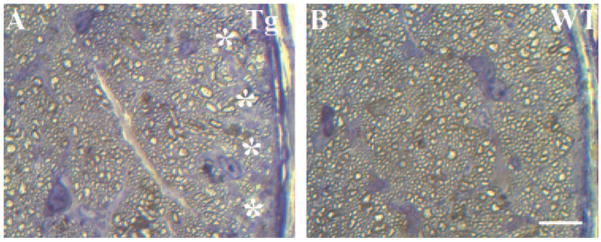
To analyze morphologic changes in the myelinated optic nerve, we cut semithin transverse sections ~1 to 2 mm behind the globe and stained them with a mixture of toluidine blue, methylene blue, and azure II (Fig. 6). In contrast to their WT littermates (Fig. 6B), the 18-month-old transgenic mice showed optic nerves (Fig. 6A) with loss of axonal profiles at the nerve periphery, disarray of fascicular organization, and degradation of myelin sheaths (n = 8, 18-month-old mice).
Figure 6.
Degeneration of the optic nerve in transgenic mice. Semithin sections through transgenic (A) and WT (B) optic nerves of 18-month-old mice were stained with toluidine blue, methylene blue, and azure II. Degenerating axons in the transgenic optic nerve were detected as accumulating darkly stained structures. Patches of empty space without axons (A,*) were observed in the peripheral areas of transgenic, but not WT, optic nerves.
Discussion
Previous studies have demonstrated that neither the absence of myocilin26 nor elevated levels of myocilin in the eye drainage structures34 lead to IOP elevation or morphologic changes in the eye. However, transgenic expression of mutated Tyr423His mouse myocilin in eye angle tissues induced moderate elevation of IOP, the loss of approximately 20% of retinal ganglion cells in the peripheral retina, and axonal degeneration in the optic nerve.19 These changes are all characteristic features of primary open-angle glaucoma in humans. Although mouse and human myocilin proteins are 82% identical,20,21 they have different C-terminal sequences. It has been demonstrated that mutated human, but not mouse, protein interacts with peroxisomal targeting signal type 1 receptor and that only mutated human myocilin induces quick elevation of IOP when overexpressed in the eye.22 These data suggest that comparable levels of mutated human myocilin, when expressed in the eye angle structures, may produce more significant damage to the eye than does mouse myocilin.22 In the present study, we test this hypothesis and describe the development of a genetic mouse model of primary open-angle glaucoma by expression of a BAC transgene carrying a clinically defined mutation of the human MYOC gene.
The human BAC MYOC transgene reproduced the expression pattern of endogenous mouse Myoc. It was expressed in the trabecular meshwork and iris of transgenic mice, as judged by Western blot analysis of dissected tissues and immunohistochemistry. We observed a two- to threefold increase in the total levels of human and mouse myocilin in the drainage structures of 18-month-old transgenic mice, compared with WT. The magnitude of this increase was similar to that observed in transgenic mice expressing mutated mouse myocilin.19
Similar to observations in cell culture experiments,10,12,13 mutated myocilin was not secreted, preventing secretion of endogenous mouse myocilin. This results in the accumulation of myocilin within cells and the absence of WT mouse and mutated human myocilin proteins in aqueous humor. Inhibition of myocilin secretion into aqueous humor was also reported in mice in which the endogenous mouse Myoc allele was replaced with the Tyr423His mutant allele.35 However, inhibition of secretion appeared more pronounced in heterozygotes carrying mutated human MYOC than in heterozygotes carrying mutated mouse Myoc (compare Fig. 1E in this article and Fig. 4G in Ref. 35). Stronger inhibition of WT myocilin secretion in our transgenic line may be explained by higher intracellular levels of mutated myocilin in aging transgenic animals compared with knock-in heterozygotes. This phenomenon may lead to the intracellular trapping of WT myocilin into complexes with mutated myocilin.
As with the expression of mutated mouse myocilin,19,35 expression of mutated human myocilin did not lead to pronounced morphologic changes in the tissues of the iridocorneal angle. Our previous experiments demonstrated that expression of mutated mouse Myoc did not induce trabecular meshwork cell death.19 However, accumulation of myocilin inside the cells of trabecular meshwork and sclera was associated with increased pathologic changes in the retina and optic nerve, which were similar to those observed in patients with glaucoma.
