Abstract
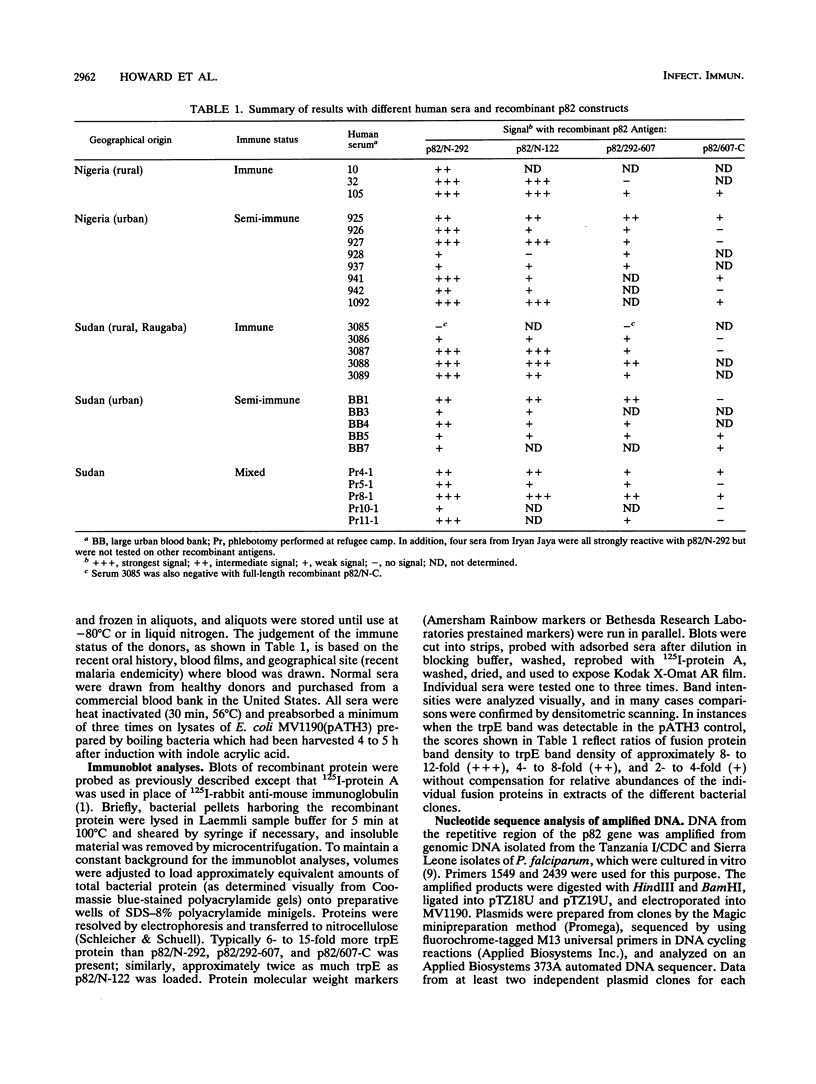
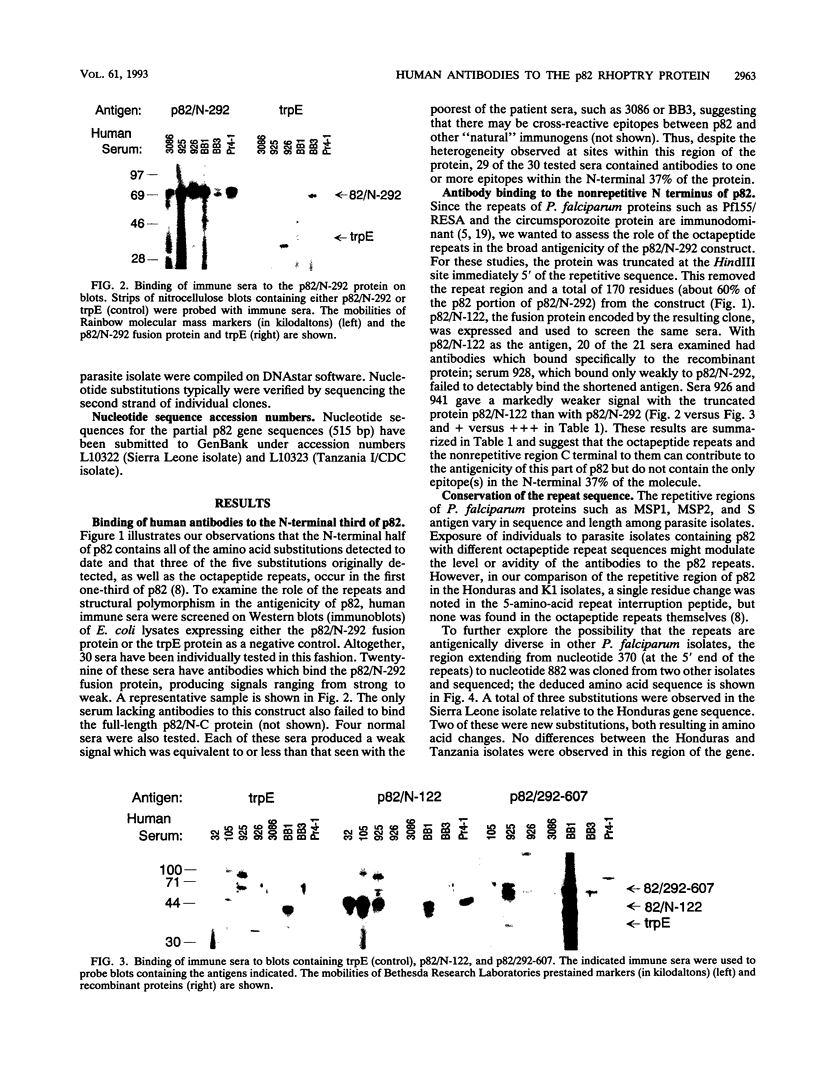
Immunization of monkeys with the 82-kDa rhoptry protein (p82) of Plasmodium falciparum can protect them against a lethal blood stage challenge, and monoclonal antibodies to p82 inhibit parasite growth in vitro. The role that a p82-specific immune response might play in human immunity to the parasite is not known. To determine to what extent humans produce antibodies to p82 following infection with P. falciparum, sera from individuals believed to be hyperimmune, semi-immune, or never infected with the parasite were examined. Portions of the p82 gene were expressed separately as fusion proteins and used on immunoblots to test for antibodies to the recombinant proteins. All but 1 of the 30 immune sera possessed antibodies to p82, while nonimmune sera produced, at best, only a marginal signal to the fusion proteins. The signal intensity produced with the human immune sera depended on the region of p82 being assayed, with the N-terminal 37% of p82 producing stronger signals than more C-terminal parts of p82. This N-terminal domain contains a tandem octapeptide repeat (consensus KSSSPSXT/V) of the structure (repeat)2-Q-T-S-G-S/L-(repeat)3. It is shown here that the sequence of this repetitive motif is conserved among four parasite isolates at both the nucleotide and amino acid levels; the five-residue repeat interruption peptide QTSGS/L separating the two sets of repeats contains the only amino acid substitution (Ser or Leu) detected in this region to date. Despite their conservation of structure, the repeats do not appear to be the only epitope recognized by the human antibodies, since sera which recognize the N-terminal fusion protein containing the repeats also bind a related protein after truncation and removal of the repeats. These results indicate that the structurally conserved p82 molecule contains multiple B-cell epitopes and is likely to be immunogenic in most individuals during natural infections with P. falciparum. These observations are consistent with the idea that antibodies to p82 generated during parasite infection have a role in the development of immunity to the organism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardeshir F., Flint J. E., Reese R. T. Expression of Plasmodium falciparum surface antigens in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2518–2522. doi: 10.1073/pnas.82.