Abstract
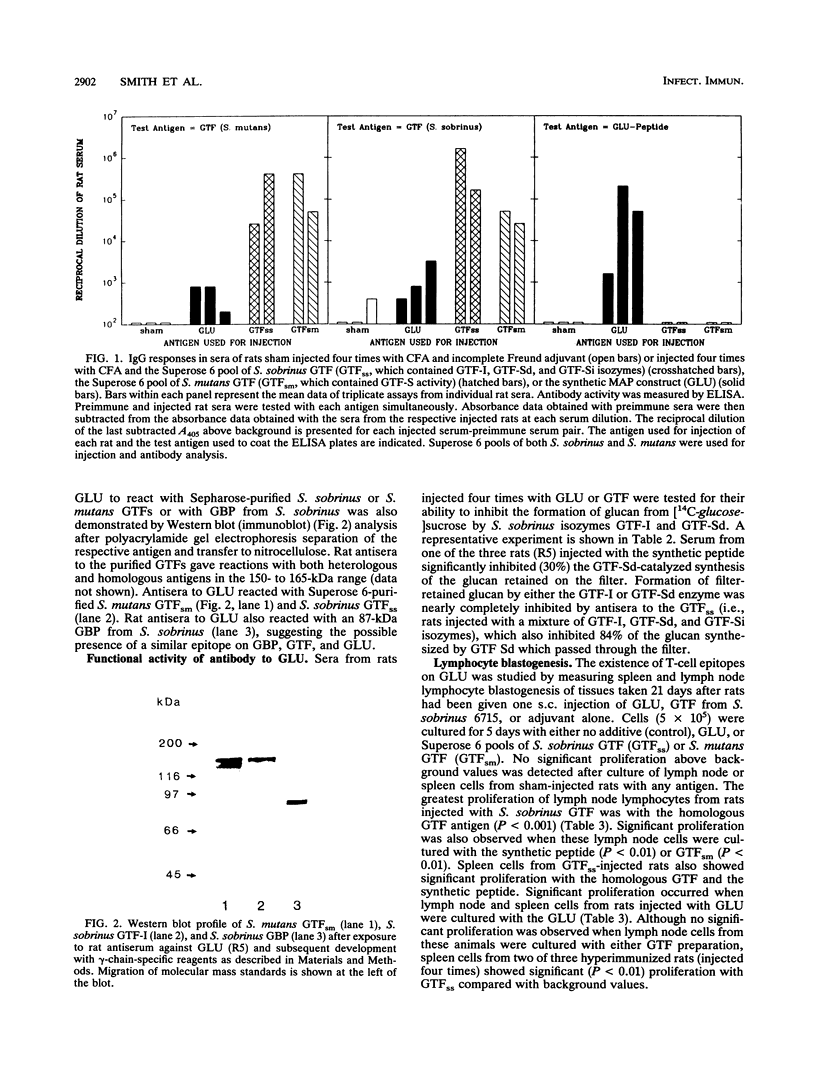
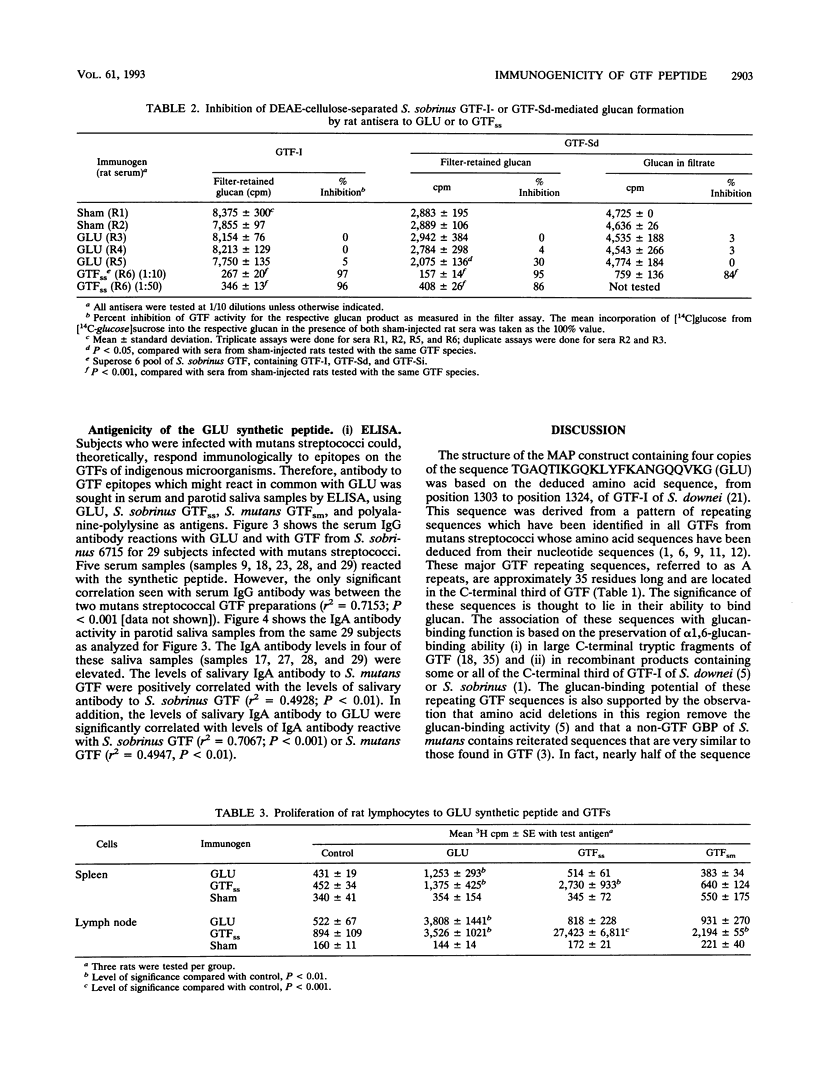
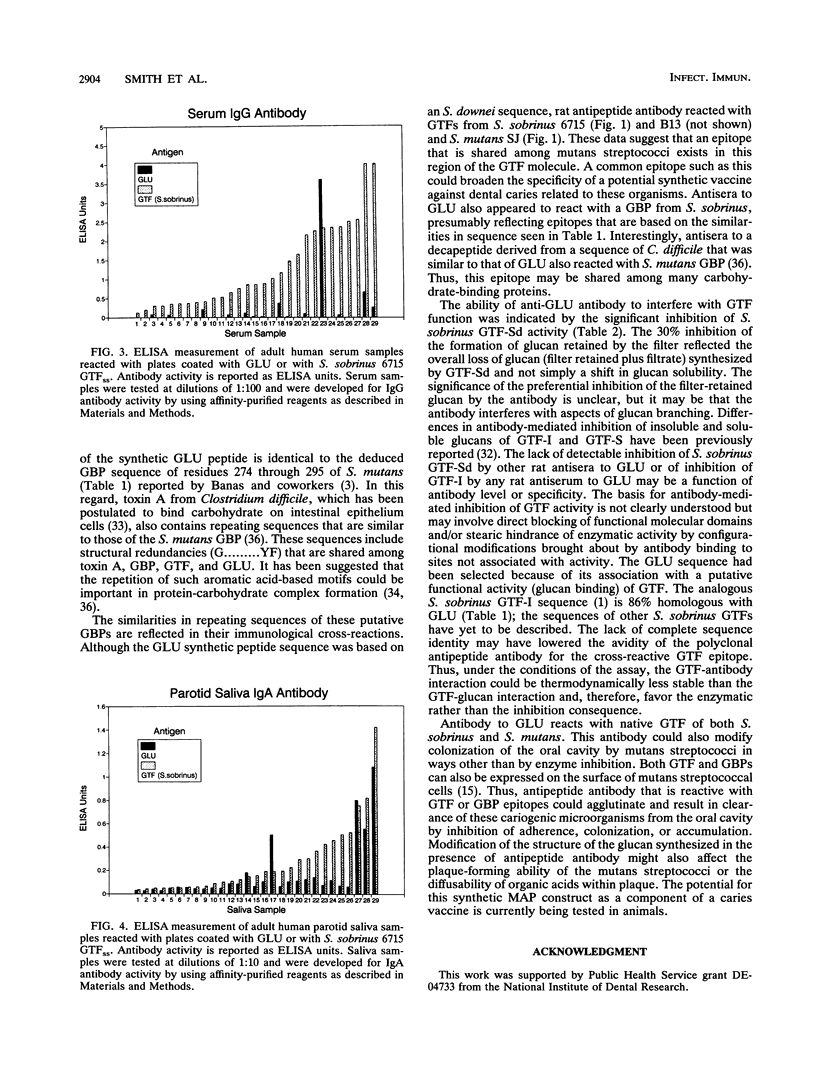
The immunogenicity and antigenicity of a multiply antigenic peptide construct containing four copies of the synthetic peptide TGAQTIKGQKLYFKANGQQVKG were measured in rodents and humans, respectively. The composition of this peptide construct (termed GLU) was derived from a major repeating sequence in the C-terminal region of mutans streptococcal glucosyltransferases that synthesize water-insoluble glucan (GTF-I). The GLU peptide elicited high levels of serum immunoglobulin G antibody to GLU after subcutaneous injection into Sprague-Dawley rats. These antisera also reacted with intact GTF isozymes from Streptococcus sobrinus and Streptococcus mutans (by enzyme-linked immunosorbent assay [ELISA] and Western blot [immunoblot] analyses) and with an 87-kDa glucan-binding protein from S. sobrinus (by Western blot). The synthesis of filter-retained glucan by GTF-Sd of S. sobrinus could be inhibited (30%) by preincubation with anti-GLU rat serum. Splenic and lymph node lymphocytes from rats injected once with S. sobrinus GTF isozymes demonstrated significant proliferation after 5 days of culture with GLU. The GLU peptide reacted with 4 of 29 human parotid saliva samples and 5 of 29 human serum samples (by ELISA). These results suggest that the GLU peptide contains B- and T-cell epitopes that are similar to those of intact mutans streptococcal GTFs and possibly certain other glucan-binding proteins as well. Furthermore, since antibody to this epitope(s) appears to inhibit GTF function, sequences within this peptide construct may have value for inclusion in a synthetic dental caries vaccine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo H., Matsumura T., Kodama T., Ohta H., Fukui K., Kato K., Kagawa H. Peptide sequences for sucrose splitting and glucan binding within Streptococcus sobrinus glucosyltransferase (water-insoluble glucan synthetase). J Bacteriol. 1991 Feb;173(3):989–996. doi: 10.1128/jb.173.3.989-996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banas J. A., Russell R. R., Ferretti J. J. Sequence analysis of the gene for the glucan-binding protein of Streptococcus mutans Ingbritt. Infect Immun. 