Introduction
The first description of haemolytic disease of the newborn (HDN) can be traced back to 1609 and was made by a French midwife, Louise Bourgeois, who, from 1600, worked at the royal court of King Henry IV and Queen Marie de Medicis1–4. In the treatise that Bourgeois wrote in 1609 she described the birth of two twins3: the first had hydrops and died immediately, while the second, initially in a better condition, rapidly became jaundiced and, after having developed neurological symptoms (kernicterus), died 3 days after being born.
Hydrops foetalis and kernicterus were correctly interpreted as two aspects of the same pathology only in 19325, when Diamond described foetal erythroblastosis secondary to severe haemolysis, although the cause was still unknown. A few years later, in 1938, Ruth Darrow correctly identified the (antibody-related) pathogenesis of HDN6, although erroneously attributing foetal haemoglobin the role of the culprit antigen, which was suggested to have induced a maternal antibody response after crossing the placenta. The true pathogenesis of the disease was definitively clarified in 1940 with the discovery of the Rhesus (Rh) blood group system by Landsteiner and Wiener7 and with the subsequent identification, in 1941, by Levine8, of the Rh(D) antigen. This antigen was, in fact, identified, in D-negative mothers, as being the cause of the immunisation occurring following transplacental passage of foetal D-positive red blood cells. The subsequent passage of maternal anti-D immunoglobulin G (IgG) across the placenta into the foetal circulation was recognised as the final event able to cause the spectrum of clinical events that characterise HDN.
It did not take long before the risk of immunisation could be quantified1,3: i) 16% in the case of a Rh(D)-negative mother and a Rh(D)-positive, ABO-compatible foetus; ii) 2% in the case of a Rh(D)-negative mother and a Rh(D)-positive, ABO-incompatible foetus (about 20% of the cases); iii) overall risk of immunisation: 13.2%.
Before 1945, about 50% of all foetuses with HDN died of kernicterus or hydrops foetalis. Subsequently, thanks to the progress in treatment, in industrialised countries the mortality decreased to 2–3%; this mortality rate was then very considerably further reduced (100-fold) with the introduction of anti-D immunoprophylaxis to prevent maternal-foetal anti-Rh(D) alloimmunisation 9.
At the beginning of the 1960s, Stern demonstrated experimentally that the administration of anti-D IgG could prevent sensitisation to the Rh(D) antigen10; in the same period, other studies clarified the mechanism of Rh iso-immunisation in pregnancy and introduced the clinical practice of passive immunisation with anti-D IgG to protect Rh(D)-negative women from sensitisation against Rh(D)-positive red blood cells11–14. The successes obtained in studies of Rh(D)-negative male volunteers formed the experimental basis for clinical trials in pregnant Rh(D)-negative women15; these trials demonstrated that post-partum immunoprophylaxis decreased the incidence of post-pregnancy anti-Rh(D) immunisation from 12–13% to 1–2%15,16.
Subsequently, in 1977, it was shown that 1.8% of Rh(D)-negative women, despite post-natal prophylaxis, continued to develop anti-D antibodies because of small transplacental haemorrhages during pregnancy17,18.
One year later, a Canadian study by Bowman et al. showed, in 1,357 Rh(D)-negative primagravida, that the incidence of Rh(D) alloimmunisation could be reduced to 0.1% by prophylaxis with antenatal anti-D IgG, in addition to post-partum prophylaxis19. There is currently sufficient evidence demonstrating that antenatal anti-D prophylaxis also reduces the risk of Rh(D) immunisation in the next pregnancy to below the level of 0.4%.
Forty years after Zipursky and Israels first proposed the use of anti-D IgG to reduce the incidence of Rh alloimmunisation in pregnancy14, immunoprophylaxis has drastically reduced the cases of Rh-induced HDN; nevertheless, this pathology continues to be relevant in 0.4 of 1,000 births (0.04%)20, for various reasons21: i) the possible occurrence of anti-D immunisation during the pregnancy (which occurs in about 1% of Rh(D)-negative women carrying a Rh(D)-positive foetus22); ii) the lack of efficacy of immunoprophylaxis because of the administration of an insufficient dose of anti-D IgG that is not congruent with the volume of the foetal-maternal haemorrhage; iii) immunoprophylaxis not administered; iv) possible errors in typing the pregnant or puerperal woman or the neonate; v) possible errors in transfusion therapy in women of child-bearing age.