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshir F., Howard R. F., Viriyakosol S., Arad O., Reese R. T. Cross-reactive asparagine-rich determinants shared between several blood-stage antigens of Plasmodium falciparum and the circumsporozoite protein. Mol Biochem Parasitol. 1990 Apr;40(1):113–128. doi: 10.1016/0166-6851(90)90085-z. [DOI] [PubMed] [Google Scholar]
- Brake D. A., Long C. A., Weidanz W. P. Adoptive protection against Plasmodium chabaudi adami malaria in athymic nude mice by a cloned T cell line. J Immunol. 1988 Mar 15;140(6):1989–1993. [PubMed] [Google Scholar]
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Collins W. E., Anders R. F., Pappaioanou M., Campbell G. H., Brown G. V., Kemp D. J., Coppel R. L., Skinner J. C., Andrysiak P. M., Favaloro J. M. Immunization of Aotus monkeys with recombinant proteins of an erythrocyte surface antigen of Plasmodium falciparum. Nature. 1986 Sep 18;323(6085):259–262. doi: 10.1038/323259a0. [DOI] [PubMed] [Google Scholar]
- Harnyuttanakorn P., McBride J. S., Donachie S., Heidrich H. G., Ridley R. G. Inhibitory monoclonal antibodies recognise epitopes adjacent to a proteolytic cleavage site on the RAP-1 protein of Plasmodium falciparum. Mol Biochem Parasitol. 1992 Oct;55(1-2):177–186. doi: 10.1016/0166-6851(92)90138-a. [DOI] [PubMed] [Google Scholar]
- Howard R. F., Reese R. T. Plasmodium falciparum: hetero-oligomeric complexes of rhoptry polypeptides. Exp Parasitol. 1990 Oct;71(3):330–342. doi: 10.1016/0014-4894(90)90038-e. [DOI] [PubMed] [Google Scholar]
- Howard R. F. The sequence of the p82 rhoptry protein is highly conserved between two Plasmodium falciparum isolates. Mol Biochem Parasitol. 1992 Apr;51(2):327–330. doi: 10.1016/0166-6851(92)90083-v. [DOI] [PubMed] [Google Scholar]