IOP is considered a major causal risk factor in primary open-angle glaucoma.35–37 We observed moderate elevation of IOP in the eyes of 18-month-old transgenic mice. This elevation was approximately 2 mm Hg in daytime and approximately 3.4 mm Hg at night. Similar elevation was observed in mice transgenic for mutant mouse myocilin, in which only daytime measurements of IOP were conducted.19 At present, we do not fully understand how the expression of mutated MYOC leads to an increase in IOP. It is interesting to note that mice in which the endogenous mouse Myoc allele was replaced with a mutant allele did not show any IOP increase in the daytime.35 These differences may be explained by different levels of mutated myocilin expression, differences in the tissue distribution of mutated myocilin protein, and environmental differences. Preliminary experiments indicate that transgenic expression of normal human myocilin in the eye-angle tissues did not lead to IOP elevation, supporting the idea that the Tyr437His mutation is critical for the observed changes (Zhou Y, Tomarev SI, unpublished data, 2006). The observed IOP increase in the BAC transgenic mice is smaller than that observed in humans with the same MYOC mutation,15 but it is significant that this modest IOP increase still causes pathologic changes in the retina and optic nerve.
Quantitative analysis of wholemount retinas after retrograde labeling with fluorescent gold indicated a 20% depletion of retinal ganglion cells in the peripheral retina of transgenic mice without significant cell loss in the central retina. The severity of retinal ganglion cell damage is similar to that observed after transgenic expression of mutated mouse myocilin.19 Large retinal ganglion cells were affected more dramatically than was the total population, showing a dramatic 55% reduction in the peripheral retinas of transgenic mice. These observations are similar to those in glaucoma in humans. Glaucoma most often affects peripheral visual function in its early stages, while deterioration of the central retina can be seen only at later stages of the disease.38 It has been demonstrated that large retinal ganglion cells die faster in the mid-peripheral retina of the glaucomatous eye.39 Preferential loss of large retinal ganglion cells has also been reported in senescent DBA2/NNia mice that have pigmentary glaucoma.40 Axonal degeneration is another hallmark of glaucoma that has been described both in glaucomatous human eyes and in animal models of glaucoma.18,19 Analysis of the retinal nerve fiber layer and optic nerve showed signs of axonal degeneration. In the optic nerve, this degeneration was more pronounced at the periphery of the optic nerve. We concluded that expression of similar physiological levels of mutated mouse or human myocilin in tissues of the iridocorneal angle produces similar damage to the eye. The difference between our results and recently published data22 may have several reasons. In the published work, adenovirus encoding mutated human myocilin was injected into the mouse vitreous, and the expression pattern of mutated myocilin may be different from the expression pattern of the endogenous Myoc gene. Mutated Tyr437His human myocilin in which the C-terminal PTS1 site was replaced by the mouse C-terminal LEM sequence, not mutated Tyr423His mouse myocilin, was used. Finally, IOP elevation was observed 4 days after viral injection, indicating that nonphysiological high levels of mutated myocilin may be produced.
In summary, moderate elevation of IOP, death of the retinal ganglion cells in the peripheral retina, and degeneration of the peripheral optic nerve observed in eyes of transgenic mice expressing mutated human myocilin, without any detectable changes in the geometry of the eye angle, are all characteristic features of primary open-angle glaucoma in humans. Transgenic mice expressing mutated human myocilin under control of its own promoter represent a genetic model of primary open-angle glaucoma that can be used to address several fundamental questions about glaucoma.
Acknowledgments
The authors thank Eric Wawrousek, Steve Lee, and Carl Haugen for help with transgenic mice production, Shyam K. Sharan for help with BAC clone modification, and Olof Sundin and Janine Davis for critical reading of the manuscript.
Supported by the National Eye Institute Intramural Program.
