Abstract
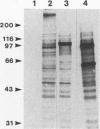
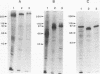
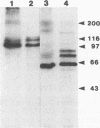
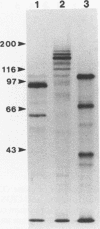
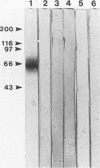
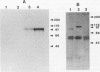
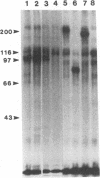
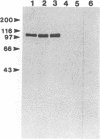
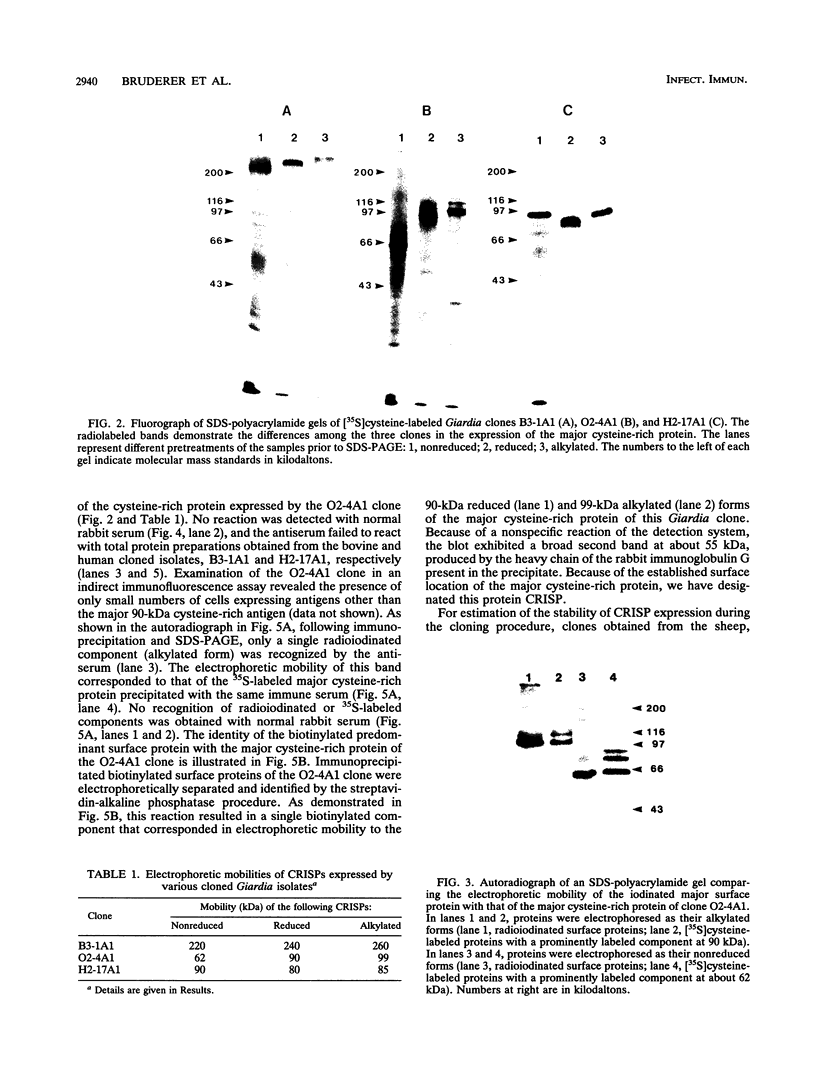
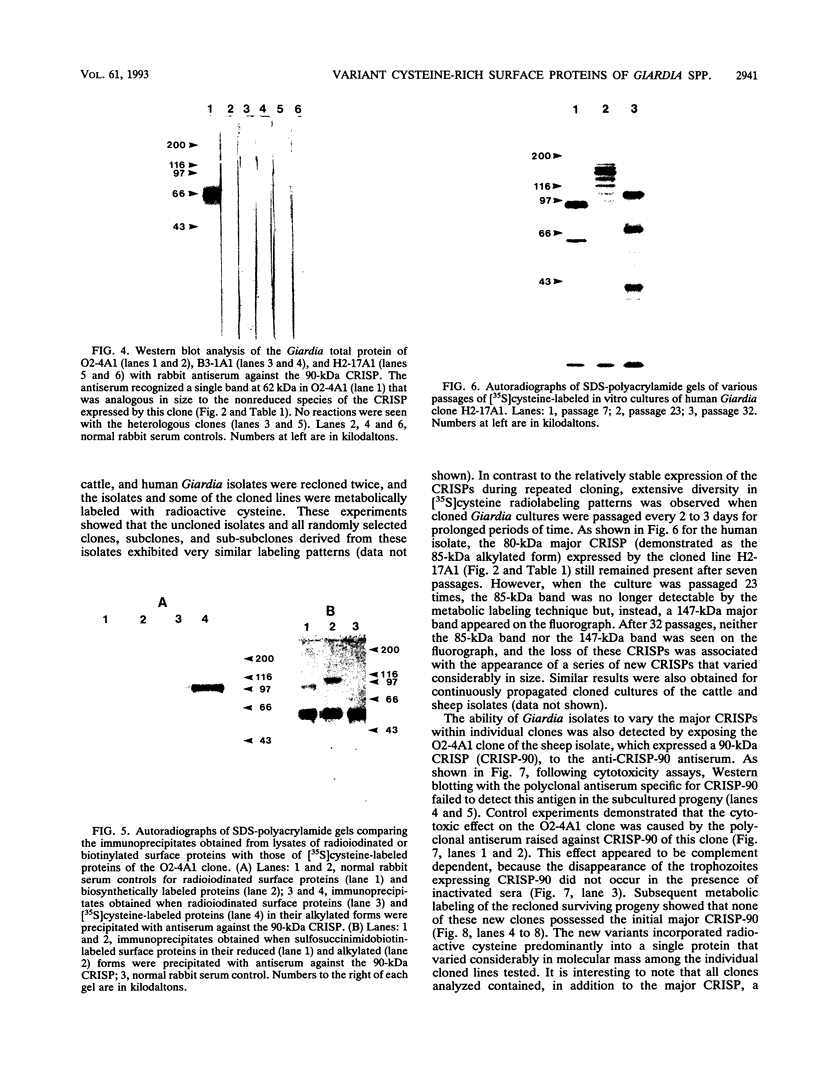
Cloned Giardia isolates obtained from a sheep, a calf, and a human possessed a major membrane protein that showed marked intraspecific variations in size as demonstrated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis following surface biotinylation and radioiodination. Metabolic labeling with [35S] cysteine and electrophoretic analysis also revealed for each cloned isolate a predominant protein that corresponded in size to the major surface protein demonstrated by surface labeling techniques. Immunoprecipitation studies with a polyclonal antiserum specifically directed against the 90-kDa major cysteine-rich protein purified from a subclone of the sheep isolate (O2-4A1) showed that the cysteine-rich protein and the major surface protein are identical. The surface location of the antigen was further corroborated by the reaction of fluorescence-labeled antibodies raised against the 90-kDa O2-4A1 cysteine-rich protein with the entire surface of live trophozoites of the homologous clone. The ability of the cloned Giardia isolates to undergo variations of their cysteine-rich surface protein (CRISP) was demonstrated by the spontaneous appearance of new CRISPs in clonally derived populations during prolonged in vitro culturing and in cultures of the O2-4A1 clone that had survived treatment with the cytotoxic anti-90-kDa CRISP antiserum specific for the surface antigen of this clone. The surviving progeny were devoid of the original CRISP, as judged by resistance to the immune serum. Subsequent cysteine metabolic labeling of the recloned surviving trophozoites demonstrated a large number of new variants, each expressing a single CRISP that varied significantly in molecular weight from those in the different cloned lines. These studies suggest that the presence of CRISPs and their variations are not restricted to Giardia isolates obtained from humans but are universal phenomena among the Giardia duodenalis types of organisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam R. D., Aggarwal A., Lal A. A., de La Cruz V. F., McCutchan T., Nash T. E. Antigenic variation of a cysteine-rich protein in Giardia lamblia. J Exp Med. 1988 Jan 1;167(1):109–118. doi: 10.1084/jem.167.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal A., Merritt J. W., Jr, Nash T. E. Cysteine-rich variant surface proteins of Giardia lamblia. Mol Biochem Parasitol. 1989 Jan 1;32(1):39–47. doi: 10.1016/0166-6851(89)90127-8. [DOI] [PubMed] [Google Scholar]
