Abstract
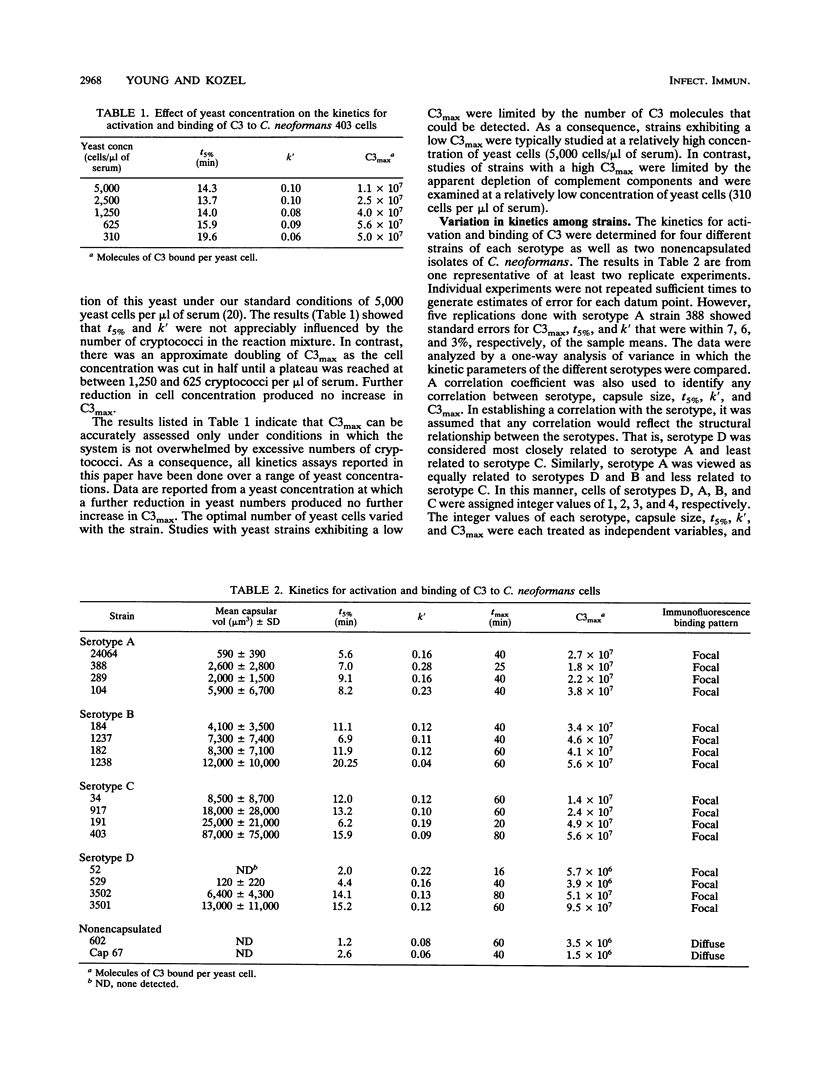
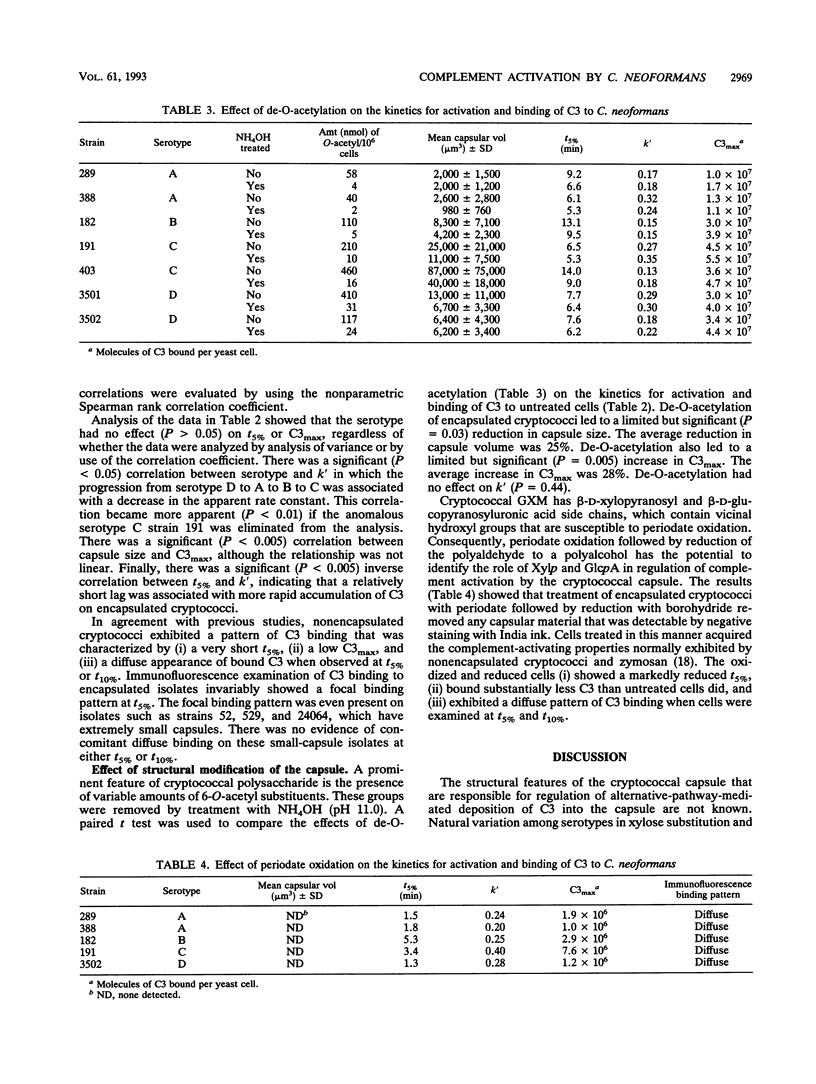
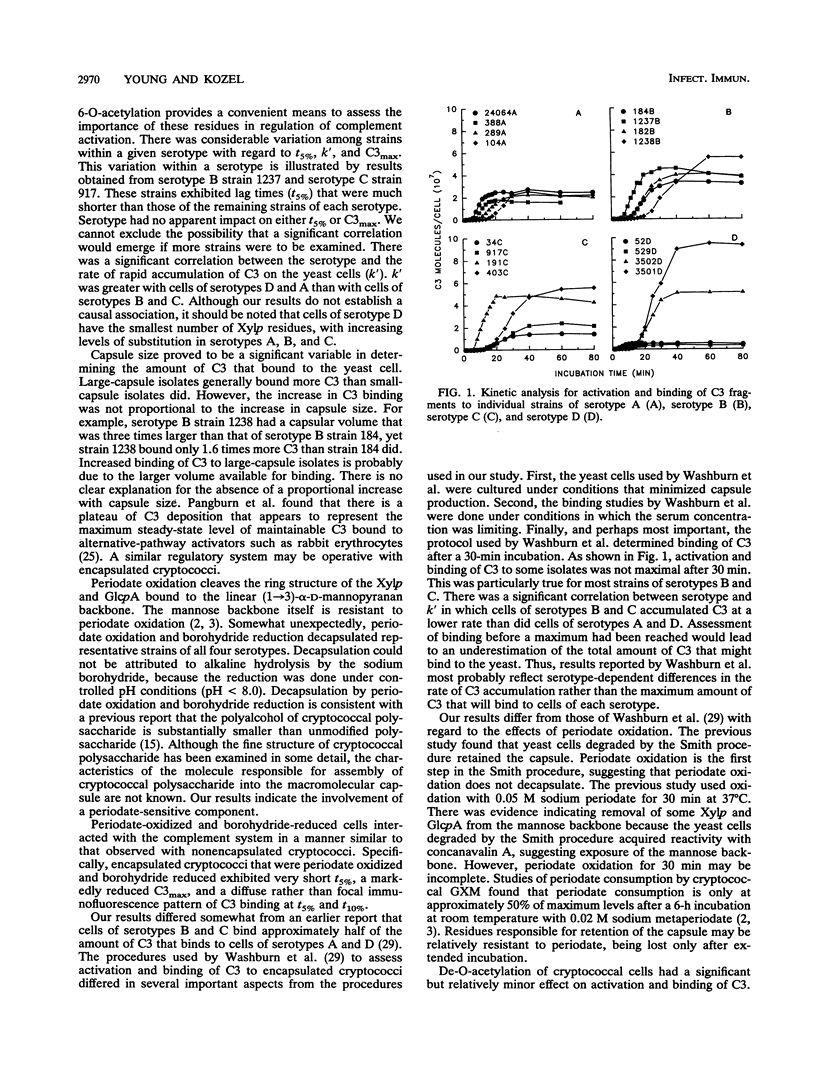
Incubation of encapsulated cells of Cryptococcus neoformans in normal human serum leads to activation of the alternative complement pathway and deposition of opsonic fragments of C3 into the capsule. We determined whether the variation in capsular structure that occurs among the four major cryptococcal serotypes was reflected in the kinetics for activation and binding of C3. We also examined the effects on activation kinetics of de-O-acetylation or periodate oxidation of the capsule. Binding kinetics were characterized in terms of the time required to deposit 5% of the maximal amount of C3 on the yeast (t5%), the first-order rate constant for amplification of C3 deposition (k'), and the maximum amount of C3 that could be deposited in the capsule (C3max). Our results showed that variations in the capsular structure that characterized each serotype had no significant influence on C3max but that the rate of C3 deposition depended significantly on the serotype. C3 accumulated at a higher rate on cells of serotypes A and D than on cells of serotypes B and C. There was a significant correlation between capsular volume and C3max, although the relationship was not linear. Periodate treatment of encapsulated cryptococci of all four serotypes led to decapsulation. Periodate-oxidized encapsulated cells displayed kinetics for activation and binding of C3 that were identical to kinetics observed with nonencapsulated cryptococci. Finally, de-O-acetylation led to a significant but relatively minor increase in C3max.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharjee A. K., Bennett J. E., Glaudemans C. P. Capsular polysaccharides of Cryptococcus neoformans. Rev Infect Dis. 1984 Sep-Oct;6(5):619–624. doi: 10.1093/clinids/6.5.619. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. On the structure of the capsular polysaccharide from Cryptococcus neoformans serotype C. Immunochemistry. 1978 Sep;15(9):673–679. doi: 10.2196/40846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. Structural studies on the major, capsular polysaccharide from Cryptococcus bacillisporus serotype B. Carbohydr Res. 1980 Jun;82(1):103–111. doi: 10.1016/s0008-6215(00)85524-x. [DOI] [PubMed] [Google Scholar]
