Abstract
Germline defects in the tuberous sclerosis 2 (TSC2) tumor suppressor gene predispose humans and rats to benign and malignant lesions in a variety of tissues. The brain is among the most profoundly affected organs in tuberous sclerosis (TSC) patients and is the site of development of the cortical tubers for which the hereditary syndrome is named. A spontaneous germline inactivation of the Tsc2 locus has been described in an animal model, the Eker rat. We report that the homozygous state of this mutation (Tsc2Ek/Ek) was lethal in mid-gestation (the equivalent of mouse E9.5–E13.5), when Tsc2 mRNA was highly expressed in embryonic neuroepithelium. During this period homozygous mutant Eker embryos lacking functional Tsc2 gene product, tuberin, displayed dysraphia and papillary overgrowth of the neuroepithelium, indicating that loss of tuberin disrupted the normal development of this tissue. Interestingly, there was significant intraspecies variability in the penetrance of cranial abnormalities in mutant embryos: the Long–Evans strain Tsc2Ek/Ek embryos displayed these defects whereas the Fisher 344 homozygous mutant embryos had normal-appearing neuroepithelium. Taken together, our data indicate that the Tsc2 gene participates in normal brain development and suggest the inactivation of this gene may have similar functional consequences in both mature and embryonic brain.
The tumor suppressor properties of the tuberous sclerosis 2 (TSC2) gene have been established in both human and rodent tumors (1–6). In humans, tuberous sclerosis (TSC) is an autosomal dominant syndrome associated with the development of hamartomas in various tissues (7). These lesions often have loss of heterozygosity or mutations at the respective TSC1 or TSC2 loci (1–4, 8, 9). This finding implies that target cells are sensitive to the loss of function of the proteins encoded by these genes and that this loss can result in tumorigenesis. Tumors in TSC patients are primarily benign, but malignant transformation does occur, affecting mainly the kidneys (10, 11). Reexpression of the TSC2 gene product, tuberin, in tumor cells defective for both TSC2 alleles has confirmed the ability of tuberin to inhibit cell proliferation and suppress tumorigenicity (12, 13).
The function of tuberin in normal cells is not clearly defined at present. The DNA sequence of TSC2 shares limited homology with Rap1GAP, which suggests that tuberin may act as a GTPase activating protein. In vitro studies demonstrated that tuberin can specifically stimulate, although only weakly, the hydrolysis of Rap1GTP (14). In vivo interaction of tuberin and Rap1 is supported by their colocalization to perinuclear organelles such as the Golgi apparatus (15); however, how TSC2 tumor suppressor function is mediated by Rap1 remains unclear. Another effector of tuberin, Rab5, was identified by using a yeast two-hybrid system (16). By interacting with rabaptin-5, tuberin significantly accelerates the rate of GTP hydrolysis of Rab5 (16), a rate-limiting step in endocytosis. In fact, cells with TSC2 mutations lack Rab5 GTPase-activating protein activity and have an increased rate of fluid-phase endocytosis (16). However, the mechanism of tuberin involvement in vesicular transport, which is important for normal function of many tissues, is not known.
The brain is the most frequently and severely affected organ in children with TSC syndrome, and disorders such as epilepsy, mental retardation, and hydrocephalus are a major source of morbidity in TSC patients (7). The underlying pathology, cortical tubers and subependymal giant cell astrocytomas (SEGAs), is characterized by focal disorganized arrays of dysplastic and proliferating cellular elements, most notably giant cells of indeterminate origin (17, 18). Although early studies have shown a lower than expected frequency of loss of heterozygosity in brain lesions (19), recent immunoanalysis of SEGAs in TSC patients has confirmed the lack of tuberin expression within the spindle and epithelioid cells in these lesions (20). Loss of tuberin function in cortical tubers would be consistent with the two-hit model (21) for TSC2 as a tumor suppressor gene; however, the mechanism by which TSC2 dysfunction causes brain lesions and the role of TSC2 in neural development in general remain poorly understood.
A naturally occurring animal model with a germline Tsc2 mutation (Eker mutation) that causes a loss of the carboxyl terminus of tuberin has been described (5, 6, 22). In the heterozygous state, these so-called Eker rats develop tumors of the kidney (renal cell carcinoma), uterus (leiomyoma), spleen (hemangiosarcoma), brain (subependymal giant cell astrocytoma), and pituitary gland (adenoma) (23–28). Many of these lesions exhibit loss of the normal Tsc2 allele and accompanying loss of functional tuberin (27, 29). Early studies of the Eker rat model suggested that the homozygous state of the Eker mutation is lethal during embryogenesis, primarily due to the smaller litters produced by heterozygous intercrosses (22, 23). Why embryos homozygous for the Eker mutation die in utero has not been determined, although gross morphological analysis suggested that they may have brain malformations (29). These studies lacked precise genotype–phenotype comparisons, currently possible because of the identification and cloning of the Eker mutation (5, 6). In the study reported here, we show that the function of the Tsc2 gene is critical for the developing embryo to proceed beyond mid-gestation. We found that this gene has a pivotal function in the normal development of the embryonic neuroepithelium in mid-gestation; however, manifestation of neuroepithelial abnormalities in homozygous mutants was greatly dependent on the genetic background in which the Eker mutation was carried, consistent with the well known variability of severity of brain lesions in TSC patients.