Footnotes
Disclosure: Y. Zhou, None; O. Grinchuk, None; S.I. Tomarev, None
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam MF, Belmouden A, Binisti P, et al. Recurrent mutations in a single exon encoding the evolutionary conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Genet. 1997;6:2091–2097. doi: 10.1093/hmg/6.12.2091. [DOI] [PubMed] [Google Scholar]
- 3.Torrado M, Trivedi R, Zinovieva R, Karavanova I, Tomarev SI. Optimedin: a novel olfactomedin-related protein that interacts with myocilin. Hum Mol Genet. 2002;11:1291–1301. doi: 10.1093/hmg/11.11.1291. [DOI] [PubMed] [Google Scholar]
- 4.Swiderski RE, Ross JL, Fingert JH, et al. Localization of MYOC transcripts in human eye and optic nerve by in situ hybridization. Invest Ophthalmol Vis Sci. 2000;41:3420–3428. [PubMed] [Google Scholar]
- 5.Yokoe H, Anholt RR. Molecular cloning of olfactomedin, an extra-cellular matrix protein specific to olfactory neuroepithelium. Proc Natl Acad Sci USA. 1993;90:4655–4659. doi: 10.1073/pnas.90.10.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fingert JH, Stone EM, Sheffield VC, Alward WL. Myocilin glaucoma. Surv Ophthalmol. 2002;47:547–561. doi: 10.1016/s0039-6257(02)00353-3. [DOI] [PubMed] [Google Scholar]
- 7.Stone EM, Fingert JH, Alward WM, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 8.Alward WL, Kwon YH, Khanna CL, et al. Variations in the myocilin gene in patients with open-angle glaucoma. Arch Ophthalmol. 2002;120:1189–1197. doi: 10.1001/archopht.120.9.1189. [DOI] [PubMed] [Google Scholar]
- 9.Gong G, Kosoko-Lasaki O, Haynatzki GR, Wilson MR. Genetic dissection of myocilin glaucoma. Hum Mol Genet. 2004;13:R91–R102. doi: 10.1093/hmg/ddh074. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson N, Andrews M, Shepard AR, et al. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet. 2001;10:117–125. doi: 10.1093/hmg/10.2.117. [DOI] [PubMed] [Google Scholar]
- 11.Gobeil S, Rodrigue MA, Moisan S, et al. Intracellular sequestration of hetero-oligomers formed by wild-type and glaucoma-causing myocilin mutants. Invest Ophthalmol Vis Sci. 2004;45:3560–3567. doi: 10.1167/iovs.04-0300. [DOI] [PubMed] [Google Scholar]
- 12.Malyukova I, Lee HS, Fariss RN, Tomarev SI. Mutated mouse and human myocilins have similar properties and do not block general secretory pathway. Invest Ophthalmol Vis Sci. 2006;47:206–212. doi: 10.1167/iovs.05-0220. [DOI] [PubMed] [Google Scholar]
- 13.Joe MK, Sohn S, Hur W, Moon Y, Choi YR, Kee C. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;312:592–600. doi: 10.1016/j.bbrc.2003.10.162. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Vollrath D. Reversal of mutant myocilin non-secretion and cell killing: implications for glaucoma. Hum Mol Genet. 2004;13:1193–1204. doi: 10.1093/hmg/ddh128. [DOI] [PubMed] [Google Scholar]
- 15.Alward WL, Fingert JH, Coote MA, et al. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A) N Engl J Med. 1998;338:1022–1027. doi: 10.1056/NEJM199804093381503. [DOI] [PubMed] [Google Scholar]
- 16.Harada T, Harada C, Nakamura K, et al. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J Clin Invest. 2007;117:1763–1770. doi: 10.1172/JCI30178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John SWM, Smith RS, Savinova OV, et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- 18.Mabuchi F, Lindsey JD, Aihara M, Mackey MR, Weinreb RN. Optic nerve damage in mice with a targeted type I collagen mutation. Invest Ophthalmol Vis Sci. 2004;45:1841–1845. doi: 10.1167/iovs.03-1008. [DOI] [PubMed] [Google Scholar]
- 19.Senatorov V, Malyukova I, Fariss R, et al. Expression of mutated mouse myocilin induces open-angle glaucoma in transgenic mice. J Neurosci. 2006;26:11903–11914. doi: 10.1523/JNEUROSCI.3020-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomarev SI, Tamm ER, Chang B. Characterization of the mouse Myoc/Tigr gene. Biochem Biophys Res Commun. 1998;245:887–893. doi: 10.1006/bbrc.1998.8541. [DOI] [PubMed] [Google Scholar]
