Abstract
Tumor transplant studies are important tools for studying cancer biology in a model organism. Transplantation is especially important for assaying tumor cell malignancy and migration capabilities, and is critical for identifying putative cancer stem cell populations. In this review, we discuss the current state of tumor transplantation studies performed in the zebrafish. We address several zebrafish-specific considerations for development of the transplant assay, including choosing recipient animals, transplant methods, and post-transplant observation. We also examine how the zebrafish is an advantageous model for transplantation, particularly with development of the translucent fish. Transplantation has already been critical for characterizing zebrafish models of leukemia, rhabdomyosarcoma, and melanoma. With further development of imaging techniques and other tools, zebrafish tumor transplantation will continue to contribute to our understanding of tumor cell biology.
Introduction
The ability of tumors to engraft after transplantation into recipient animals has been known for decades.1 A malignant population of tumor cells is not only capable of primary engraftment, but also capable of serial transplantation in subsequent recipient animals. Transplantation has been used to validate animal models of cancer2,3 and test malignancy of human tumor samples.4 Transplantation has also been important for drug development, as chemical treatment of transplanted cells has been used to screen for potential therapeutics that might inhibit tumor malignancy.5 Identifying transplantation capabilities of tumor cells has addressed several key aspects of tumor biology, particularly malignancy, metastasis, and cancer stem cell biology. Studies over 50 years ago demonstrated that, in many cases, human tumor cells that successfully transplant in rodents are more likely to be malignant in patients.6 A malignant tumor is expected to engraft and develop into a tumor in the recipient animal. This assay for malignancy may also have implications for metastasis, as it looks for tumor cells to migrate from the site of injection and form a new tumor. Additionally, transplantation of tumors in murine models has been crucial for identifying cancer stem cell populations.7
Over the past few years, the zebrafish has emerged as a tumor model that complements studies performed in the murine system. The transplantation assay in the zebrafish has developed to be a robust assay. In many ways, the zebrafish as a model system is particularly advantageous for tumor transplantation assays. Zebrafish fecundity provides high numbers of donor and recipient fish, and generating large numbers of transgenic fish is feasible.8 In addition, the generation of a transparent adult fish is particularly beneficial for post-transplant observation.9 Yet, technical improvements can be made for further development to improve zebrafish transplantation methods.
Uses of the Transplant Assay in Cancer Biology
Transplantation of tumor cells from one animal to another provides information about the malignancy of the tumor. Cells from a tumor that propagate post-transplant have acquired the ability to self-renew and generate more tumor cells. Transplantation as an assay has been especially important for testing blood neoplasias to differentiate a myeloproliferative or lymphoproliferative disorder from leukemia,2,10 which is capable of propagation. In this sense, transplantation as an assay can differentiate between a hyperplastic and malignant growth.
Transplantation can also identify putative cancer stem cells, rare populations of a tumor that can form a new tumor. Testing this requires isolation of a subset of tumor cells from the bulk mass, and then utilization of limiting dilution transplant assays to determine whether the isolated population is more transplantable than the bulk tumor. These assays have been extensively tested in mouse models, primarily in leukemia, to identify a cancer stem cell population.11 Despite advances in this field, transplantation as an assay for cancer stem cells has recently been called into question, since modification of the transplant procedure itself can dramatically alter the results of the assay in some tumor models.12 Regardless, transplantability is helpful in understanding tumor cell properties.
Studying tumor dissemination post-transplantation also aids in the understanding of migration, homing, and vasculature induction properties of tumor cells. Tumor cells injected into the tail vein of a mouse enter the blood stream, at which point migration and metastatic potential of the tumor cells can be studied.13 Immunocompromised mice can serve as recipients for human tumor cells, allowing similar types of studies on human samples.14 Xenotransplantation provides an opportunity to study human tumor cells in vivo.