MATERIALS AND METHODS
Tissue Samples.
The Long–Evans strain on which the Eker mutation was carried has been described previously (22). These animals were bred to Fisher 344 rats that were subsequently back-crossed onto the Fisher background for 10 generations. Tsc2Ek/+ rats were intercrossed and timed-pregnant females were killed by using CO2. Long–Evans Eker rat breeding colony was maintained at the Science Park-Research Division, University of Texas MD Anderson Cancer Center, Smithville, TX, and timed-pregnant study animals were transported to the Institute for Cellular and Molecular Biology, Department of Microbiology, University of Texas, Austin, TX, during early pregnancy for later embryo dissection and analysis between embryonic days 10–21. Embryos at the equivalent of mouse gestational stages E8–E18 were dissected and washed in PBS. Reichert’s membrane, embryo-derived tissue, or both were removed and frozen in liquid nitrogen for genotyping. The embryos were fixed in 4% paraformaldehyde at 4°C overnight, washed three times for 5 min in PBS containing 0.1% Tween 20, and stored in absolute methanol at −20°C. For synthesis of Tsc2 cDNA, testes from Eker rats that did not carry the Tsc2 mutation (Tsc2+/+) were quick frozen in liquid nitrogen for RNA extraction.
PCR.
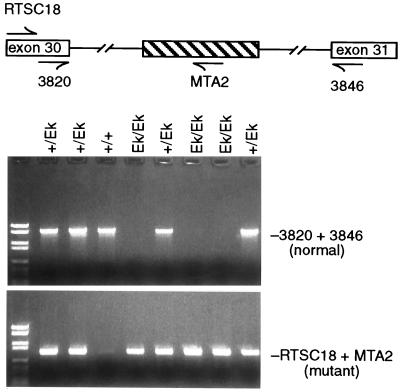
Genomic DNA from embryonic tissues was extracted with a QIAamp Tissue kit from Qiagen. A PCR-based strategy was used to determine the genotype of the collected embryos (Fig. 1). Primers 3820 5′-CGCTCTAACACAGGTGAGTGC-3′ and 3846 5′-AACCACGGCCGAGTCTGAGAGG-3′, specific to Tsc2 exons 30 and 31, were designed to amplify a 1.7-kb PCR product from the wild-type allele, and primers RTSC18 5′-GTCTAATGCCCTTATGGCTG-3′ and MTA2 5′-TCCTCCTGAAGCTGAAGAGT-3′, specific to Tsc2 exon 30 and Eker mutation in intron 31 (5, 30), were used to amplify a 892-bp PCR product from the mutant allele. The PCR conditions were 93°C for 1 min, 58°C for 1 min, and 72°C for 2 min for 35 cycles.
Figure 1.
Strategy for genotyping of embryos. Wild-type- (3820 and 3846) or mutant- (RTSC18 and MTA2) specific primers were used to amplify embryonic DNA by PCR. Presence of the normal and mutant alleles was demonstrated by visualization of the corresponding PCR fragments with ethidium bromide.
Riboprobe Construction.
Total RNA from normal rat testes was obtained by standard CsCl centrifugation (31). To obtain a fragment of the Tsc2-coding region for riboprobe construction, 2 μg of total RNA was reverse transcribed (RT) with Moloney murine leukemia virus reverse transcriptase (GIBCO/BRL) by following the manufacturer’s conditions. The 715-bp RT-PCR product was amplified by using the primers TSC2Hind 5′-CTTCAGACCAAGCTTTATAC-3′and TSC2Kpn 5′-TCACACACCATGGGAACCG-3′, that contained HindIII and KpnI restriction sites, respectively, and directionally subcloned into the pBluescript II KS (±). The presence of the 715-bp Tsc2 insert in the clone TSC2–1794 was confirmed by sequencing using the fmol DNA Sequencing System kit (Promega). HindIII and KpnI were used to linearize plasmid DNA to generate antisense and sense Tsc2 riboprobe templates, respectively. Riboprobes were synthesized and labeled with 35S by using the Riboprobe Combination System kit (Promega) according to the manufacturer’s instructions. The labeled RNA probes were purified with Quick Spin Columns (Boehringer Mannheim).
In Situ Hybridization.
