Abstract
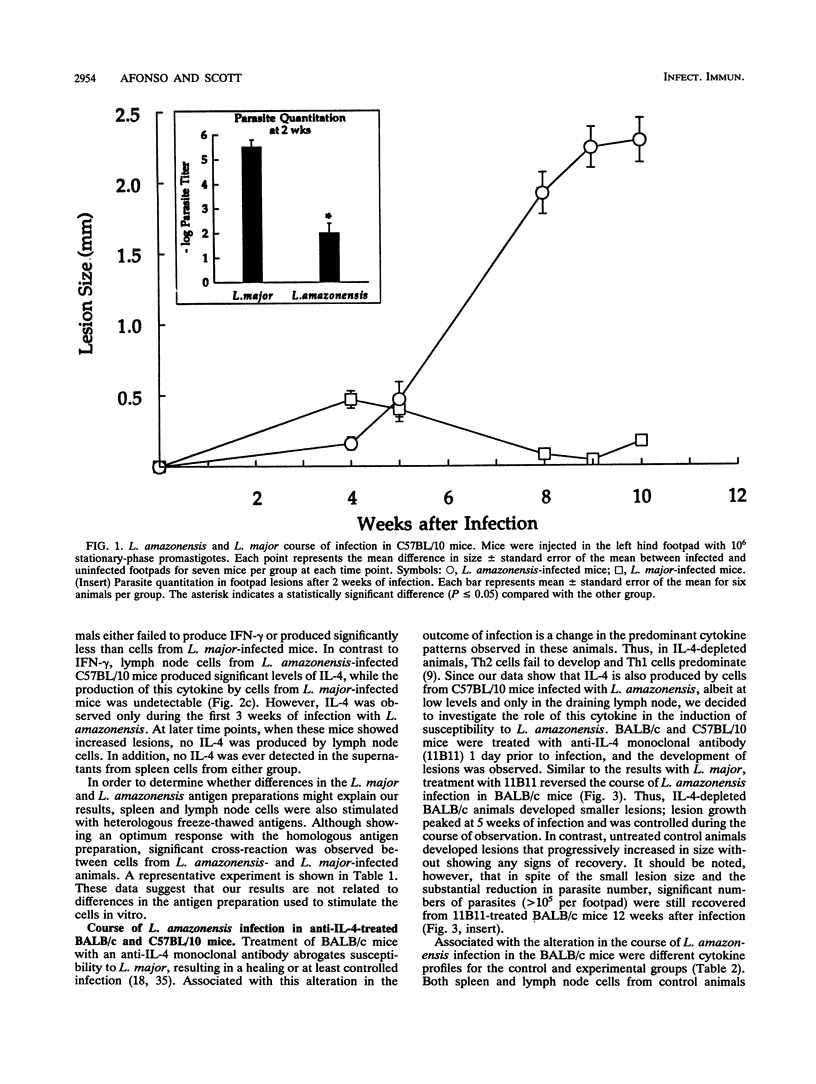
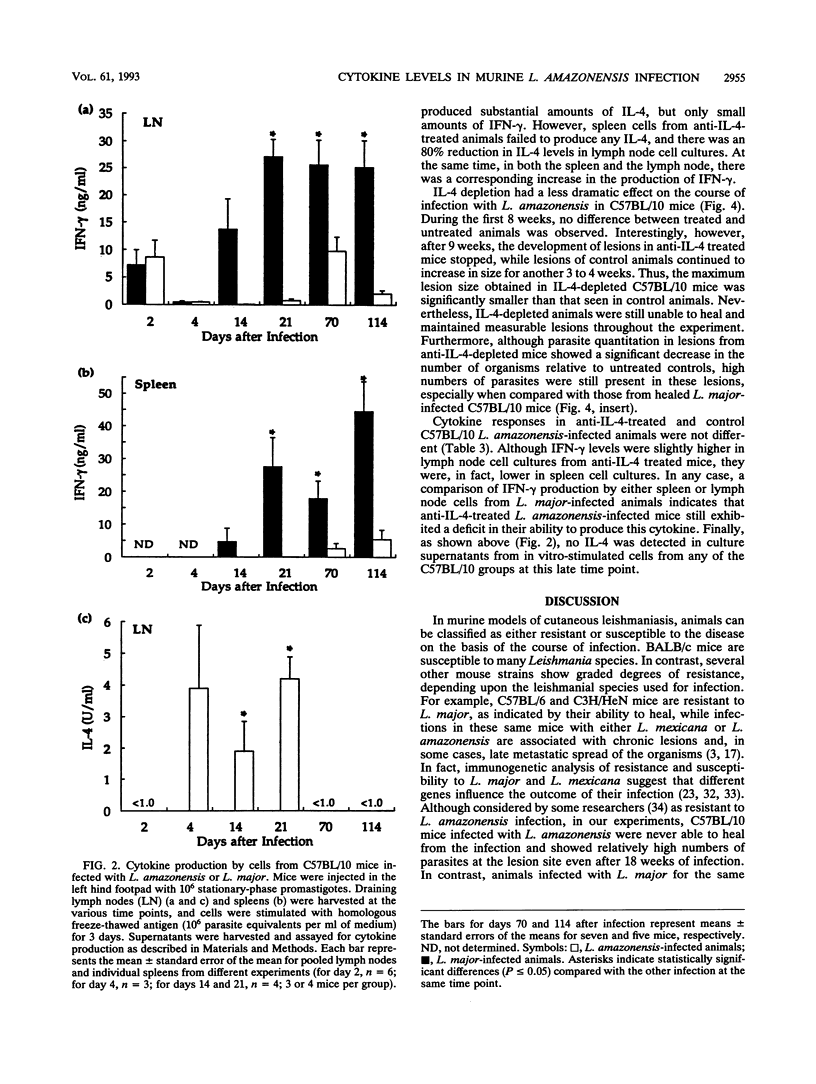
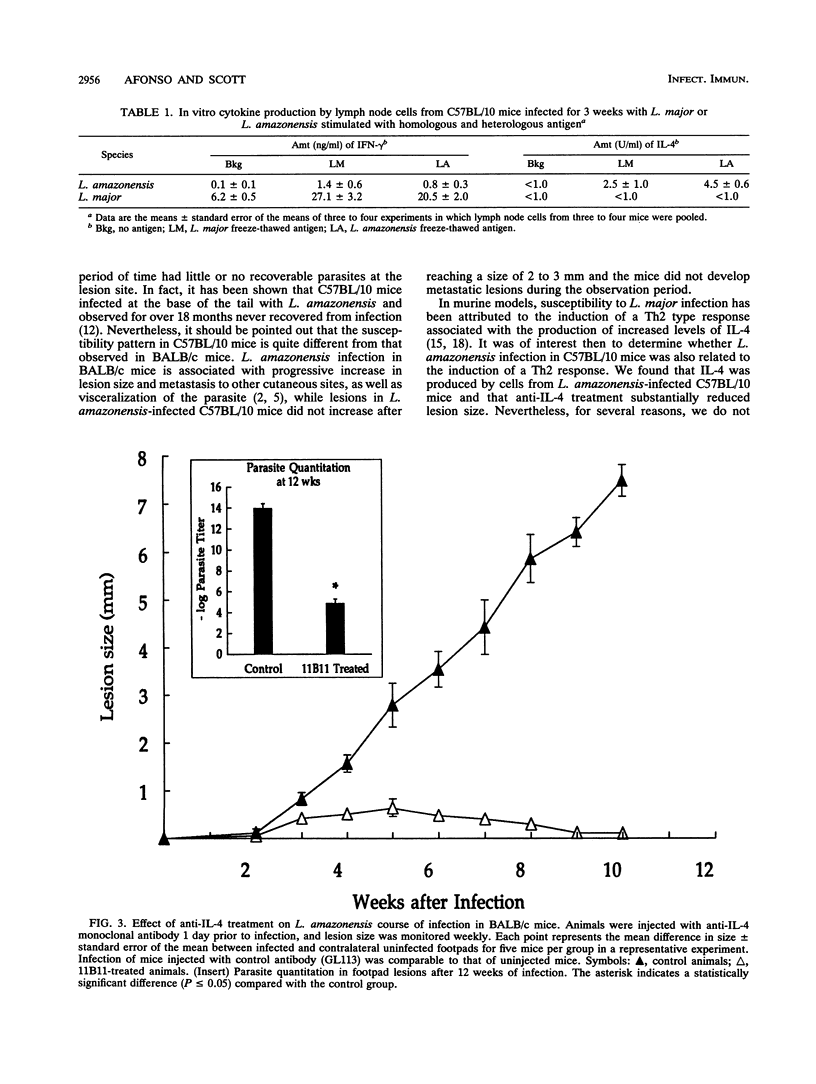
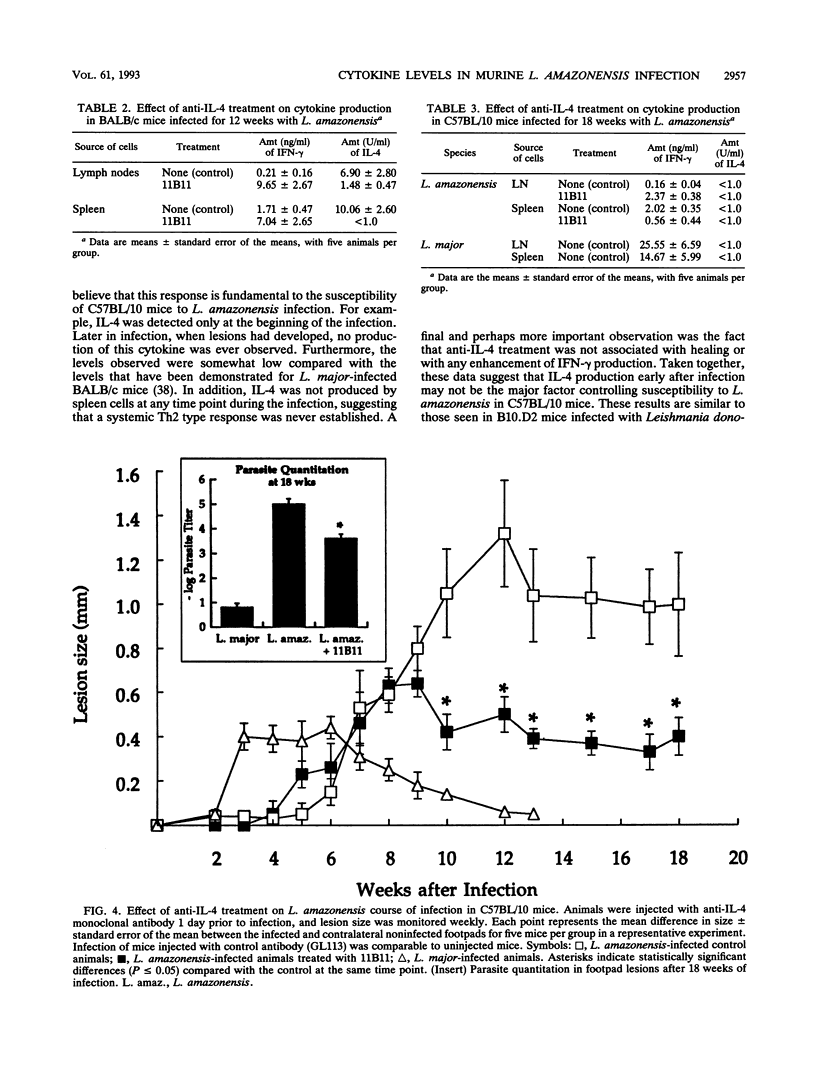
Leishmaniae are protozoans which, depending upon both the host and parasite species, can cause either a healing or nonhealing infection. While C57BL/10 mice are able to heal following infection with Leishmania major, they fail to heal following infection with Leishmania amazonensis. In order to address the role of Th1 and Th2 cell responses in the outcome of these infections in C57BL/10 mice, gamma interferon (IFN-gamma) and interleukin-4 (IL-4) production was assessed. While cells from L. major-infected C57BL/10 mice produced high levels of IFN-gamma, cells from L. amazonensis-infected animals produced little or no IFN-gamma. On the other hand, IL-4 was produced only by cells from L. amazonensis-infected C57BL/10 mice, but this production was restricted to the first few weeks of infection. Later in infection, when lesions were evident, no IL-4 was detected. Treatment of BALB/c mice with a monoclonal antibody (11B11) directed against IL-4 induced a dramatic reduction in L. amazonensis lesions. This reduction was associated with a decrease in IL-4 levels and an increase in IFN-gamma production. However, only a slight reduction in lesion sizes and parasite numbers was observed when anti-IL-4-treated C57BL/10 mice were infected with L. amazonensis. These results suggest that IL-4 may have an important role in mediating susceptibility to L. amazonensis in BALB/c mice, as previously demonstrated for L. major. More importantly, however, the data suggest that susceptibility to L. amazonensis in C57BL/10 mice is due to the absence of a Th1 cell response, rather than to the presence of a Th2 cell response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiri P., Locksley R. M., Parslow T. G., Sadick M., Rector E., Ritter D., McKerrow J. H. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992 Apr 16;356(6370):604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- Andrade Z. A., Reed S. G., Roters S. B., Sadigursky M. Immunopathology of experimental cutaneous leishmaniasis. Am J Pathol. 1984 Jan;114(1):137–148. [PMC free article] [PubMed] [Google Scholar]
- Barral-Netto M., Barral A., Brownell C. E., Skeiky Y. A., Ellingsworth L. R., Twardzik D. R., Reed S. G. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science. 1992 Jul 24;257(5069):545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- Barral-Netto M., Cardoso S. A., Barral A. Different patterns of disease in two inbred mouse strains infected with a clone of Leishmania mexicana amazonensis. Acta Trop. 1987 Mar;44(1):5–11. [PubMed] [Google Scholar]
- Barral A., Petersen E. A., Sacks D. L., Neva F. A. Late metastatic Leishmaniasis in the mouse. A model for mucocutaneous disease. Am J Trop Med Hyg. 1983 Mar;32(2):277–285. doi: 10.4269/ajtmh.1983.32.277. [DOI] [PubMed] [Google Scholar]
- Bretscher P. A., Wei G., Menon J. N., Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes "susceptible" mice resistant to Leishmania major. Science. 1992 Jul 24;257(5069):539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- Chantry D., Turner M., Abney E., Feldmann M. Modulation of cytokine production by transforming growth factor-beta. J Immunol. 1989 Jun 15;142(12):4295–4300. [PubMed] [Google Scholar]