Antenatal prophylaxis
Rationale
Systematic anti-D prophylaxis was proposed in the 1970s with the aim of reducing the percentage of Rh(D)-negative women who could be sensitised during pregnancy, despite the use of post-partum prophylaxis. This percentage ranged from 0.9–2.2% in a series of studies carried out between 1977 and 198917–19,23–28, and from 0.8–1.5% in subsequent studies carried out between 1995 and 199929–32; this latter modest reduction is probably due to intercurrent changes in obstetric care and the wider use of anti-D prophylaxis following sensitising events during pregnancy33.
The D antigen has been demonstrated to be present on foetal red blood cells from the 7th week of pregnancy onwards34. There is a direct, proportional relationship between the volume of Rh(D)-positive red blood cells to which the Rh(D)-negative subject is exposed and the incidence of anti-Rh immunisation, although it has been estimated that 0.1 mL is already sufficient to induce the formation of antibodies14,33.
During the first trimester of a normal pregnancy, foetal red blood cells can be found in the maternal circulation of about 3% of women, albeit in amounts less than 0.1 mL35. As the pregnancy proceeds, the frequency and volume of foetal red blood cells in the maternal circulation increase, such that during the second and third trimesters these cells can be detected in 12% and 45% of women, respectively. At the time of delivering an ABO-compatible neonate, foetal red blood cells can be found in the maternal circulation in as many as 50% of cases12. However, the main sensitising event for Rh(D)-negative women, in whom prevention is based on post-natal prophylaxis, occurs at the end of the pregnancy, with detachment of the placenta during delivery33.
Other sensitising events, in the form of occult or silent transplacental haemorrhages, are probably the principal cause of the approximately 1% residual rate of immunisation. These events provide the rationale for the strategy, adopted in many countries, of systemic antenatal anti-D prophylaxis for all non-immunised Rh(D)-negative pregnant women, as a complementary measure to post-partum prophylaxis36.
Numerous studies have demonstrated that the antenatal administration of anti-D IgG does not have adverse effects on the foetus, even though small amounts of anti-D can cross the placenta and, by binding to foetal D-positive red blood cells, can cause weak positivity at birth in the direct Coombs’ test16,23,37–40.
Antenatal anti-D prophylaxis is generally recommended from the 28th week of pregnancy onwards, because transplacental haemorrhages large enough to cause sensitisation do not occur until the third trimester and, therefore, anti-D antibodies usually develop after the 28th week of gestation17,41,42; studies showing that 92% of Rh(D)-negative women who develop anti-D do so after the 28th week of gestation lend support to this recommendation16,37.
Anti-D prophylaxis has a strong immunosuppressive effect, such that subsequent exposure to the D antigen does not cause a secondary immune response, but rather a primary response, as if the immune system has never encountered the D antigen43. Numerous mechanisms of action have been proposed to explain the efficacy of this strategy, including : (i) accelerated clearance of D-positive cells, (ii) epitope masking, (iii) inhibition due to antibodies against FcγRIIB (the specific activating receptor for the Fc fragment of IgG) or anti-idiotype44, (iv) inhibition of immature dendritic cells, and (v) inhibition of B-cell clones specific for the D antigen43; this last, recently proposed, mechanism was suggested by the finding of an increase in the levels of transforming growth factor-β and prostaglandin E2, found in a cohort of pregnant women who were given antenatal prophylaxis. However, the accelerated destruction of D-positive red blood cells remains, according to some authors, the main mechanism of action45.
Dose
A dose of 20 μg (100 UI) of anti-D IgG protects against 1 mL of D-positive red blood cells or 2 mL of whole blood10,20. However, the World Health Organisation (WHO) and the British Medical Research Council consider that 25 μg (125 UI) are needed to protect against the same volume of D-positive red blood cells46; according to Bowman and Pollock, this is equivalent to having a concentration of anti-D IgG in the maternal circulation of 2.4 ng/mL26.
There are essentially two approaches to antenatal prophylaxis: i) a single injection of 300 μg (1,500 UI) in the 28th week of pregnancy19,28,47, or ii) two injections of 100–125 μg (500–625 UI), one in the 28th week, the other in the 34th week24,32,40.