Zebrafish Transplant Methods
Choosing a recipient fish
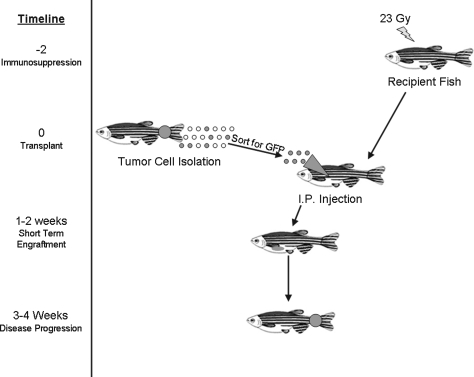
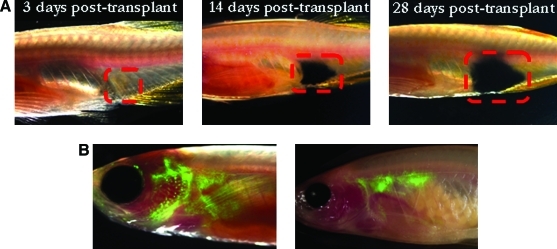
Choosing a recipient animal for the donor tumor cells is a crucial question in both zebrafish and murine systems (Fig. 1). Because the major readout from most transplant studies is engraftment, the sensitivity of this readout can dramatically alter the results. Initial tumor transplants in the fish used wild-type recipients, and whether a recipient has engrafted may be subjective if it is based solely on visual identification of a growing mass. For this reason, transplants into wild-type animals may require sacrifice and histological examination of each recipient. The recently developed transparent zebrafish named casper enables a much more sensitive technique for visual identification of engrafted tumors. Casper fish, a cross between the nacre and roy lines, lack melanocytes and iridophores, allowing for increased translucency in the adult fish.9 As a result, when tumors are transplanted into this line, it is much easier to observe engraftment, tumor growth, and metastasis. For example, pigmented zebrafish melanoma cells can be scored after transplant into the casper fish, and tumor size can be measured over time without sacrificing the fish (Fig. 2A). Additionally, since pigmented cells can interfere with fluorescent signal, transplant into casper fish will allow imaging of fluorescently labeled cells in the adult (Fig. 2B). Using confocal microscopy, single-cell resolution of transplanted green fluorescent protein (GFP)-positive cells is possible.
FIG. 1.
Steps of the zebrafish transplantation assay.
FIG. 2.
Transplantation in the casper fish.15 (A) Imaging of melanoma development post-transplant. Melanoma from an mitf:NRAS;p53−/− zebrafish was transplanted into the casper fish, and images were taken 3, 14, and 28 days post-transplant. (B) Homing of hematopoietic cells post-transplant. Whole kidney marrow expressing β-actin green fluorescent protein was transplanted into irradiated casper fish. On the left, cells at 2 weeks post-transplant are beginning to home to the thymus and kidney. On the right, by 4 weeks post-transplant, cells are localized to the kidney.
Transgenic fish with fluorescently labeled tissues are another option for a recipient. To identify the effect of tumor cells on vasculature, cells can be transplanted into fli1-enhanced green fluorescent protein-positive embryos or adult fish, and vasculature development and angiogenesis can be monitored post-transplant.15 This approach is a powerful method to track fluorescently labeled tissues.
Irradiation/immune ablation
An important consideration with the recipient animal is to prevent rejection and allow engraftment. As few isogenic strains have been developed in the zebrafish,16 irradiation is often used to suppress the immune system of adult fish and prevent rejection of transplanted cells. Single doses of 20–25 Gy are sublethal, and at least 90% of fish tolerate this dose.17 Tumors engraft at doses as low as 20 Gy given 2 days before irradiation.3 The immune system of larval fish is also a consideration for assay development. During development, immature T cells arise in the thymus by 3–4 days postfertilization (dpf ),18 and the zebrafish immune system is functional starting at 28 days.19 To ablate T cells, larval fish from 5 days to 1 month of age can be treated in 25 to 250 μg/mL of dexamethasone at 1 to 3 days before transplant.15,18 A 15 Gy dose of gamma-irradiation will also ablate T cells in 6-day-old embryos to 1-month-old larvae.15 For transplants into zebrafish embryos, immunosuppression has not been used, as T cells and B cells are not present until 3–4 dpf.20
Cell isolation and transplant methods
Cells directly isolated from a zebrafish tumor can be used for transplant into an immunosuppressed recipient fish. In our lab, we generally inject 100,000 cells into each adult recipient, though these numbers can vary from 1000 to 3,000,000 cells.2,9 For transplant, one option is to disaggregate the primary tumor and then transplant a defined number of cells directly from the whole tumor population. Because whole tumors contain a mixture of malignant cells, stroma, and blood, a more direct assay for malignant cells is to sort cells by fluorescence-activated cell sorting, allowing transplant of specific cell populations. Fluorescence-activated cell sorting can be used to separate different blood cell populations17 or fluorescently labeled cell populations21 before transplant. By sorting populations, the malignant capability of each population can be determined. A third option is to remove tumor cells and use an inducible expression system to activate an oncogene ex vivo before transplantation, providing spatial and temporal control. Alternatively, chemical treatment ex vivo before transplantation can be used to test potential cancer therapeutics.