- Chatelain R., Varkila K., Coffman R. L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992 Feb 15;148(4):1182–1187. [PubMed] [Google Scholar]
- Childs G. E., Lightner L. K., McKinney L., Groves M. G., Price E. E., Hendricks L. D. Inbred mice as model hosts for cutaneous leishmaniasis. I. Resistance and susceptibility to infection with Leishmania braziliensis, L. mexicana, and L. aethiopica. Ann Trop Med Parasitol. 1984 Feb;78(1):25–34. doi: 10.1080/00034983.1984.11811769. [DOI] [PubMed] [Google Scholar]
- Convit J., Pinardi M. E., Rondón A. J. Diffuse cutaneous leishmaniasis: a disease due to an immunological defect of the host. Trans R Soc Trop Med Hyg. 1972;66(4):603–610. doi: 10.1016/0035-9203(72)90306-9. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R. T., Oswald I. P., James S. L., Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992 Mar 15;148(6):1792–1796. [PubMed] [Google Scholar]
- Green S. J., Crawford R. M., Hockmeyer J. T., Meltzer M. S., Nacy C. A. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990 Dec 15;145(12):4290–4297. [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Grimaldi G., Jr, Moriearty P. L., Hoff R. Leishmania mexicana: immunology and histopathology in C3H mice. Exp Parasitol. 1980 Aug;50(1):45–56. doi: 10.1016/0014-4894(80)90006-5. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Chan-Liew W. L. Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 1980 Winter;2(4):303–314. doi: 10.1111/j.1365-3024.1980.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Kaye P. M., Curry A. J., Blackwell J. M. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine-induced rate of cure in murine visceral leishmaniasis. J Immunol. 