In pharmacokinetic studies, the half-life of the IgG was estimated to be, on average, 17–22 days (range: 11–29)48,49; a recently published pharmacokinetic study46 reported that both protocols are effective and able to ensure, 12 weeks after administration, the minimum residual concentration of 25 μg (2.4 ng/mL) of anti-D IgG indicated by the WHO as a protective level. This is probably an overcautious estimate because it is extremely unlikely that the volume of a transplacental haemorrhage in the antenatal period exceeds 1 mL of foetal red blood, which is equivalent to the volume of foetal-maternal haemorrhage occurring at the time of delivery in 98% of women46,50.
A recent systematic review of the literature on the clinical efficacy of anti-D prophylaxis during pregnancy33 found 11 studies that compared the outcome of Rh(D)-negative women (and their babies)19,23–32 not immunised to D antigen who underwent antenatal prophylaxis in the 28th week of pregnancy or subsequently, with the outcome of Rh(D)-negative controls (and their babies) who did not receive prophylaxis. Table I reports the characteristics of the studies analysed in the review, which showed that the percentage of sensitised women decreased from 1.9–2.2% to 0–0.2% with the use of antenatal prophylaxis; the data also showed that the main clinical benefit that a Rh(D)-negative woman can have from antenatal prophylaxis is the possibility of avoiding HDN in subsequent pregnancies with Rh(D)-positive foetuses.
Table I.
Summary of the results of the clinical efficacy of antenatal anti-D prophylaxis (adapted from: Jones ML et al.33).
| Study [Study desing] | Dosage | Patient selection | Anti-D prophylaxis group | Control group | ||
|---|---|---|---|---|---|---|
| r/n | % Sensitised or sensibilised (95% CI) | r/n | % Sensitised or sensibilised (95% CI) | |||
| Bowman et al. (1978) [NRCT]19 | 2 x 1500 IU (28 and 34 weeks) | Primigravidae | 1/1357 | 0.1 (0.1 to 0.2) | 45/2768 | 1.6 (1.6 to 2.1) |
| Bowman and Pollock (1978) [NRCT]23 | 1 x 1500 IU (28 weeks) | Unselected | 11/1805 | 0.6 (0.3 to 1.0) | 62/3533 | 1.8 (1.3 to 2.2) |
| Tovey et al. (1983) [NRCT]24 | 2 x 500 IU (28 and 34 weeks) | Primigravidae | 5/1238 | 0.4 (0.1 to 0.8) | 30/2000 | 1.5 (1.0 to 2.0) |
| Hermann et al. (1984) [NRCT]25 | 1 x 1250 IU (34 weeks) | Primigravid primiparae | 4/236 | 1.7 (0.0 to 3.3) | 5/286 | 1.7 (0.2 to 3.3) |
| Multigravid primiparae | 1/332 | 0.3 (0.3 to 0.9) | 7/359 | 1.9 (0.2 to 2.6) | ||
| Unselected primiparae | 5/568 | 0.9 (0.1 to 1.6) | 12/645 | 1.9 (0.8 to 2.9) | ||
| Bowman and Pollock (1987) [NRCT]26 | 1 x 1500 IU (28 weeks) | Unselected | 30/9295 | 0.3 (0.2 to 0.4) | 62/3533 | 1.8 (1.3 to 2.2) |
| Huchet et al. (1987) [Quasi RCT]27 | 2 x 500 IU (28 and 34 weeks) | Primiparae | 0/461 | 0.0 (0.0 to 0.0) | 4/454 | 0.9 (0.0 to 1.7) |
| Multiparae | 1/138 | 0.7 (0.7 to 2.1) | 3/136 | 2.2 (0.3 to 4.7) | ||
| Unselected | 1/599 | 0.2 (0.2 to 0.5) | 7/590 | 1.2 (0.3 to 2.1) | ||
| Trolle (1989) [NRCT]28 | 1 x 1500 IU (28 weeks) | Unselected | 0/291 | 0.0 (0.0 to 0.0) | 6/322 | 1.9 (0.4 to 3.3) |
| Lee and Rawlinson (1995) [RCT]29 | 2 x 250 IU (28 and 34 weeks) | Primigravidae | 5/513 | 1.0 (0.1 to 1.8) | 9/595 | 1.5 (0.5 to 2.5) |
| Mayne et al. (1997) [Before and after study]30 | 2 x 500 IU (28 and 34 weeks) | Primiparae | 4/1425 | 0.3 (0.0 to 0.6) | 16/1426 | 1.1 (0.6 to 1.7) |
| Parsons et al. (1998) [NRCT]31 | Not stated* (28 weeks) | Not stated | 72/9684 | 0.7 (0.6 to 0.9) | No data | 0.8 (not given) |
| Mackenzie et al. (1999) [Controlled before and after study]32 | 2 x 500 IU (28 and 34 weeks) | Primiparae | 12/3320 | 0.4 (0.2 to 0.6) | 26/3146 | 0.8 (0.5 to 1.1) |
NRCT = non randomised controlled trial.; RCT = randomised controlled trial; n = number of deliveries of RhD-positive babies to RhD-negative women; r = number of sensitised RhD-negative women in the trial group.