Some mouse and human tumor cells are also capable of engraftment in 2 dpf zebrafish embryos and blastulas. For transplantation into 2 dpf embryos, between 50 and 2000 cells can be injected into the yolk or near the vasculature plexus, though there is contrasting evidence regarding the upper limit of cells that can be injected without causing toxicity.22,23 These cells can also be labeled with CiM-DiI or other fluorescent labels. For transplantation into embryos at the blastula stage, 1 to 100 cells are sufficient for engraftment.24,25
Tumor cells can be injected into either the zebrafish embryo or adult. Previous experiments in the adult zebrafish involve tumor transplantation of cells into the intraperitoneal space of sublethally irradiated fish.2,9 Intracardiac injection has been used for hematopoietic cell transplantation26 and may be extended to tumor transplantation. Retroorbital injection techniques are also being developed (R. White, personal communication). Human or mouse cells are generally transplanted into the yolk of the 2-day-old embryo or into the blastula.24,25
Post-transplant observation
Observation of recipient animals post-transplant will determine if engraftment has occurred. This is the crucial limitation of all transplantation studies, since a tumor transplanted into the peritoneal cavity may not be externally visible, yet still can cause the death of the recipient. In general, engraftment in a wild-type adult fish can be seen around 10–14 days post-transplant. If the adult casper fish is the recipient and transplanted cells are pigmented or labeled with GFP, then engraftment can be seen at approximately 5 days posttransplant. When setting up a new transplantation model, it is important to correlate engraftment by visual scoring with histological examination of sacrificed fish. If the concordance between visual and histological examination is high, then visual scoring is faster and more amenable to high-throughput approaches.
If engraftment has occurred, continued growth and disease development can also be observed. This can be assayed by observation of transplant cells if they are fluorescent or pigmented, especially if the casper fish is the recipient. If this is not the case, fish can be monitored for external signs of disease development.27 The timeline for long-term engraftment is often 3–4 weeks, but this may differ for each tumor model. Again, monitoring long-term engraftment is much easier if the casper fish is the recipient, so transplanted cells can be observed over time without sacrificing the transplanted fish. If a tumor has developed in the recipient fish, this can be removed and transplanted into a new recipient to test whether the tumor cells can serially transplant, a hallmark of self-renewal ability.
One of the complications of the transplantation assay is that “no engraftment” can result either when the cell population is not capable of engrafting or when the injection itself is not successful. When developing the transplantation assay, it may be useful to have an independent marker of successful injection, especially if the transplanted cells are not labeled or pigmented. Previous studies have used fluorescent microspheres as a readout of successful injection.15
In the case where embryos are the transplant recipient, they can often survive at least 1 week post-transplantation for observation. Engraftment can be observed soon after transplant in the embryo, even immediately in blastula transplants. The effects of transplantation into a 2 dpf embryo on the vasculature can be seen by 3 dpf.23 Transplant into the zebrafish embryo can also be used to study developmental signaling pathways, as the zebrafish is an excellent model for studying early development.25
Zebrafish Tumor Transplants to Date
Leukemias and myeloproliferative disorders
One of the first fish tumor models developed as a stable transgenic zebrafish line was Myc-induced T cell acute lymphoblastic leukemia (ALL) (Table 1). When transplanted, lymphoblasts from these tumors were able to home to the thymus.2 Tel-AML1 induces B-ALL, and transplantation of these leukemic kidney marrow cells leads to disease formation 6–9 weeks post-transplant.28 Notch1 overexpression in Rag2-positive cells induces T-ALL that can propagate in a recipient animal.29 More recently, T-ALL models have been developed where tumors can be serially transplanted, and engrafted hosts die in a matter of weeks.30 These studies demonstrate that transplanted leukemia cells can engraft and perpetuate the tumor in zebrafish models of leukemia.
Table 1.