1991 Apr 15;146(8):2763–2770. [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- McElrath M. J., Kaplan G., Nusrat A., Cohn Z. A. Cutaneous leishmaniasis. The defect in T cell influx in BALB/c mice. J Exp Med. 1987 Feb 1;165(2):546–559. doi: 10.1084/jem.165.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S. T-cell-mediated activation of macrophages. Curr Opin Immunol. 1991 Jun;3(3):330–335. doi: 10.1016/0952-7915(91)90033-w. [DOI] [PubMed] [Google Scholar]
- Neal R. A., Hale C. A comparative study of susceptibility of inbred and outbred mouse strains compared with hamsters to infection with New World cutaneous leishmaniases. Parasitology. 1983 Aug;87(Pt 1):7–13. doi: 10.1017/s0031182000052379. [DOI] [PubMed] [Google Scholar]
- Nelson B. J., Ralph P., Green S. J., Nacy C. A. Differential susceptibility of activated macrophage cytotoxic effector reactions to the suppressive effects of transforming growth factor-beta 1. J Immunol. 1991 Mar 15;146(6):1849–1857. [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Oswald I. P., Gazzinelli R. T., Sher A., James S. L. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992 Jun 1;148(11):3578–3582. [PubMed] [Google Scholar]
- Pérez H., Labrador F., Torrealba J. W. Variations in the response of five strains of mice to Leishmania mexicana. Int J Parasitol. 1979 Feb;9(1):27–32. doi: 10.1016/0020-7519(79)90062-6. [DOI] [PubMed] [Google Scholar]
- Roberts M., Alexander J., Blackwell J. M. Genetic analysis of Leishmania mexicana infection in mice: single gene (Scl-2) controlled predisposition to cutaneous lesion development. J Immunogenet. 1990 Feb-Apr;17(1-2):89–100. doi: 10.1111/j.1744-313x.1990.