- Cherniak R., Jones R. G., Reiss E. Structure determination of Cryptococcus neoformans serotype A-variant glucuronoxylomannan by 13C-n.m.r. spectroscopy. Carbohydr Res. 1988 Jan 15;172(1):113–138. doi: 10.1016/s0008-6215(00)90846-2. [DOI] [PubMed] [Google Scholar]
- Cherniak R. Soluble polysaccharides of Cryptococcus neoformans. Curr Top Med Mycol. 1988;2:40–54. doi: 10.1007/978-1-4612-3730-3_2. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Activation of the alternative complement pathway due to resistance of zymosan-bound amplification convertase to endogenous regulatory mechanisms. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1683–1687. doi: 10.1073/pnas.74.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Fromtling R. A., Shadomy H. J., Jacobson E. S. Decreased virulence in stable, acapsular mutants of cryptococcus neoformans. Mycopathologia. 1982 Jul 23;79(1):23–29. doi: 10.1007/BF00636177. [DOI] [PubMed] [Google Scholar]
- Horstmann R. D., Pangburn M. K., Müller-Eberhard H. J. Species specificity of recognition by the alternative pathway of complement. J Immunol. 1985 Feb;134(2):1101–1104. [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Hermerath C. A. Binding of cryptococcal polysaccharide to Cryptococcus neoformans. Infect Immun. 1984 Mar;43(3):879–886. doi: 10.1128/iai.43.3.879-886.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Highison B., Stratton C. J. Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect Immun. 1984 Feb;43(2):574–579. doi: 10.1128/iai.43.2.574-579.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S., Guerlain A. S., Highison B. A., Highison G. J. Strain variation in phagocytosis of Cryptococcus neoformans: dissociation of susceptibility to phagocytosis from activation and binding of opsonic fragments of C3. Infect Immun. 1988 Nov;56(11):2794–2800. doi: 10.1128/iai.56.11.2794-2800.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Wilson M. A., Murphy J. W. Early events in initiation of alternative complement pathway activation by the capsule of Cryptococcus neoformans. Infect Immun. 