- Aggarwal A., Nash T. E. Antigenic variation of Giardia lamblia in vivo. Infect Immun. 1988 Jun;56(6):1420–1423. doi: 10.1128/iai.56.6.1420-1423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews R. H., Adams M., Boreham P. F., Mayrhofer G., Meloni B. P. Giardia intestinalis: electrophoretic evidence for a species complex. Int J Parasitol. 1989 Apr;19(2):183–190. doi: 10.1016/0020-7519(89)90006-4. [DOI] [PubMed] [Google Scholar]
- Andrews R. H., Chilton N. B., Mayrhofer G. Selection of specific genotypes of Giardia intestinalis by growth in vitro and in vivo. Parasitology. 1992 Dec;105(Pt 3):375–386. doi: 10.1017/s0031182000074540. [DOI] [PubMed] [Google Scholar]
- Bertram M. A., Meyer E. A., Lile J. D., Morse S. A. A comparison of isozymes of five axenic Giardia isolates. J Parasitol. 1983 Oct;69(5):793–801. [PubMed] [Google Scholar]
- Das S., Traynor-Kaplan A., Reiner D. S., Meng T. C., Gillin F. D. A surface antigen of Giardia lamblia with a glycosylphosphatidylinositol anchor. J Biol Chem. 1991 Nov 5;266(31):21318–21325. [PubMed] [Google Scholar]
- Gillin F. D., Hagblom P., Harwood J., Aley S. B., Reiner D. S., McCaffery M., So M., Guiney D. G. Isolation and expression of the gene for a major surface protein of Giardia lamblia. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4463–4467. doi: 10.1073/pnas.87.12.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. Antibody probing of western blots which have been stained with india ink. Anal Biochem. 1986 Aug 1;156(2):315–319. doi: 10.1016/0003-2697(86)90259-9. [DOI] [PubMed] [Google Scholar]
- Gottstein B., Nash T. E. Antigenic variation in Giardia lamblia: infection of congenitally athymic nude and scid mice. Parasite Immunol. 1991 Nov;13(6):649–659. doi: 10.1111/j.1365-3024.1991.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley W. L., Finkelstein E., Holst B. D. Identification of surface proteins on bovine leukocytes by a biotin-avidin protein blotting technique. J Immunol Methods. 1985 Dec 17;85(1):195–202. doi: 10.1016/0022-1759(85)90287-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meloni B. P., Thompson R. C., Strandén A. M., Köhler P., Eckert J. Critical comparison of Giardia duodenalis from Australia and Switzerland using isoenzyme electrophoresis. Acta Trop. 1991 Dec;50(2):115–124. doi: 10.1016/0001-706x(91)90004-4. [DOI] [PubMed] [Google Scholar]
- Meyer E. A. The epidemiology of Giardiasis. Parasitol Today. 1985 Oct;1(4):101–105. doi: 10.1016/0169-4758(85)90004-3. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Aggarwal A., Adam R. D., Conrad J. T., Merritt J. W., Jr Antigenic variation in Giardia lamblia. J Immunol. 1988 Jul 15;141(2):636–641. [PubMed] [Google Scholar]