1990 Mar;58(3):667–673. doi: 10.1128/iai.58.3.667-673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Gilpin M. L., Russell R. R. Nucleotide sequence of a glucosyltransferase gene from Streptococcus sobrinus MFe28. J Bacteriol. 1987 Sep;169(9):4271–4278. doi: 10.1128/jb.169.9.4271-4278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Huang T. T., Russell R. R. Sequence analysis of the glucosyltransferase A gene (gtfA) from Streptococcus mutans Ingbritt. Infect Immun. 1988 Jun;56(6):1585–1588. doi: 10.1128/iai.56.6.1585-1588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffard P. M., Simpson C. L., Milward C. P., Jacques N. A. Molecular characterization of a cluster of at least two glucosyltransferase genes in Streptococcus salivarius ATCC 25975. J Gen Microbiol. 1991 Nov;137(11):2577–2593. doi: 10.1099/00221287-137-11-2577. [DOI] [PubMed] [Google Scholar]
- Gilmore K. S., Russell R. R., Ferretti J. J. Analysis of the Streptococcus downei gtfS gene, which specifies a glucosyltransferase that synthesizes soluble glucans. Infect Immun. 1990 Aug;58(8):2452–2458. doi: 10.1128/iai.58.8.2452-2458.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun. 1988 Aug;56(8):1999–2005. doi: 10.1128/iai.56.8.1999-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun. 1989 Jul;57(7):2079–2085. doi: 10.1128/iai.57.7.2079-2085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Asakawa H., Okahashi N., Hamada S. Sucrose-dependent cell adherence and cariogenicity of serotype c Streptococcus mutans. J Gen Microbiol. 1986 Oct;132(10):2873–2883. doi: 10.1099/00221287-132-10-2873. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Alberts M., Stein J. Monoclonal antibodies to the extracellular glucosyltransferases from Streptococcus sobrinus 6715. Infect Immun. 1987 Aug;55(8):1900–1905. doi: 10.1128/iai.55.8.1900-1905.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Hamelik R. M., Smith E. E. Purification of dextran-binding protein from cariogenic Streptococcus mutans. Biochem Biophys Res Commun. 1977 Sep 9;78(1):273–278. doi: 10.1016/0006-291x(77)91250-5. [DOI] [PubMed] [Google Scholar]