The in situ hybridization was performed by the method of Simmons et al. (32) with the modifications described below. The procedure was performed overnight in a humid chamber at 60°C in hybridization buffer (50% formamide/10% dextran sulfate/0.3 M NaCl/1× Denhardt’s solution/1 mM EDTA/10 mM Tris⋅HCl, pH 8.0/7.5 μg/ml yeast tRNA/50 mM DTT) containing 107 cpm/ml labeled riboprobe. The slides were washed in 4× SSC, treated with RNase A (20 μg/ml) followed by a series of increasing stringency SSC washes, with a final wash in 0.5× SSC containing 1 mM DTT. After the second dehydration series, the slides were coated with Kodak NTB-2 emulsion (Eastman Kodak) and exposed for 3 weeks at 4°C. Then the slides were analyzed by bright- and dark-field microscopy.
RESULTS
To investigate the function of the Tsc2 gene during embryogenesis, we crossed rats heterozygous for the Eker Tsc2 mutation. Independent experiments were performed with two strains of rats (Long–Evans and Fisher 344) that carry the Eker mutation. Embryos at the equivalent of mouse gestational age E8–E18 (days 10–20 in the rat) were genotyped and examined for developmental defects. A portion of the Reichert’s membrane or embryo-derived tissue was obtained from individual embryos to determine whether they were homozygous wild-type (Tsc2+/+), homozygous mutant (Tsc2Ek/Ek), or heterozygous (Tsc2Ek/+). Fig. 1 shows the PCR strategy used to determine the carrier status of individual embryos. The primers located in exons 30 and 31 (3820 and 3846) amplified only the wild-type allele, resulting in a 1.7-kb PCR product, whereas primers RTSC18 and MTA2, located in exon 30 and the Eker mutation site, respectively, specifically amplified a 892-bp product from the mutant allele. Therefore those embryos with 1.7-kb PCR product were wild-type homozygotes, those with both the 1.7-kb and 892-bp PCR products were heterozygotes, and those with only the 892-bp PCR product were mutant homozygotes.
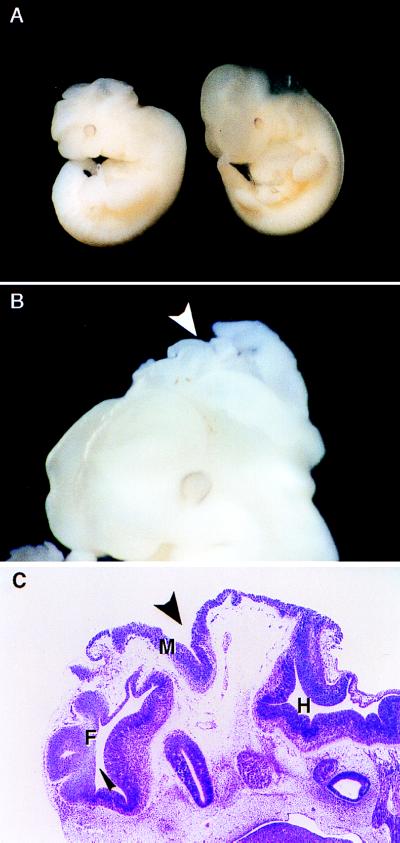
As shown in Table 1, the appearance of morphological defects segregated with homozygosity of the Eker mutation at the Tsc2 locus. As early as E9.5, all Tsc2Ek/Ek embryos of the Long–Evans strain had abnormalities. All of the 12 Tsc2Ek/Ek embryos examined between E9.5 and E13 had abnormal morphology, whereas 55 of 56 Tsc2Ek/+ heterozygotes and 16 of 17 Tsc2+/+ wild-type embryos were grossly normal, indicating a low (<3%) background frequency of morphological defects in this strain. What was consistent among the Tsc2Ek/Ek embryos was an abnormal shape of the head and the fact that they were generally smaller than their wild-type or heterozygous littermates. In addition to these abnormalities, two dramatic exencephalic (cartilage skull is defective, causing exposure of the brain) cases were observed. One of these embryos at E10.5 is shown in Fig. 2 A and B. The penetrance of the dysraphic phenotype (defective fusion of the neural folds) was variable because only two of the embryos examined in this study exhibited such abnormalities. Histological sections of the embryo shown in Fig. 2 A and B confirmed that the midbrain was entirely open to the amniotic cavity with the neural tissue exposed and degenerative (Fig. 2C). Interestingly, the genetic background on which the Eker mutation was carried profoundly affected the penetrance of the cranial abnormalities observed. In contrast to the Long–Evans embryos, the Tsc2Ek/Ek embryos of the Fisher 344 strain appeared normal with respect to head development, and the neural tube was completely closed in all embryos after E11 (data not shown).
Table 1.