- Nash T. E., Aggarwal A. Cytotoxicity of monoclonal antibodies to a subset of Giardia isolates. J Immunol. 1986 Apr 1;136(7):2628–2632. [PubMed] [Google Scholar]
- Nash T. E., Banks S. M., Alling D. W., Merritt J. W., Jr, Conrad J. T. Frequency of variant antigens in Giardia lamblia. Exp Parasitol. 1990 Nov;71(4):415–421. doi: 10.1016/0014-4894(90)90067-m. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Conrad J. T., Merritt J. W., Jr Variant specific epitopes of Giardia lamblia. Mol Biochem Parasitol. 1990 Aug;42(1):125–132. doi: 10.1016/0166-6851(90)90120-b. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Gillin F. D., Smith P. D. Excretory-secretory products of Giardia lamblia. J Immunol. 1983 Oct;131(4):2004–2010. [PubMed] [Google Scholar]
- Nash T. E., Herrington D. A., Levine M. M., Conrad J. T., Merritt J. W., Jr Antigenic variation of Giardia lamblia in experimental human infections. J Immunol. 1990 Jun 1;144(11):4362–4369. [PubMed] [Google Scholar]
- Nash T. E., Keister D. B. Differences in excretory-secretory products and surface antigens among 19 isolates of Giardia. J Infect Dis. 1985 Dec;152(6):1166–1171. doi: 10.1093/infdis/152.6.1166. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Merritt J. W., Jr, Conrad J. T. Isolate and epitope variability in susceptibility of Giardia lamblia to intestinal proteases. Infect Immun. 1991 Apr;59(4):1334–1340. doi: 10.1128/iai.59.4.1334-1340.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash T. E., Mowatt M. R. Characterization of a Giardia lamblia variant-specific surface protein (VSP) gene from isolate GS/M and estimation of the VSP gene repertoire size. Mol Biochem Parasitol. 1992 Apr;51(2):219–227. doi: 10.1016/0166-6851(92)90072-r. [DOI] [PubMed] [Google Scholar]
- Nash T. Surface antigen variability and variation in Giardia lamblia. Parasitol Today. 1992 Jul;8(7):229–234. doi: 10.1016/0169-4758(92)90119-m. [DOI] [PubMed] [Google Scholar]
- Sommer I., Mehlhorn H., Rüger W. Biochemical and immunological characterization of major surface antigens of Sarcocystis muris and S. suicanis cyst merozoites. Parasitol Res. 1991;77(3):204–211. doi: 10.1007/BF00930859. [DOI] [PubMed] [Google Scholar]
- Strandén A. M., Eckert J., Köhler P. Electrophoretic characterization of Giardia isolated from humans, cattle, sheep, and a dog in Switzerland. J Parasitol. 1990 Oct;76(5):660–668. [PubMed] [Google Scholar]
- Weiss J. B., van Keulen H., Nash T. E. Classification of subgroups of Giardia lamblia based upon ribosomal RNA gene sequence using the polymerase chain reaction. Mol Biochem Parasitol. 1992 Aug;54(1):73–86. doi: 10.1016/0166-6851(92)90096-3. [DOI] [PubMed] [Google Scholar]