Summary of Zebrafish Tumor Transplants to Date
| Model | Publication | Recipient fish | Immunosuppression | Transplanted cells | Detection | Disease development |
|---|---|---|---|---|---|---|
| Myc-induced T-ALL | Langenau et al., 2003 | (1) 2 dpf embryos (2) AB adults |
(1) n/a (2) 25 Gy | WKM i.p. (1) 103 to 104 cells (2) 106 cells | GFP positive by 7 days | Homing to the thymus and tumor development starting at 14 days |
| BRAF-induced melanoma | (1) Patton et al., 2005 | (1) Tu adults | (1) 20 Gy | (1) 3 × 106 cells i.p. | By 1–2 weeks | (1) Melanoma by 3 weeks |
| (2) White et al., 2008 | (2) Casper | (2) 23 Gy | (2) 2 × 105 cells i.p. or i.c. | (2) Tumor dissemination by 5 to 28 days | ||
| Melanoma xenotransplant | Lee et al., 2005 | WT blastula-stage embryos | n/a | 1–100 cells | Immediately | n/a |
| Human melanoma cells | Topczewska et al., 2006 | WT blastula-stage embryos | n/a | 50–100 cells | Immediately | Masses form by 3 days |
| Human melanoma cell line | Haldi et al., 2006 | WT 2 dpf embryos | n/a | 50–100 cells labeled with CM-DiI | By 5 dpf | Melanoma masses by 10 days |
| Tel-AML1-induced B-ALL | Sabaawy et al., 2006 | WT adults | 25 Gy | 5 × 105 WKM cells i.p. | Signs of disease by 6–9 weeks | |
| Notch1-induced T-ALL | Chen et al., 2007 | AB fish | 23 Gy | 106 WKM cells i.p. | GFP cells at 7 days | Lymphoid cell infiltration by 21 days |
| Adenocarcinoma cell line xenotransplant | Nicoli et al., 2007 | WT 2 dpf embryos | n/a | 1000–2000 cells labeled with CM-DiI or DiO | Increased vasculature by 3 dpf | |
| KRAS-induced rhabdomyosarcoma | Langenau et al., 2007 | AB fish | 23 Gy | 10–2 × 104 FACS sorted tumor cells i.p. | By 7 days | Tumor formation by 14 days |
| KRAS-induced MPD | Le et al., 2007 | AB fish | 23 Gy | 3 × 105 WKM cells i.p. | Disease by 2 months, but serial transplant did not induce disease | |
| Human cancer cell lines | Stoletov et al., 2007 | AB fish, fli1-EGFP fish | Dexamethasone | 50–300 fluorescently labeled cells i.p. | Immediately | Microscopic tumors |
| NRAS-induced melanoma | White et al., 2008 | Casper fish | 23 Gy | 2 × 105 tumor cells i.p. or i.c. | GFP positive cells at 5 days | Tumors begin to form at 14 days |
| Ras-transformed murine epithelial cells | Marques et al., 2009 | Tu, fli1-EGFP | n/a | Cells injected into yolk | Immediately | If TGFβ stimulated, tumors begin to form at 3 days |
| Primary human pancreatic tumor cells | Marques et al., 2009 | Tu, fli1-EGFP | n/a | Cells injected into yolk or via orthotopic injection | Immediately | Invasion and micrometasis by 24 hours |
| Heritable T-ALL | Frazer et al., 2009 | WT fish | 25 Gy | 2.5 × 103 to 1 × 106 GFP+ tumor cells i.p. | Engrafted hosts began to die at 11–13 days | Disease capable of serial transplantation |
Many groups have previously tested transplantation in both the embryo and adult zebrafish. This table compares the methods used for each type of tumor cell transplanted, including numbers of cells transplanted and post-transplant phenotype. Irradiation, when used for immunosuppression, was performed 2 days before transplant. Dexamethasone was given at 10 μg/mL for 2 days before and 1 day post-transplant. i.p., intraperitoneal injection; i.c., intracardiac injection; WKM, whole kidney marrow; dpf, days postfertilization; WT, wild type; MPD, myeloproliferative disorder; GFP, green fluorescent protein; EGFP, enhanced green fluorescent protein; TGF, transforming growth factor; n/a, not applicable.
In a KRAS-induced model of myeloproliferative disorder (MPD), the disease could be transplanted one time, but further serial transplants did not result in MPD or leukemia.27 These results established that oncogenic KRAS alone was not enough to confer self-renewal properties and malignancy in blood cells, in agreement with experiments performed in a mouse model of MPD.10 Interestingly, ex vivo induction of KRAS before transplantation also induced MPD after primary transplant. This demonstrates how transplantation provides an additional approach for tissue-specific and temporally controlled oncogene induction, which may be useful in tumor models with particularly potent oncogenes or developmental regulators.