Most probably the standard Canadian dose of 1500 IU.
A meta-analysis, carried out on two of the 11 studies included in the review, demonstrated a notable reduction in relative risk of sensitisation in women treated with the antenatal prophylaxis; indeed, the odds ratio (OR) was 0.37 (95% CI: 0.2–0.65), while the absolute reduction in risk of sensitisation in mothers at risk (carrying a Rh(D)-positive foetus) was 0.6%; the number of women who had to be treated to avoid one case of sensitisation (i.e. the NNT, number needed to treat) was 278.
A meta-analysis by the Cochrane Collaboration in 200042 also included only two studies27,29, for a total of more than 4,500 women treated with immunoprophylaxis in the 28th and 34th weeks of pregnancy; the doses of anti-D IgG used were 100 μg (500 UI) x 2 in the French trial27, and 50 μg (250 UI) x 2 in the British trial29. Three outcomes were evaluated and for each of these the reduction in the relative risk was statistically significant. As far as concerns positivity for the Kleihauer test, which detects foetal red blood cells in the maternal circulation, the OR was 0.6 (95% CI: 0.41–0.88) during pregnancy and 0.6 (95% CI: 0,46–0,79) also after the delivery of a Rh(D)-positive baby. The OR for the incidence of anti-D alloimmunisation was 0.42 (95% CI: 0.15–1.17) both during pregnancy and after the birth of a Rh(D)-positive baby; the OR became 0.41 (95% CI: 0.16–1.04) within 12 months of the birth, while the OR for women at a first pregnancy, within 12 months after the delivery, was 0.11 (95% CI: 0.01–2.04). The reduction in the relative risk of neonatal jaundice was considerably greater, with an OR of 0.26 (95% CI: 0.03–2.3).
According to the Cochrane reviewers, as a result of antenatal prophylaxis [100 μg (500 UI) of anti-D IgG in the 28th to 34th weeks], the risk of alloimmunisation of Rh(D)-negative pregnant women drops from about 1% to 0.2% and the probability of immunisation in subsequent pregnancies is also reduced; the NNT to avoid one case of sensitisation is 213.
International recommendations
Various international recommendations (table II) suggest antenatal anti-D prophylaxis (i.m. or i.v.) in unsensitised Rh(D)-negative women as a complement to post-partum prophylaxis.
The Society of Obstetricians and Gynaecologists of Canada (SOGC) suggests 300 μg in the 28th week (grade of recommendation: A) or two doses of 100–120 μg in the 28th and 34th weeks of pregnancy20.
The Italian Society of Transfusion Medicine and Immunohaematology (SIMTI), together with the Italian Society of Gynaecology and Obstetrics (SIGO) recommends 250–300 μg in the 28th week21.
The American Society of Clinical Pathologists (ASCP) suggests 300 μg in the 28th–30th week16.
The American College of Obstetrician and Gynaecologists (ACOG) recommends prophylaxis51, around the 28th week, but does not specify the dose (grade of recommendation: A).
The U.S. Preventive Services Task Force (USPSTF) (USA) recommends 300 μg in the 24th–28th week52.
The National Institute for Clinical Excellence (NICE) (UK), in guidelines published in 2002 and revised in 2005, indicates 100 μg (500 UI) in the 28th and 34th weeks53; the NICE states that this scheme is equivalent to a single dose of 300 μg (1,500 UI) in the 34th week.