- Koerner T. J., Hill J. E., Myers A. M., Tzagoloff A. High-expression vectors with multiple cloning sites for construction of trpE fusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- Ladda R., Aikawa M., Sprinz H. Penetration of erythrocytes by merozoites of mammalian and avian malarial parasites. J Parasitol. 1969 Jun;55(3):633–644. [PubMed] [Google Scholar]
- Marchuk D., Drumm M., Saulino A., Collins F. S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991 Mar 11;19(5):1154–1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig R. S., Vanderberg J. P., Most H., Orton C. Specificity of protective immunity produced by x-irradiated Plasmodium berghei sporozoites. Nature. 1969 May 3;222(5192):488–489. doi: 10.1038/222488a0. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Dayal R. Immunity to asexual erythrocytic stages of Plasmodium falciparum: role of defined antigens in the humoral response. Immunol Rev. 1982;61:245–269. doi: 10.1111/j.1600-065x.1982.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Ramirez E., Lambert P. H., Miescher P. A. Inhibition of P. falciparum growth in human erythrocytes by monoclonal antibodies. Nature. 1981 Jan 22;289(5795):301–303. doi: 10.1038/289301a0. [DOI] [PubMed] [Google Scholar]
- Ridley R. G., Takacs B., Etlinger H., Scaife J. G. A rhoptry antigen of Plasmodium falciparum is protective in Saimiri monkeys. Parasitology. 1990 Oct;101(Pt 2):187–192. doi: 10.1017/s0031182000063228. [DOI] [PubMed] [Google Scholar]
- Ridley R. G., Takacs B., Lahm H. W., Delves C. J., Goman M., Certa U., Matile H., Woollett G. R., Scaife J. G. Characterisation and sequence of a protective rhoptry antigen from Plasmodium falciparum. Mol Biochem Parasitol. 1990 Jun;41(1):125–134. doi: 10.1016/0166-6851(90)90103-s. [DOI] [PubMed] [Google Scholar]
- Schofield L., Bushell G. R., Cooper J. A., Saul A. J., Upcroft J. A., Kidson C. A rhoptry antigen of Plasmodium falciparum contains conserved and variable epitopes recognized by inhibitory monoclonal antibodies. Mol Biochem Parasitol. 1986 Feb;18(2):183–195. doi: 10.1016/0166-6851(86)90037-x. [DOI] [PubMed] [Google Scholar]
- Zavala F., Cochrane A. H., Nardin E. H., Nussenzweig R. S., Nussenzweig V. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J Exp Med. 1983 Jun 1;157(6):1947–1957. doi: 10.1084/jem.157.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]