Solid tumors
Transplantation assays have also been used to study zebrafish solid tumors. Cells from zebrafish BRAFV600E;p53−/− melanomas regenerate highly invasive tumors after serial transplantation.3 Transplantation of these tumors into transparent adult zebrafish demonstrated metastatic capability, as cells were able to disseminate far from the transplantation site.9 Transplantation of a zebrafish RAS-induced embryonal rhabdomyosarcoma was used to identify a cancer stem cell population. In this model, populations of cells were labeled with α-actin GFP (a late marker of muscle cell differentiation) or Rag2-dsRed (an early marker of differentiation). Transplantation of sorted populations showed that Rag2+ (red) cells were more capable of engrafting in primary and serial transplants, so enrichment for Rag2 expression enriches for a cancer stem cell population. Additionally, limiting dilution assays were used to determine that only 10 Rag2+ cells are required for transplantation, whereas other cell populations required a much higher number of cells.21
Xenotransplantation
Human tumor cells lines can be transplanted into zebrafish embryos for study of tumor cell migration, metastasis, and effect on vasculogenesis. Several groups have shown that transplantation of human tumor cell lines into zebrafish embryos or larvae induces increased vasculature formation and endothelial cell gene expression,15,22,23 providing a model by which to study tumor-induced vascularization. Transplant into embryos at the blastula stage shows whether tumor cells are capable of engraftment, invasion, and developmental effects.24,26 More recently, fluorescently labeled pancreatic tumor cells and transforming growth factor β-stimulated transformed mammary epithelial human cells (but not pancreatic tumor cells) were shown to be capable of invading and metastasizing into other tissues when injected into 48 h postfertilization embryos.32 Primary human pancreatic cancer cells also demonstrated invasiveness post-transplant that could be modulated by protease inhibitor treatment. These types of experiments demonstrate how the zebrafish can be used to study and compare proangiogenic and metastatic behavior of human tumor cells. It is still important to note that a limitation of xenotransplants to date is the inability to successfully engraft in adult fish. Addressing some of the immunological issues around adult transplantation (see below) will address this issue in the future.
Advantages of Using the Zebrafish System
Numbers
One advantage of the zebrafish as a model is the ability to easily generate and maintain large numbers of fish, and this greatly increases the throughput of the transplant assay. It is reasonable to generate large numbers of fish with tumors, and even more feasible to generate hundreds of recipient fish. For adult transplantation assays, 100–200 or more transplants can be completed in a single day, which is generally more than what is standard for a mouse model of transplantation, where often only 2–3 animals are injected per experimental group.5 In embryo transplant experiments, hundreds to thousands of recipient embryos can be generated each day, although practically only about 300–700 one-cell-stage embryos can be reasonably injected per day.
Generation of transgenics
The ease of making transgenics in the zebrafish, particularly with fluorescent markers, is advantageous for study of tumor transplantability. Although targeted knockdown technology is still under development in the zebrafish,33 creating transgenic lines is a powerful tool in zebrafish biology. With the Tol2 transposase-based system of transgenesis, injection of a few hundred embryos is enough to create a stable transgenic line.8 This allows generation of a variety of tumor models and, if needed, a large number of mosaic fish. It is also feasible to generate inducible models, where an oncogene can be expressed under control of a responsive promoter. Using a heat-shock or alternative promoter, an oncogene can be induced ex vivo before transplantation.27 An oncogene can also be activated at a specific stage of development, or tissue-specific promoters can be used to limit oncogene expression to the tissue of interest. These methods provide spatial and temporal control over oncogene expression, decreasing toxicity and potentially creating a more accurate model of human disease.
In addition to facilitating tumor model generation, fluorescence can also be used to label cells expressing the transgene, providing a simple way to observe or sort tumor cells. Fluorescent transgenics can also label populations of interest within a tumor by co-injection of constructs with an oncogene of interest and a fluorescent marker driven by the same promoter.34 This may be useful to label cells expressing a specific transcription factor or in a particular differentiation state.
Sensitivity of post-transplant observation
The translucent zebrafish embryo has been critical for studying development, and this is also beneficial for the recipient in the transplant assay. Fluorescently tagged transplant cells can be scored in the zebrafish embryo during early development.24 With the development of the casper line, fluorescently tagged or pigmented cells can easily be imaged in the adult fish as well. The single-cell resolution possible in the zebrafish casper line exceeds the resolution currently possible in mouse transplant models.9 Since the fish does not need to be sacrificed for analysis, observation over extended periods of time is feasible. This makes the transparent zebrafish particularly advantageous for post-transplant monitoring.
Chemical treatment
Unique to the zebrafish system, whole organism treatment is possible by direct administration to the water. In both embryos and adult zebrafish, this method of chemical treatment can be used to modulate regenerative angiogenesis.35,36 This treatment provides an approach to test the importance of angiogenesis in tumor formation post-transplantation, and other processes can be assayed in a similar matter. As chemical treatment of zebrafish embryos has proven to be a useful high-throughput screening tool,31,37 treatment of embryos before or after transplant may also prove effective.