The same indications are included in the recent guidelines from the British Committee for Standards in Haematology (BCSH)54, which replaced the preceding recommendations by Lee et al. in 199955.
The National Health and Medical Research Council (NHMRC) (Australia) suggests 125 μg (625 UI) in the 28th and 34th weeks56.
The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) confirms the above recommendation57.
The French recommendations are for a dose of 300 μg in the 28th (± 1) week of pregnancy58.
The Dutch authorities recommend a single dose of 200 μg in the 30th week59.
The Spanish Society of Blood Transfusion (SETS) and the Spanish Society of Obstetrics and Gynaecology indicate a single dose of 300 μg in the 28th week60.
Table II.
Recommended antenatal anti-D prophylaxis in the main international recommendations
| Recommended dose | Week | |
|---|---|---|
| Canada (SOGC)20 | 300 (or 100–120) | 28 (or 28 and 34) |
| Italy (SIMTI-SIGO)21 | 250–300 | 28 |
| USA (ASCP)16 | 300 | 28–30 |
| USA (ACOG)51 | Not specified | 28 |
| USA (USPSTF)52 | 300 | 24–28 |
| UK (NICE)53 | 100 | 28 and 34 |
| UK (BCSH)54 | 100 | 28 and 34 |
| Australia (NHMRC)56 | 125 | 28 and 34 |
| Australia (RANZCOG) 57 | 125 | 28 and 34 |
| France (CNGOF)58 | 300 | 28 |
| The Netherlands (CHI) 59 | 200 | 30 |
| Spain (SETS-SEOG)60 | 300 | 28 |
SOGC: Society of Obstetricians and Gynaecologists of Canada
SIMTI: Società Italiana di Medicina Trasfusionale e Immunoematologia [Italian Society of Transfusion Medicine and Immunohaematology]
SIGO: Società Italiana di Ginecologia e Ostetricia [Italian Society of Obstetrics and Gynaecology]
ASCP: American Society of Clinical Pathologists
ACOG: The American College of Obstetricians and Gynecologists
USPSTF: U.S. Preventive Services Task Force
NICE: National Institute of Clinical Excellence
BCSH: British Committee for Standards in Haematology
NHMRC: National Health and Medical Research Council
RANZCOG: Royal Australian and New Zealand College of Obstetricians and Gynaecologists
CNGOF: Collège National des Gynècologues et Obstétriciens Francais [French College of Obstetricians and Gynaecologists]
CHI: Council of Health Insurances
SETS: Sociedad Española de Transfusión Sanguínea [Spanish Society of Blood Transfusion]
SEOG: Sociedad Española de Obstetricia y Ginecología [Spanish Society of Obstetrics and Gynaecology]
Prophylaxis should not be given to women with a “weak” D phenotype, in that such women are actually D-positive21; however, the use of anti-D IgG in these women does not have significant side effects.
Conclusions
HDN has become a rare disorder since the introduction of anti-D prophylaxis. The foetal Rh(D) antigen is already well developed after 30–40 days of gestation and the risk of antenatal foeto-maternal haemorrhage, already possible from the 6th week61, increases exponentially as a pregnancy progresses22,35. Although the risk of foetal blood entering the maternal circulation is high, the average volume of is very small, being 0.07 mL in the first trimester, 0.08 mL in the second and 0.13 mL in the third62. A Rh(D)-negative woman not immunised and who does not receive prophylaxis has, in every pregnancy, a 16% risk of becoming immunised to a Rh(D)-positive foetus63. However, the global success rate of post-natal Rh immunoprophylaxis has now reached 98.4 – 99%1,3.
Given that anti-D prophylaxis is effective for about 12 weeks, as indicated by pharmacokinetic studies on anti-D IgG46, and given the relatively low risk of significant foeto-maternal haemorrhage before the 28th week of gestation, antenatal prophylaxis is carried out from the 28th week onwards41.
The currently available evidence demonstrates that antenatal anti-D prophylaxis, besides being a cost-effective strategy36,64, is able to further reduce the incidence of sensitisation to D antigen down to about 0.2%33,42; a variety of prophylactic regimens are used in different countries, although 300 μg of anti-D IgG in the 28th week is the dose most commonly indicated in international recommendations16,20,52,58.