Tsc2 genotype/phenotype correlation in Long–Evans rats
| Age | Litters, n | No. of embryos
|
|||||
|---|---|---|---|---|---|---|---|
| +/+
|
+/Ek
|
Ek/Ek
|
|||||
| N | A | N | A | N | A | ||
| E18 | 1 | 0 | 0 | 7 | 0 | 0 | 0 (3) |
| E13 | 2 | 3 | 0 | 8 | 0 | 0 | 3 (1) |
| E11 | 3 | 4 | 0 | 21 | 0 (2) | 0 | 5 (3) |
| E10.5 | 1 | 4 | 1 | 5 | 0 | 0 | 2 |
| E9.5 | 4 | 5 | 0 | 14 | 1 (3) | 0 | 2 (3) |
| Total | 11 | 16 | 1 | 55 | 1 (5) | 0 | 12 (10) |
| (17%) | (61%) | (22%) | |||||
N, normal morphology; A, characteristic cranial abnormalities. Parentheses indicate resorbed embryos in these groups. Status of Tsc2 gene was determined by PCR of DNA from Reichert’s membrane or tail tissue of individual embryos.
Figure 2.
Wild-type and Tsc2Ek/Ek mutant embryos. (A) Gross appearance of wild-type (left) and mutant (right) E10.5 embryos. Mutant embryo is smaller and has an abnormal head shape. (B) Head of E10.5 mutant embryo exhibiting dysraphia with protruding folds of neuroepithelium indicated by arrow. (C) Sagittal section of the embryo in B; narrow arrow indicates prominent folding of forebrain (F) and wide arrow indicates opening of midbrain (M) to the amniotic cavity. Hindbrain (H) and choroid plexus were morphologically normal.
Loss of Tsc2 function arrested embryonic development at E13 and resulted in the death of both Long–Evans and Fisher 344 Tsc2Ek/Ek homozygotes. The numbers of embryos with mutant and normal phenotypes at various gestational ages are shown in Tables 2 and 3. During early development, the ratio of Tsc2+/+ to Tsc2Ek/+ to Tsc2Ek/Ek embryos of the Long–Evans strain roughly followed the 1:2:1 ratio predicted for Mendelian inheritance of a single gene. However, the number of viable Tsc2Ek/Ek embryos decreased from 25% at E9 to 0% at E13, indicating that expression of Tsc2 was required for normal development beyond mid-gestation. The development of nonviable embryos, which were present at later gestational periods but which had not yet been resorbed, appeared to be arrested and had not progressed beyond E13. Although they did not display gross morphological abnormalities including head defects, Fisher 344 embryos homozygous for the Eker mutation died during the same mid-gestational period (Table 3).
Table 2.
Frequency of mutant and wild-type phenotypes in Long–Evans embryos
| E8 | E8.5 | E9 | E9.5 | E10.5 | E11.0 | E11.5 | E13.0 | E18.0 | |
|---|---|---|---|---|---|---|---|---|---|
| Litters, n | 1 | 1 | 1 | 4 | 3 | 4 | 3 | 2 | 1 |
| Embryos with normal appearance, n | 10 | 10 | 9 | 22 | 23 | 38 | 28 | 12 | 7 |
| Mutants, n | |||||||||
| Viable | 2 | 3 | 4 | 5 | 5 | 3 | 6 | 0 | 0 |
| Nonviable | 0 | 0 | 0 | 1 | 1 | 4 | 3 | 3 | 0 |
| Resorptions, n | 2 | 1 | 3 | 7 | 1 | 8 | 6 | 6 | 7 |
| Percentage of viable mutants | 14 | 21 | 25 | 14 | 17 | 7 | 14 | 0 | 0 |
Table 3.
Viability of Fisher 344 embryos carrying the Eker mutation
| Litters, n | Genotype
|
|||
|---|---|---|---|---|
| +/+ | +/Ek | Ek/Ek | ||
| E12.5 | 5 | 12 (5) | 25 (5) | 11 (5) |
| 21% | 61% | 18% | ||
| E13.5 | 3 | 12 (2) | 17 (1) | 6 (6) |
| 38% | 62% | 0% | ||
Parentheses indicate number of nonviable embryos based on the lack of a beating heart.