- McCabe M. M. Purification and characterization of a primer-independent glucosyltransferase from Streptococcus mutans 6715-13 mutant 27. Infect Immun. 1985 Dec;50(3):771–777. doi: 10.1128/iai.50.3.771-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., Childers N. K. Development and outlook for a caries vaccine. Crit Rev Oral Biol Med. 1990;1(1):37–54. doi: 10.1177/10454411900010010401. [DOI] [PubMed] [Google Scholar]
- Mooser G., Hefta S. A., Paxton R. J., Shively J. E., Lee T. D. Isolation and sequence of an active-site peptide containing a catalytic aspartic acid from two Streptococcus sobrinus alpha-glucosyltransferases. J Biol Chem. 1991 May 15;266(14):8916–8922. [PubMed] [Google Scholar]
- Mooser G., Iwaoka K. R. Sucrose 6-alpha-D-glucosyltransferase from Streptococcus sobrinus: characterization of a glucosyl-enzyme complex. Biochemistry. 1989 Jan 24;28(2):443–449. doi: 10.1021/bi00428a006. [DOI] [PubMed] [Google Scholar]
- Mooser G., Wong C. Isolation of a glucan-binding domain of glucosyltransferase (1,6-alpha-glucan synthase) from Streptococcus sobrinus. Infect Immun. 1988 Apr;56(4):880–884. doi: 10.1128/iai.56.4.880-884.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R., Shiroza T., Kuramitsu H. K., Ferretti J. J. Homology of glucosyltransferase gene and protein sequences from Streptococcus sobrinus and Streptococcus mutans. J Dent Res. 1988 Mar;67(3):543–547. doi: 10.1177/00220345880670030401. [DOI] [PubMed] [Google Scholar]
- Shiroza T., Ueda S., Kuramitsu H. K. Sequence analysis of the gtfB gene from Streptococcus mutans. J Bacteriol. 1987 Sep;169(9):4263–4270. doi: 10.1128/jb.169.9.4263-4270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A., Ebersole J. L. Local and systemic antibody response to oral administration of glucosyltransferase antigen complex. Infect Immun. 1980 May;28(2):441–450. doi: 10.1128/iai.28.2.441-450.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A., Ebersole J. L. Salivary IgA antibody to glucosyltransferase in man. Clin Exp Immunol. 1985 Aug;61(2):416–424. [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A. Effect of local deposition of antigen on salivary immune responses and reaccumulation of mutans streptococci. J Clin Immunol. 