It is not, however, possible to completely eliminate the risk of sensitisation because, despite increased adherence to antenatal immunoprophylaxis65, and although most foeto-maternal haemorrhages able to cause immunisation occur in the last trimester of pregnancy, sensitisation does occur before the 28th week in a small percentage of women; alternatively, there may be cases in which intramuscular administration of the anti-D IgG is insufficient to provide passive prophylaxis.
Furthermore, the risk of intrauterine foetal death of Rh(D)-positive foetuses of Rh(D)-negative mothers appears to be increased despite antenatal immunoprophylaxis, as indicated by a recent, retrospective observational study carried out in Israel on more than 140,000 deliveries occurring between 1988 and 200366; although the subject undoubtedly needs further investigation, this retrospective study seems to suggest a possible pathogenic scenario not limited to maternal-foetal serological alloimmunisation but also involving the maternal-foetal interface (i.e. the placenta), which could undergo immune-mediated damage leading to intrauterine foetal death.
The IgG subclasses with anti-D activity are IgG1 and IgG3; the anti-D IgG are produced by industrial fractionation from pools of donor plasma containing a high titre of anti-D antibodies; chromatographic procedures, which are well suited to processing small quantities of plasma and guarantee an excellent yield, are used for the fractionation67.
Antenatal prophylaxis with anti-D IgG does not have side effects on the foetus and can be considered complementary to post-partum prophylaxis, but the decision on whether to undertake a programme of prophylaxis during pregnancy can be influenced by the commercial availability of anti-D IgG. The raw material is a limited resource because the hyperimmune plasma comes exclusively from the USA, the only country that still carries out active immunisation of donors which, until about 10 years ago, was also performed in Switzerland and Slovenia (Italian National Blood Centre, unpublished data).
An economic analysis carried out in the United Kingdom36,64 demonstrated that the cost per year of life gained from using antenatal anti-D prophylaxis for women in their first pregnancy was low compared to that of other interventions normally offered by the health care service in that country; the same prophylaxis, given to all pregnant women, although costing more, maintained a relatively low cost per year of life gained, thus confirming that antenatal anti-D prophylaxis is a decidedly cost-effective strategy.
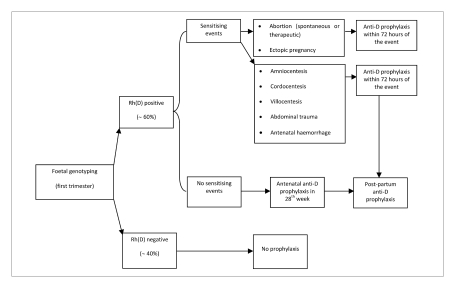
Methods of foetal genotyping on maternal plasma68–74 are able to detect foetal DNA sequences in the maternal plasma; used on a large scale, these methods could enable elimination of unnecessary antenatal prophylaxis with anti-D IgG in Rh(D)-negative mothers of Rh(D)-negative foetuses, who represent about 40% of all cases, thus sparing this blood derivative and reserving its appropriate use exclusively to those women at risk of maternal-foetal alloimmunisation (Figure 1). In addition to this benefit, a further advantage of foetal genotyping would be that of knowing whether, in a women already immunised in an early stage of a pregnancy, the foetus of the current pregnancy is Rh(D)-negative or positive, providing important information for the management of the pregnancy.
Figure 1.
Anti-D prophylaxis in Rh(D) negative pregnant women: possible scenario following the introduction of foetal genotyping
The possible use of monoclonal or recombinant anti-D antibodies in clinical practice remains very uncertain75. These antibodies could, in the future, be an alternative to the prophylactic use of plasma-derived polyclonal anti-D IgG76–78; unfortunately, however, none of the products developed by industry so far has been demonstrated to be effective in the numerous phase I clinical trials that have been carried out79,80. During the last 20 years, 19 monoclonal or recombinant antibodies, produced by different cell lines, have been tested in humans75, but the in vivo clinical efficacy and side effects were very heterogeneous. Most of the side effects are probably related to the type of glycosylation to which the IgG molecule is subjected in the various different cell systems used for its production75,80.