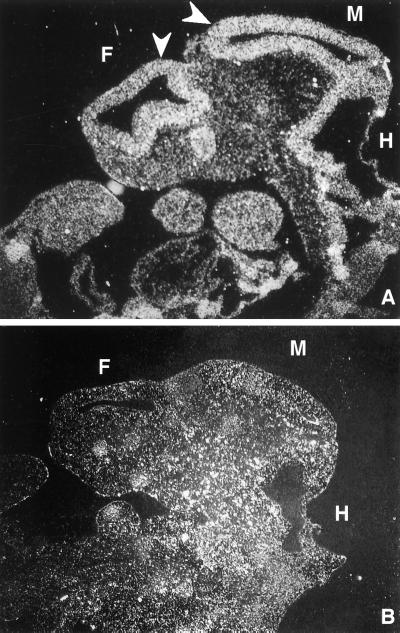
Expression of Tsc2 during this early period of development when embryonic lethality occurs, has not been described previously, and in situ hybridization experiments were performed to determine the pattern of expression of this gene at E10.5. That tuberin is involved in brain development during mid-gestation was supported by the high level of Tsc2 gene expression in neuroepithelium, relative to adjacent tissues, detected by hybridization of a 35S-labeled rat Tsc2 riboprobe to wild-type rat embryos. Antisense probe Tsc2–1794 hybridized strongly to the neuroepithelium lining the forebrain, midbrain, and hindbrain (Fig. 3A), although other embryonic structures were labeled as well, consistent with the ubiquitous expression pattern of this gene previously reported (33). In contrast, the sense probe Tsc2–1794 used as a control showed no increased labeling of the neuroepithelium over background (Fig. 3B).
Figure 3.
In situ hybridization. Sagittal section of E10.5 wild-type embryo showing localization of Tsc2 expression by hybridization of antisense (A) and sense (B) Tsc2–1794 riboprobes to the developing neuroepithelium. F, forebrain; M, midbrain; H, hindbrain. Arrows indicate the neuroepithelium, which was intensely labeled by using antisense Tsc2 riboprobe.
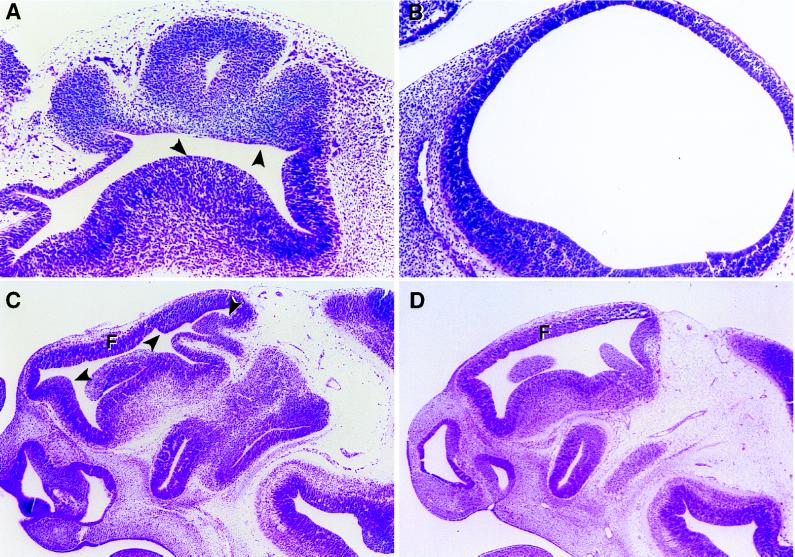
To determine the nature of the embryonic defects at the cellular level, embryos, including the abnormal embryo shown in Fig. 2A, were analyzed histologically. Because the phenotypic window appeared to be broad and the effects of loss of Tsc2 function were variable, a time course analysis was performed to describe the phenotype of loss of Tsc2 function in early embryos. A total of four pairs of normal and abnormal matched embryos at E9.5, E10.5 (2 pairs), and E11.5, were serially sectioned and examined for morphological defects. A subtle phenotype defined as an abnormal shape and size of the head was first identified at late E9.5 (data not shown). Careful examination revealed that the head was smaller with respect to body size and the superficial demarcations between forebrain and midbrain vesicles were not discernible. At E10.5, both mutant embryos examined were characterized by wrinkled, “deflated” telencephalic vesicles, which appeared to have not properly expanded. Histopathologically, E10.5 and E11.5 mutant embryos were generally very similar, and therefore these embryos are described together. Several landmark features of the developing brain were normal: the infundibulum, the Rathke’s pouch, the optic stalk, and the eye when visible. The neural epithelial layer in the midbrain and hindbrain of these embryos was less affected although it was convoluted and misshapen. In dramatic contrast, the forebrains always had overgrown neuroepithelium and resembled “deflated balloons” (Fig. 4 A and C), unlike the rounded structures observed in normal embryos (Fig. 4B), even though there was no obvious communication to the exterior that could account for loss of intraventricular pressure in these cavities. In the hindbrain, the choroid plexus, the structure responsible for the secretion of cerebrospinal fluids into the ventricular cavities regulating intraventricular pressure, appeared normal in mutant embryos (Fig. 2C). However, abnormal expansion of the telencephalic vesicles could not be ruled out because there was no evidence for the presence of chroid plexus in these vesicles due to the major disorganization of the forebrain in the mutant embryos. As shown in Fig. 4 A and C, the papillary overgrowth of the neuroepithelium in the ventricular cavity that was evident in mutant embryos was suggestive of hamartomas of the brain in patients affected with TSC. The neuroepithelium in these embryos appeared to be cytologically normal, although excessive in amount, suggesting a relaxation of growth constraints had occurred as a result of loss of tuberin function in this tissue.