1990 Sep;10(5):273–281. doi: 10.1007/BF00916703. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A. Oral immunization of humans with Streptococcus sobrinus glucosyltransferase. Infect Immun. 1987 Nov;55(11):2562–2569. doi: 10.1128/iai.55.11.2562-2569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman M. A., Smith D. J. Effects of local immunization with glucosyltransferase fractions from Streptococcus mutans on dental caries in rats and hamsters. J Immunol. 1977 Feb;118(2):710–720. [PubMed] [Google Scholar]
- Taubman M. A., Smith D. J., King W. F., Eastcott J. W., Bergey E. J., Levine M. J. Immune properties of glucosyltransferases from S. sobrinus. J Oral Pathol. 1988 Nov;17(9-10):466–470. doi: 10.1111/j.1600-0714.1988.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Taubman M. A., Smith D. J. Significance of immune responses to oral antigens in dental diseases. Adv Exp Med Biol. 1992;327:273–286. doi: 10.1007/978-1-4615-3410-5_29. [DOI] [PubMed] [Google Scholar]
- Tucker K. D., Wilkins T. D. Toxin A of Clostridium difficile binds to the human carbohydrate antigens I, X, and Y. Infect Immun. 1991 Jan;59(1):73–78. doi: 10.1128/iai.59.1.73-78.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Hefta S. A., Paxton R. J., Shively J. E., Mooser G. Size and subdomain architecture of the glucan-binding domain of sucrose:3-alpha-D-glucosyltransferase from Streptococcus sobrinus. Infect Immun. 1990 Jul;58(7):2165–2170. doi: 10.1128/iai.58.7.2165-2170.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren B. W., Russell R. R., Tabaqchali S. Antigenic cross-reactivity and functional inhibition by antibodies to Clostridium difficile toxin A, Streptococcus mutans glucan-binding protein, and a synthetic peptide. Infect Immun. 1991 Sep;59(9):3151–3155. doi: 10.1128/iai.59.9.3151-3155.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Eichel-Streiber C., Sauerborn M., Kuramitsu H. K. Evidence for a modular structure of the homologous repetitive C-terminal carbohydrate-binding sites of Clostridium difficile toxins and Streptococcus mutans glucosyltransferases. J Bacteriol. 1992 Oct;174(20):6707–6710. doi: 10.1128/jb.174.20.6707-6710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]