Areas for Future Development in Zebrafish Transplantation
Preventing death post-transplant
Even at sublethal doses of irradiation, the death rate of zebrafish post-transplant is often substantially higher than 10%. Optimization of irradiation dose, including split dose treatments, may decrease the death rate (T. Bowman and J. DeJong, unpublished observations). Additionally, post-transplant care may need to be optimized because irradiated and immunosuppressed fish are susceptible to infection. Maintaining transplant recipients in a clean facility with fewer microorganisms may significantly decrease infection-related death.
It is also possible that alternative and less toxic forms of immunosuppression can be used. These include testing drugs similar to dexamethasone before transplant into adult fish. Another alternative is to generate fish lacking T-cell receptors, lacking specific cytokines like interleukin-2, or having an otherwise impaired immune system. Similar to NOD/SCID mice, these fish would be less likely to reject a transplant, particularly a xenograft of mouse or human cells. Alternatively, typing of MHC genes in the zebrafish may allow for matching of donor and recipients, helping to circumvent graft rejection.38 It is also possible that development of these methods will allow engraftment of xenotransplants in the adult.
Variability between strains
Transplants in mouse are often between isogenic mice, decreasing the possibility of transplant rejection by the recipient mouse. Without isogenic zebrafish strains, transplant rejection and graft versus host disease can cause increased death and decreased engraftment. Unlike in mouse models where numerous isogenic lines exist, isogenic clonal zebrafish lines have just recently been developed. Serial transplant of chemically induced tumors is feasible using the clonal CG1 line.16 If transgenic tumor models can be generated in these fish lines, they will be increasingly useful for transplant studies. Until that becomes more widespread, it is crucial to determine the ideal recipient line for each study (i.e., AB, Tu, WIK, or others). In mice, there is evidence that the background genotype of the animal can strongly affect tumor behavior,39 and it is likely to be similar in the zebrafish as well.
Injection site
The location of the injection site in zebrafish also substantially differs from mouse models. While most zebrafish tumors are transplanted via intraperitoneal injection, mouse transplants often occur at or near the tissue of interest. Development of methods to transplant in specific tissues will likely improve overall engraftment rates. Orthotopic injection methods should be developed, as this method of injection is better than subcutaneous injection to assess engraftment and metastatic potential in mouse models.40 Although testing migration ability from the peritoneal cavity may be a model of metastasis in some ways, transplant closer to the tissue of interest will likely better represent human tumor cell biology.
Temporal and spatial control
Although there are currently several inducible expression systems available for zebrafish modeling, there are still systems used in the mouse that have not been fully developed for zebrafish tumor models. Tamoxifen-inducible expression may provide higher efficiency and better temporal control.41 The Gal4/UAS inducible system widely used in yeast and Drosophila has more recently been developed in the zebrafish system.42 Use of these systems for inducible tumor models will provide an additional level of control in the transplant fish assay.
Imaging techniques
Fluorescence-based imaging provides single-cell resolution in the zebrafish system, but other imaging methods are not well developed, particularly bioluminescence- and luciferase-based assays. These types of approaches are useful in mouse models for measuring tumor growth over time.43 Application of these tools to the fish system will provide increased alternatives for post-transplant analysis without sacrificing the recipient fish.
Summary
Transplantation of tumors has already taught us a significant amount of zebrafish tumor cell biology. Tumor malignancy has been demonstrated in multiple zebrafish tumor models by tumor propagation in a transplant recipient. Using fluorescently labeled transgenics, cancer stem cell populations enriched for tumor regeneration capability have been identified in rhabdomyosarcoma. A zebrafish model of melanoma is shown to have metastatic capability when transplanted into translucent fish. Human tumor cells are able to induce vasculogenesis in the zebrafish embryo. These experiments were successful in large part due to the advantages of the zebrafish as a model system. Beyond the ability to maintain large numbers of fish and make a variety of transgenic lines, the generation of the transparent adult fish has greatly enhanced transplantation in the fish. As this tumor assay is further developed, tumor transplantation in the zebrafish will be increasingly important in our understanding of tumor biology.
Acknowledgments
We would like to acknowledge Katie Kathrein, David Langenau, and Richard White for critical review and helpful feedback for this article. We would also like to thank Zon lab members for much transplant-related discussion.
Disclosure Statement
L.I.Z. is a founder and stockholder of Fate, Inc. and a scientific advisor for Stemgent.