Figure 4.
Neuroepithelial abnormalities in mutant embryos. (A) Forebrain of an E10.5 Tsc2Ek/Ek mutant embryo. (B) Forebrain of wild-type embryo at E10.5, which is more rounded and inflated than the forebrain of mutant embryo shown in A. (C) Head of Tsc2Ek/Ek embryo at E11.5 with papillary overgrowth of midbrain neuroepithelium, indicated by arrows. Forebrain is misshapen and disorganized. (D) Head of wild-type embryo at E11.5. (A and C) Arrows indicate areas of thickening and folding of neuroepithelium in mutant embryos. F, forebrain.
DISCUSSION
The phenotype–genotype comparison reported in this study revealed that loss of function of the Tsc2 tumor suppressor gene had dramatic effects on both Long–Evans and Fisher 344 Tsc2Ek/Ek embryos. Embryonic lethality occurred in both strains at the same stage of embryogenesis, and all of the Long–Evans embryos were morphologically abnormal. Papillary overgrowth of the neuroepithelium was observed in these embryos in mid-gestation, and the most severely affected embryos had dysraphia. We found that Tsc2 mRNA was expressed in the neuroepithelium very early, at E10.5, and appeared to persist in the neuroepithelium at later stages of brain development (33, 34). In the permissive Long–Evans strain, loss of functional tuberin at this stage of development resulted in various cranial defects including papillary overgrowth of neuroepithelium. These findings suggest that tuberin participates in brain development in mid-gestation when the telencephalic vesicles differentiate and lack of tuberin at this stage may trigger inappropriate proliferation, which prevents further differentiation. In Long–Evans Tsc2Ek/+ embryos, brain development appeared to be normal, implying that the abnormalities observed in Tsc2Ek/Ek embryos resulted from the absence of a functional Tsc2 allele. This finding is consistent with the two-hit model for TSC2 inactivation in SEGAs in TSC patients which is further suggested by lack of tuberin expression in these lesions (20).
Crino et al. (35) have detected in cortical tubers a number of proteins that are normally expressed in immature neurons, suggesting that these brain lesions may result from disrupted maturation of the neuroepithelium due to the loss of tuberin function in these cells. Focal dysplasia of the cerebral cortex (cortical tubers) characterized by disorganized cortical lamination and morphologically abnormal neurons and giant cells, and proliferative SEGAs are frequently diagnosed in children affected with TSC (9). Our finding that there was overgrowth and papillary projections of neuroepithelium in Tsc2Ek/Ek embryos suggests that neural precursors that lack functional tuberin and undergo abnormal proliferation or differentiation may give rise to the cortical tubers and SEGAs that are frequently diagnosed in children affected with TSC.
Since tuberous sclerosis was first described at the end of the 19th century, it has been known as a disease with highly variable clinical manifestations (7). The differences in the spectrum of clinical findings are believed to result from individual differences in genetic or environmental factors. In rats, the genetic background on which the Eker mutation was carried appeared to have a profound effect on the development of the observed neuroepithelial abnormalities. The neuroepithelium of homozygous mutant Fisher 344 rat embryos appeared grossly and histologically unaffected by the loss of tuberin, whereas all Long–Evans homozygous mutant embryos had neuroepithelial alterations. The variation in the penetrance of the neuroepithelial defects would be consistent with the influence of modifier genes that can modulate the impact of loss of tuberin function. This finding is supported by observation that the expression of the tumor phenotype in Tsc2Ek/+ rats is modified by the genetic background. For example, the size and multiplicity of renal tumors were significantly different between the Fisher 344 and Brown Norway rats carrying the Eker mutation (R. S. Yeung, unpublished data). Modifier genes have been demonstrated for other tumor suppressor genes, such as the adenomatous polyposis coli (APC)min tumor suppressor gene, the murine homologue of the human APC gene, whose activity is modulated by the recently identified gene mom-1 (36). The existence of murine strain-specific modifiers also has been recognized previously for such diverse tumor types as lung carcinoma (37), colon cancer (38), and thymic lymphoma (39) as well as for embryonic lethality (40). Our data strongly suggest that genes that can modify the activity of TSC2 also exist, the products of which may be useful therapeutic targets for countering the loss of tuberin function observed in lesions that develop in patients affected with TSC.