The drafting and adoption of national recommendations on the systematic use of antenatal immunoprophylaxis must, however, take into consideration both clinical and organisational implications81, and the potential financial impact of using this blood derivative which, already in the 3 years from 2002 to 2005, led to a 56% increase in costs, for a 15% rise in sales (Italian National Blood Centre, unpublished data), caused presumably also by the increase in the sale price of the product.
References
- 1.Bowman J. Thirty-five years of Rh prophylaxis. Transfusion. 2003;43:1661–6. doi: 10.1111/j.0041-1132.2003.00632.x. [DOI] [PubMed] [Google Scholar]
- 2.Dunn PM. Louise Bourgeois (1563–1636): royal midwife of France. Arch Dis Child Fetal Neonatal Ed. 2004;89:F185–7. doi: 10.1136/adc.2003.037929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman J. Rh-immunoglobulin: Rh prophylaxis. Best Pract Res Clin Haematol. 2006;19:27–34. doi: 10.1016/j.beha.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Bourgeois L.Observations Diverses sur la Sterilité Perte de Fruiot Fecondité Accouchements et Maladies des Femmes et Enfants Nouveaux Naiz. Paris; 1609. [DOI] [PubMed]
- 5.Diamond LK, Blackfan KD, Baty JM. Erythroblastosis fetalis and its association with universal edema of the fetus, icterus gravis neonatorum and anemia of the newborn. J Pediatr. 1932;1:269–309. [Google Scholar]
- 6.Darrow RR. Icterus gravis (erythroblastosis neonatorum, examination of etiologic considerations) Arch Pathol. 1938;25:378–417. [Google Scholar]
- 7.Landsteiner K, Wiener AS. An agglutinable factor in human blood recognized by immune sera for Rhesus blood. Proc Soc Exp Biol Med. 1940;43:223–9. [Google Scholar]
- 8.Levine P, Katzin EM, Burnham L. Isoimmunization in pregnancy: its possible bearing on the etiology of erythroblastosis fetalis. JAMA. 1941;116:825–7. [Google Scholar]
- 9.Urbaniak S. The scientific basis of antenatal prophylaxis. Br J Obstet Gynaecol. 1998;105(Suppl 18):11–8. doi: 10.1111/j.1471-0528.1998.tb10286.x. [DOI] [PubMed] [Google Scholar]
- 10.Stern K, Goodman HS, Berger M. Experimental isoimmunization to hemo-antigens in man. J Immunol. 1961;97:1245–57. [Google Scholar]
- 11.Clarke CA, Donohoe WTA, McConnell RB, et al. Further experimental studies on the prevention of Rh haemolytic disease. Br Med J. 1963;1:979–84. doi: 10.1136/bmj.1.5336.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen F, Zuelzer WW, Gustafson DC, et al. Mechanisms of isoimmunization. I. The transplacental passage of fetal erythrocytes in homospecific pregnancies. Blood. 1964;23:621–46. [PubMed] [Google Scholar]
- 13.Freda VJ, Gorman JC, Pollack W. Successful prevention of experimental Rh sensitization in man with an anti-Rh gamma2-globulin antibody preparation: a preliminary report. Transfusion. 1964;4:26–32. doi: 10.1111/j.1537-2995.1964.tb02824.x. [DOI] [PubMed] [Google Scholar]
- 14.Zipursky A, Israels LG. The pathogenesis and prevention of Rh immunization. Can Med Assoc J. 1967;97:1245–57. [PMC free article] [PubMed] [Google Scholar]
- 15.Pollack W, Gorman JG, Freda VJ, et al. Results of clinical trials of RhoGAM in women. Transfusion. 1968;8:151–3. doi: 10.1111/j.1537-2995.1968.tb04895.x. [DOI] [PubMed] [Google Scholar]
- 16.Hartwell EA. Use of Rh immune globulin. Am J Clin Pathol. 1998;110:281–92. doi: 10.1093/ajcp/110.3.281. [DOI] [PubMed] [Google Scholar]
- 17.McMaster Conference on Prevention of Rh immunization28–30 September, 1977Vox Sang 1979; 3650–64. [PubMed] [Google Scholar]