- 21.Fingert JH, Ying L, Swiderski RE, et al. Characterization and comparison of the human and mouse GLC1A glaucoma genes. Genome Res. 1998;8:377–384. doi: 10.1101/gr.8.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shepard AR, Jacobson N, Millar JC, et al. Glaucoma-causing myocilin mutants require the peroxisomal targeting signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum Mol Genet. 2007;16:609–617. doi: 10.1093/hmg/ddm001. [DOI] [PubMed] [Google Scholar]
- 23.Swaminathan S, Ellis HM, Waters LS, et al. Rapid engineering of bacterial artificial chromosomes using oligonucleotides. Genesis. 2001;29:14–21. doi: 10.1002/1526-968x(200101)29:1<14::aid-gene1001>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 24.Court DL, Swaminathan S, Yu D, et al. Mini-lambda: a tractable system for chromosome and BAC engineering. Gene. 2003;315:63–69. doi: 10.1016/s0378-1119(03)00728-5. [DOI] [PubMed] [Google Scholar]
- 25.Filippopoulos T, Matsubara A, Danias J, et al. Predictability and limitations of non-invasive murine tonometry: comparison of two devices. Exp Eye Res. 2006;83:194–201. doi: 10.1016/j.exer.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Kim BS, Savinova OV, Reedy MV, et al. Targeted disruption of the myocilin gene (Myoc) suggests that human glaucoma-causing mutations are gain of function. Mol Cell Biol. 2001;21:7707–7713. doi: 10.1128/MCB.21.22.7707-7713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sacca SC, Rolando M, Marletta A, Macri A, Cerqueti P, Ciurlo G. Fluctuations of intraocular pressure during the day in open-angle glaucoma, normal-tension glaucoma and normal subjects. Ophthalmologica. 1998;212:115–119. doi: 10.1159/000027290. [DOI] [PubMed] [Google Scholar]
- 28.Savinova OV, Sugiyama F, Martin JE, et al. Intraocular pressure in genetically distinct mice: an update and strain survey. BMC Genet. 2001;2:12. doi: 10.1186/1471-2156-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda A, Tsujiya S, Higashide T, et al. Circadian intraocular pressure rhythm is generated by clock genes. Invest Ophthalmol Vis Sci. 2006;47:4050–4052. doi: 10.1167/iovs.06-0183. [DOI] [PubMed] [Google Scholar]
- 30.Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002;451:115–126. doi: 10.1002/cne.10323. [DOI] [PubMed] [Google Scholar]
- 31.Coombs J, van der List D, Wang GY, Chalupa LM. Morphological properties of mouse retinal ganglion cells. Neuroscience. 2006;140:123–136. doi: 10.1016/j.neuroscience.2006.02.079. [DOI] [PubMed] [Google Scholar]
- 32.Lin B, Wang SW, Masland RH. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron. 2004;43:475–485. doi: 10.1016/j.neuron.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Ochs S, Pourmand R, Jersild RA, Friedman RN. The origin and nature of beading: a reversible transformation of the shape of nerve fibers. Prog Neurobiol. 1997;52:391–426. doi: 10.1016/s0301-0082(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 34.Gould DB, Miceli-Libby L, Savinova OV, et al. Genetically increasing myoc expression supports a necessary pathologic role of abnormal proteins in glaucoma. Mol Cell Biol. 2004;24:9019–9025. doi: 10.1128/MCB.24.20.9019-9025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gould DB, Reedy M, Wilson LA, Smith RS, Johnson RL, John SW. Mutant myocilin nonsecretion in vivo is not sufficient to cause glaucoma. Mol Cell Biol. 2006;26:8427–8436. doi: 10.1128/MCB.01127-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 37.Weih LM, Mukesh BN, McCarty CA, Taylor HR. Association of demographic, familial, medical, and ocular factors with intraocular pressure. Arch Ophthalmol. 2001;119:875–880. doi: 10.1001/archopht.119.6.875. [DOI] [PubMed] [Google Scholar]
- 38.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–464. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 40.Filippopoulos T, Danias J, Chen B, Podos SM, Mittag TW. Topographic and morphologic analyses of retinal ganglion cell loss in old DBA/2NNia mice. Invest Ophthalmol Vis Sci. 2006;47:1968–1974. doi: 10.1167/iovs.05-0955. [DOI] [PubMed] [Google Scholar]