Although the cranial abnormalities observed in Long–Evans Tsc2Ek/Ek embryos were not observed in Tsc2Ek/Ek Fisher 344 embryos, embryonic lethality occurred in the same developmental stage in both strains. This suggests that defects in addition to those observed in the brain had occurred in other organ systems or developmental processes. However, abnormalities in other embryonic structures of the trunk and posterior region of the trunk were not observed in the histological analysis. Whether the cranial abnormalities were directly responsible for death of the Long–Evans mutant embryos cannot be determined, as dysraphia is not always lethal in utero. Embryos suffering from open-brain defects have lived through birth (41), much longer than mid-gestation, when Tsc2Ek/Ek embryos die. Therefore, although it appears that the penetrance of neuroepithelial abnormalities can be modified by host genetic factors, lethality is always a consequence of loss of Tsc2 function. In fact, it was shown that introduction of a wild-type Tsc2 transgene into Eker rats was able to rescue embryonic lethality in Tsc2Ek/Ek embryos (42), consistent with the apparent requirement for tuberin function for embryogenesis to proceed beyond E13 of development.
Acknowledgments
The authors thank Drs. Rick Finnell and Ayesh Perera for critical reviewing of the manuscript and valuable suggestions. The excellent technical assistance of K. Sowell, X. Yuan, and C. Renner is greatly appreciated. The authors thank Dr. Maureen Goode for editing the manuscript and Michelle Gardiner for help in manuscript preparation. This work was supported by a National Institutes of Health Grant CA61889 and National Tuberous Sclerosis Association Grant 95–3 (to R.S.Y.), National Institutes of Health Grants HD30658 and HD10668 (to K.A.), Grant CA 63613 (to C.L.W.), Grant CA16672 to the Veterinary Division, Science Park-Research Division, University of Texas MD Anderson Cancer Center, and National Institute of Environmental Health Sciences Grant ES07784.
ABBREVIATIONS
- TSC
tuberous sclerosis
- SEGAs
subependymal giant cell astrocytomas
- RT
reverse transcription
- E
embryonic equivalent day
References
- 1.European Tuberous Sclerosis Consortium. Cell. 1993;75:1305–1315. [Google Scholar]
- 2.Green A J, Smith M, Yates J R. Nat Genet. 1994;6:193–196. doi: 10.1038/ng0294-193. [DOI] [PubMed] [Google Scholar]
- 3.Wilson P J, Ramesh V, Kristiansen A, Bove C. Hum Mol Genet. 1996;8:249–256. doi: 10.1093/hmg/5.2.249. [DOI] [PubMed] [Google Scholar]
- 4.Sepp T, Yates J R W, Green A J. J Med Genet. 1996;33:962–964. doi: 10.1136/jmg.33.11.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung R S, Xiao G H, Jin F, Lee W C, Testa J R, Knudson A. Proc Natl Acad Sci USA. 1994;91:11413–11416. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi T, Hirayama Y, Kobayashi E, Kubo Y, Hino O. Nat Genet. 1995;9:70–74. doi: 10.1038/ng0195-70. [DOI] [PubMed] [Google Scholar]
- 7.Gomez M R. Tuberous Sclerosis. 2nd Ed. New York: Raven; 1988. [Google Scholar]
- 8.Slegtenhorst M, Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, Ouweland A, Halley D, Young J, et al. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 9.Carbonara C, Long L, Grosso E, Mazzucco G, Borrone C, Carre M L, Brisigotti M, Filippi G, Scabar A, Giannotti A, et al. Genes Chromosomes Cancer. 1996;15:18–25. doi: 10.1002/(SICI)1098-2264(199601)15:1<18::AID-GCC3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Bjornsson J, Short M P, Kwiatkowski D J, Henske E P. Am J Pathol. 1996;149:1201–1208. [PMC free article] [PubMed] [Google Scholar]
- 11.Cook J A, Oliver K, Mueller R F, Sampson J. J Med Genet. 1996;33:480–484. doi: 10.1136/jmg.33.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin F, Wienecke R, Guang-Hui X, Maize J C, DeClue J E, Yeung R S. Proc Natl Acad Sci USA. 1996;93:9154–9159. doi: 10.1073/pnas.93.17.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orimoto K, Tsuchiya H, Kobayashi T, Matsuda T, Hino O. Biochem Biophys Res Commun. 1996;219:70–75. doi: 10.1006/bbrc.1996.0183. [DOI] [PubMed] [Google Scholar]