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Roberts M., Alexander J., Blackwell J. M. Influence of Lsh, H-2, and an H-11-linked gene on visceralization and metastasis associated with Leishmania mexicana infection in mice. Infect Immun. 1989 Mar;57(3):875–881. doi: 10.1128/iai.57.3.875-881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M. M., Xavier M. T., Previato L. M., Barcinski M. A. Characterization of cellular immune response to chemically defined glycoconjugates from Leishmania mexicana subsp. amazonensis. Infect Immun. 1986 Jan;51(1):80–86. doi: 10.1128/iai.51.1.80-86.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravia N. G., Weigle K., Segura I., Giannini S. H., Pacheco R., Labrada L. A., Goncalves A. Recurrent lesions in human Leishmania braziliensis infection--reactivation or reinfection? Lancet. 1990 Aug 18;336(8712):398–402. doi: 10.1016/0140-6736(90)91945-7. [DOI] [PubMed] [Google Scholar]
- Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991 Nov 1;147(9):3149–3155. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Pearce E., Cheever A. W., Coffman R. L., Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989 Dec;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Scott P., Sher A. A spectrum in the susceptibility of leishmanial strains to intracellular killing by murine macrophages. J Immunol. 1986 Feb 15;136(4):1461–1466. [PubMed] [Google Scholar]
- Scott P. The role of TH1 and TH2 cells in experimental cutaneous leishmaniasis. Exp Parasitol. 1989 Apr;68(3):369–372. doi: 10.1016/0014-4894(89)90120-3. [DOI] [PubMed] [Google Scholar]
- Silva J. S., Morrissey P. J., Grabstein K. H., Mohler K. M., Anderson D., Reed S. G. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992 Jan 1;175(1):169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]