References
- 1.Seller MH. Animal models for bone-marrow transplantation. J Med Genet. 1970;7:305–309. doi: 10.1136/jmg.7.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langenau DM. Traver D. Ferrando AA. Kutok JL. Aster JC. Kanki JP, et al. Myc-induced T cell leukemia in transgenic zebrafish. Science. 2003;299:887–890. doi: 10.1126/science.1080280. [DOI] [PubMed] [Google Scholar]
- 3.Patton EE. Widlund HR. Kutok JL. Kopani KR. Amatruda JF. Murphey RD, et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15:249–254. doi: 10.1016/j.cub.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 4.Inohara H. Matsunaga T. Nomura T. Growth and metastasis of fresh human benign and malignant tumors in the head and neck regions transplanted into scid mice. Carcinogenesis. 1992;13:845–849. doi: 10.1093/carcin/13.5.845. [DOI] [PubMed] [Google Scholar]
- 5.Kruczynski A. Hill BT. Classic in vivo cancer models: three examples of mouse models used in experimental therapeutics. Curr Protoc Pharmacol. 2002:5.24.1–5.24.16. doi: 10.1002/0471141755.ph0524s15. [DOI] [PubMed] [Google Scholar]
- 6.Greene HS. The transplantation of tumors to the brains of heterologous species. Cancer Res. 1951;11:529–534. [PubMed] [Google Scholar]
- 7.Dick JE. Lapidot T. Biology of normal and acute myeloid leukemia stem cells. Int J Hematol. 2005;82:389–396. doi: 10.1532/IJH97.05144. [DOI] [PubMed] [Google Scholar]
- 8.Kawakami K. Transposon tools and methods in zebrafish. Dev Dyn. 2005;234:244–254. doi: 10.1002/dvdy.20516. [DOI] [PubMed] [Google Scholar]
- 9.White RM. Sessa A. Burke C. Bowman T. LeBlanc J. Ceol C, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–189. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan IT. Kutok JL. Williams IR. Cohen S. Kelly L. Shigematsu H, et al. Conditional expression of oncogenic K-ras from its endogenous promoter induces a myeloproliferative disease. J Clin Invest. 2004;113:528–538. doi: 10.1172/JCI20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapidot T. Sirard C. Vormoor J. Murdoch B. Hoang T. Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 12.Quintana E. Shackleton M. Sabel MS. Fullen DR. Johnson TM. Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elkin M. Vlodavsky I. Tail vein assay of cancer metastasis. Curr Protoc Cell Biol. 2001 doi: 10.1002/0471143030.cb1902s12. Chapter 19: Unit 19.2. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura M. Suemizu H. Novel metastasis models of human cancer in NOG mice. Curr Top Microbiol Immunol. 2008;324:167–177. doi: 10.1007/978-3-540-75647-7_11. [DOI] [PubMed] [Google Scholar]
- 15.Stoletov K. Montel V. Lester RD. Gonias SL. Klemke R. High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc Natl Acad Sci USA. 2007;104:17406–17411. doi: 10.1073/pnas.0703446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizgireuv IV. Revskoy SY. Transplantable tumor lines generated in clonal zebrafish. Cancer Res. 2006;66:3120–3125. doi: 10.1158/0008-5472.CAN-05-3800. [DOI] [PubMed] [Google Scholar]
- 17.Traver D. Paw BH. Poss KD. Penberthy WT. Lin S. Zon LI. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- 18.Langenau DM. Ferrando AA. Traver D. Kutok JL. Hezel JP. Kanki JP, et al. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc Natl Acad Sci USA. 2004;101:7369–7374. doi: 10.1073/pnas.0402248101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam SH. Chua HL. Gong Z. Lam TJ. Sin YM. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev Comp Immunol. 2004;28:9–28. doi: 10.1016/s0145-305x(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 20.Traver D. Herbomel P. Patton EE. Murphey RD. Yoder JA. Litman GW, et al. The zebrafish as a model organism to study development of the immune system. Adv Immunol. 2003;81:253–330. [PubMed] [Google Scholar]
- 21.Langenau DM. Keefe MD. Storer NY. Guyon JR. Kutok JL. Le X, et al. Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes Dev. 2007;21:1382–1395. doi: 10.1101/gad.1545007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haldi M. Ton C. Seng WL. McGrath P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis. 2006;9:139–151. doi: 10.1007/s10456-006-9040-2. [DOI] [PubMed] [Google Scholar]
- 23.Nicoli S. Ribatti D. Cotelli F. Presta M. Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res. 2007;67:2927–2931. doi: 10.1158/0008-5472.CAN-06-4268. [DOI] [PubMed] [Google Scholar]