- 14.Wienecke R, Konig A, DeClue J. J Biol Chem. 1995;270:16409–16414. doi: 10.1074/jbc.270.27.16409. [DOI] [PubMed] [Google Scholar]
- 15.Wienecke R, Maize J C, Shoarinejad F, Vass W C, Reed J, Bonifacino J S, Resau J H, de Gunzburg J, Yeung R S, DeClue J E. Oncogene. 1996;13:913–923. [PubMed] [Google Scholar]
- 16.Xiao G H, Shoarinejad F, Jin F, Golemis E A, Yeung R S. J Biol Chem. 1997;272:6097–6100. doi: 10.1074/jbc.272.10.6097. [DOI] [PubMed] [Google Scholar]
- 17.Bender B L, Yunis E J. Ultrastruct Pathol. 1980;1:287–299. doi: 10.3109/01913128009141432. [DOI] [PubMed] [Google Scholar]
- 18.Hirose T, Scheithauer B W, Lopes M B, S, Gerber H A, Altermatt H J, Hukee M J, Vanden Berg S R, Charlesworth J C. Acta Neuropathol. 1995;90:387–399. doi: 10.1007/BF00315012. [DOI] [PubMed] [Google Scholar]
- 19.Henske E P, Scheithauer B W, Short M P, Wollmann R, Nahmias J, Hornigold M, van Slegtenhorst M, Welsh C T, Kwiatkowski J. Am J Hum Genet. 1996;59:400–406. [PMC free article] [PubMed] [Google Scholar]
- 20.Henske E P, Wessner L, Golden J, Scheithauer B W, Volkmeyer A, Zuang P, Klein-Szanto A J, Kwiatkowski D J, Yeung R S. Am J Path. 1997;151:1639–1647. [PMC free article] [PubMed] [Google Scholar]
- 21.Knudson A G. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eker R, Mossige J. Nature (London) 1961;189:858–859. [Google Scholar]
- 23.Eker R, Mossige J, Johannessen J V, Aars H. Diagn Histopathol. 1981;4:99–110. [PubMed] [Google Scholar]
- 24.Everitt J, Goldsworthy T L, Wolf D S, Walker C. J Urol. 1992;148:1932–1936. doi: 10.1016/s0022-5347(17)37087-8. [DOI] [PubMed] [Google Scholar]
- 25.Hino O, Mitani H, Katsuyama H, Kubo Y. Cancer Lett (Shannon, Irel) 1994;83:117–121. doi: 10.1016/0304-3835(94)90307-7. [DOI] [PubMed] [Google Scholar]
- 26.Yeung R S, Katsetos C D, Klein-Szanto A J. Am J Path. 1997;151:1477–1486. [PMC free article] [PubMed] [Google Scholar]
- 27.Yeung R S, Xiao G H, Everitt J I, Jin F, Walker C. Mol Carcinog. 1995;14:28–36. doi: 10.1002/mc.2940140107. [DOI] [PubMed] [Google Scholar]
- 28.Kubo Y, Mitani H, Hino O. Cancer Res. 1994;54:2633–2635. [PubMed] [Google Scholar]
- 29.Hino O, Klein-Szanto A J, Freed J J, Testa J R, Brown D Q, Vilensky M, Yeung R S, Tartof K D, Knudson A G. Proc Natl Acad Sci. 1993;90:327–331. doi: 10.1073/pnas.90.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao G H, Jin F, Yueng R S. Oncogene. 1995;11:81–87. [PubMed] [Google Scholar]
- 31.Ausubel F M, editor. Current Protocols in Molecular Biology. New York: Wiley; 1994. [Google Scholar]
- 32.Simmons D M, Arriza J L, Swanson L W. J Histotechnol. 1989;12:169–181. [Google Scholar]
- 33.Geist R, Gutman D H. Cell Growth Differ. 1995;6:1477–1483. [PubMed] [Google Scholar]
- 34.Geist R T, Reddy A J, Zhang J, Gutman D H. Neurobiol Dis. 1996;3:111–120. doi: 10.1006/nbdi.1996.0011. [DOI] [PubMed] [Google Scholar]
- 35.Crino P B, Trojanowski J Q, Dichter M A, Eberwine J. Proc Natl Acad Sci USA. 1996;93:14152–14157. doi: 10.1073/pnas.93.24.14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dietrich W F, Lander E S, Smith J S, Moser A R, Gould K A, Luongo C, Borenstein N, Dove W. Cell. 1993;75:631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- 37.Kemp C J, Drinkwater N R. Cancer Res. 1989;49:5044–5047. [PubMed] [Google Scholar]
- 38.Moen C J, Snoek M, Hart A A, Demant P. Oncogene. 1992;7:563–566. [PubMed] [Google Scholar]
- 39.Obata M, Nishimori H, Ogawa K, Lee G H. Oncogene. 1996;13:1599–1604. [PubMed] [Google Scholar]
- 40.Bonyadi M, Rusholme S A, Cousins F M, Su H C, Biron C A, Farrall M, Akhurst R J. Nat Genet. 1997;15:207–211. doi: 10.1038/ng0297-207. [DOI] [PubMed] [Google Scholar]
- 41.Willnow T E, Hilpert J, Armstrong S A, Rohlmann A, Hammer R E, Burns D K, Herz J. Proc Natl Acad Sci USA. 1996;93:8460–8464. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi T, Mitani H, Takahashi R, Hirabayashi, Ueda M, Tamura H, Hino O. Proc Natl Acad Sci USA. 1997;94:3990–3993. doi: 10.1073/pnas.94.8.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]