1991 Sep;59(9):3101–3110. doi: 10.1128/iai.59.9.3101-3110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Wilson M. A., Pfrommer G. S., Schlageter A. M. Activation and binding of opsonic fragments of C3 on encapsulated Cryptococcus neoformans by using an alternative complement pathway reconstituted from six isolated proteins. Infect Immun. 1989 Jul;57(7):1922–1927. doi: 10.1128/iai.57.7.1922-1927.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Wilson M. A., Welch W. H. Kinetic analysis of the amplification phase for activation and binding of C3 to encapsulated and nonencapsulated Cryptococcus neoformans. Infect Immun. 1992 Aug;60(8):3122–3127. doi: 10.1128/iai.60.8.3122-3127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Polacheck I., Bennett J. E. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C). J Clin Microbiol. 1982 Mar;15(3):535–537. doi: 10.1128/jcm.15.3.535-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J Exp Med. 1980 Oct 1;152(4):1102–1114. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. C3b deposition during activation of the alternative complement pathway and the effect of deposition on the activating surface. J Immunol. 1983 Oct;131(4):1930–1935. [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981 Sep 1;154(3):856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Twose T. M., Paterson D. S., Sim E. The covalent-binding reaction of complement component C3. Biochem J. 1981 Jan 1;193(1):115–127. doi: 10.1042/bj1930115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn R. G., Bryant-Varela B. J., Julian N. C., Bennett J. E. Differences in Cryptococcus neoformans capsular polysaccharide structure influence assembly of alternative complement pathway C3 convertase on fungal surfaces. Mol Immunol. 1991 Apr-May;28(4-5):465–470. doi: 10.1016/0161-5890(91)90160-l. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H., Fey G. H. Human complement component C3: cDNA coding sequence and derived primary structure. Proc Natl Acad Sci U S A. 1985 Feb;82(3):708–712. doi: 10.1073/pnas.82.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]