- 24.Lee LM. Seftor EA. Bonde G. Cornell RA. Hendrix MJ. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dyn. 2005;233:1560–1570. doi: 10.1002/dvdy.20471. [DOI] [PubMed] [Google Scholar]
- 25.Topczewska JM. Postovit LM. Margaryan NV. Sam A. Hess AR. Wheaton WW, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 26.Traver D. Winzeler A. Stern HM. Mayhall EA. Langenau DM. Kutok JL, et al. Effects of lethal irradiation in zebrafish and rescue by hematopoietic cell transplantation. Blood. 2004;104:1298–1305. doi: 10.1182/blood-2004-01-0100. [DOI] [PubMed] [Google Scholar]
- 27.Le X. Langenau DM. Keefe MD. Kutok JL. Neuberg DS. Zon LI. Heat shock-inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc Natl Acad Sci USA. 2007;104:9410–9415. doi: 10.1073/pnas.0611302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabaawy HE. Azuma M. Embree LJ. Tsai HJ. Starost MF. Hickstein DD. TEL-AML1 transgenic zebrafish model of precursor B cell acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2006;103:15166–15171. doi: 10.1073/pnas.0603349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J. Jette C. Kanki JP. Aster JC. Look AT. Griffin JD. NOTCH1-induced T-cell leukemia in transgenic zebrafish. Leukemia. 2007;21:462–471. doi: 10.1038/sj.leu.2404546. [DOI] [PubMed] [Google Scholar]
- 30.Frazer JK. Meeker ND. Rudner L. Bradley DF. Smith AC. Demarest B, et al. Heritable T-cell malignancy models established in a zebrafish phenotypic screen. Leukemia. 2009;23:1825–1835. doi: 10.1038/leu.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson RT. Shaw SY. Peterson TA. Milan DJ. Zhong TP. Schreiber SL, et al. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol. 2004;22:595–599. doi: 10.1038/nbt963. [DOI] [PubMed] [Google Scholar]
- 32.Marques IJ. Weiss FU. Vlecken DH. Nitsche C. Bakkers J. Lagendijk AK, et al. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer. 2009;9:128. doi: 10.1186/1471-2407-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amacher SL. Emerging gene knockout technology in zebrafish: zinc-finger nucleases. Brief Funct Genomics Proteomics. 2008;7:460–464. doi: 10.1093/bfgp/eln043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langenau DM. Keefe MD. Storer NY. Jette CA. Smith AC. Ceol CJ, et al. Co-injection strategies to modify radiation sensitivity and tumor initiation in transgenic Zebrafish. Oncogene. 2008;27:4242–4248. doi: 10.1038/onc.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan J. Bayliss PE. Wood JM. Roberts TM. Dissection of angiogenic signaling in zebrafish using a chemical genetic approach. Cancer Cell. 2002;1:257–267. doi: 10.1016/s1535-6108(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 36.Bayliss PE. Bellavance KL. Whitehead GG. Abrams JM. Aegerter S. Robbins HS, et al. Chemical modulation of receptor signaling inhibits regenerative angiogenesis in adult zebrafish. Nat Chem Biol. 2006;2:265–273. doi: 10.1038/nchembio778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.North TE. Goessling W. Walkley CR. Lengerke C. Kopani KR. Lord AM, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostatis. Nature. 2007;447:1007–1012. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook JG. Figueroa F. Beck S. A genome-wide survey of major histocompatibility complex (MHC) genes and their paralogues in zebrafish. BMC Genomics. 2005;6:152. doi: 10.1186/1471-2164-6-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park YG. Zhao X. Lesueur F. Lowy DR. Lancaster M. Pharoah P, et al. Sipa1 is a candidate for underlying the metastasis efficiency modifier locus Mtes1. Nat Genet. 2005;37:1055–1062. doi: 10.1038/ng1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerbel RS. Cornil I. Theodorescu D. Importance of orthotopic transplantation procedures in assessing the effects of transfected genes on human tumor growth and metastasis. Cancer Metastasis Rev. 1991;10:201–215. doi: 10.1007/BF00050792. [DOI] [PubMed] [Google Scholar]
- 41.Hans S. Kaslin J. Freudenreich D. Brand M. Temporally-controlled site-specific recombination in zebrafish. PLoS One. 2009;4:e4640. doi: 10.1371/journal.pone.0004640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halpern ME. Rhee J. Goll MG. Akitake CM. Parsons M. Leach SD. Gal4/UAS transgenic tools and their application to zebrafish. Zebrafish. 2008;5:97–110. doi: 10.1089/zeb.2008.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato A. Klaunberg B. Tolwani R. In vivo bioluminescence imaging. Comp Med. 2004;54:631–634. [PubMed] [Google Scholar]