- 18.Scott JR, Beer AE, Guy LR, et al. Pathogenesis of Rh immunization in primigravidas. Fetomaternal versus maternofetal bleeding. Obstet Gynecol. 1977;49:9–14. [PubMed] [Google Scholar]
- 19.Bowman JM, Chown B, Lewis M, Pollock JM. Rh isoimmunization during pregnancy: antenatal prophylaxis. Can Med Assoc J. 1978;118:623–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Fung Kee Fung K, Eason E, Crane J, et al. Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2003;25:765–73. doi: 10.1016/s1701-2163(16)31006-4. [DOI] [PubMed] [Google Scholar]
- 21.Biffoni F, D’Angiolino A, Massaro AL, et al. Recommendations for the management of haemolytic disease of the newborn. Blood Transfus. 2006;4:237–50. [Google Scholar]
- 22.Bowman JM. The prevention of Rh immunization. Transfus Med Rev. 1988;2:129–50. doi: 10.1016/s0887-7963(88)70039-5. [DOI] [PubMed] [Google Scholar]
- 23.Bowman JM, Pollock JM. Antenatal prophylaxis of Rh isoimmunization: 28-weeks’-gestation service program. Can Med Assoc J. 1978;118:627–30. [PMC free article] [PubMed] [Google Scholar]
- 24.Tovey LA, Townley A, Stevenson BJ, Taverner J. The Yorkshire antenatal anti-D immunoglobulin trial in primigravidae. Lancet. 1983;2:244–6. doi: 10.1016/s0140-6736(83)90232-5. [DOI] [PubMed] [Google Scholar]
- 25.Hermann M, Kjellman H, Ljunggren C. Antenatal prophylaxis of Rh immunization with 250 micrograms anti-D immunoglobulin. Acta Obstet Gynecol Scand Suppl. 1984;124:1–15. doi: 10.3109/00016348409157011. [DOI] [PubMed] [Google Scholar]
- 26.Bowman JM, Pollock JM. Failures of intravenous Rh immune globulin prophylaxis: an analysis of the reasons for such failures. Transfus Med Rev. 1987;1:101–12. doi: 10.1016/s0887-7963(87)70010-8. [DOI] [PubMed] [Google Scholar]
- 27.Huchet J, Dallemagne S, Huchet C, et al. Ante-partum administration of preventive treatment of Rh-D immunization in rhesus-negative women. Parallel evaluation of transplacental passage of fetal blood cells. Results of a multicenter study carried out in the Paris Region. J Gynecol Obstetr Biol Reprod. 1987;16:101–11. [PubMed] [Google Scholar]
- 28.Trolle B. Prenatal Rh-immune prophylaxis with 300 micrograms immune globulin anti-D in the 28th week of pregnancy. Acta Obstet Gynecol Scand. 1989;68:45–7. doi: 10.3109/00016348909087688. [DOI] [PubMed] [Google Scholar]
- 29.Lee D, Rawlison VI. Multicentre trial of antepartum low-dose anti-D immunoglobulin. Transfus Med. 1995;5:15–9. doi: 10.1111/j.1365-3148.1995.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 30.Mayne S, Parker JH, Harden TA, et al. Rate of Rh D sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ. 1997;315:1588. doi: 10.1136/bmj.315.7122.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parson ML, Van den Hof MC, Armson BA, et al. A comparison of the rate of Rh(D) alloimmunisation between Nova Scotia and Scotland [abstract] Br J Obstet Gynaecol. 1998;105(Suppl 18):39. [Google Scholar]
- 32.MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynaecol. 1999;106:492–7. doi: 10.1111/j.1471-0528.1999.tb08304.x. [DOI] [PubMed] [Google Scholar]
- 33.Jones ML, Wray J, Wight J, et al. A review of the clinical effectiveness of routine antenatal anti-D prophylaxis for rhesus-negative women who are pregnant. Br J Obstet Gynaecol. 2004;111:892–902. doi: 10.1111/j.1471-0528.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- 34.Bergstrom H, Nilsson LA, Nilsson L, et al. Demonstration of Rh antigens in a 38-day-old fetus. Am J Obstet Gynecol. 1967;99:130–3. doi: 10.1016/s0002-9378(16)34502-1. [DOI] [PubMed] [Google Scholar]
- 35.Bowman JM, Pollock J, Penston L. Fetomaternal transplacental hemorrage during pregnancy and after delivery. Vox Sang. 1986;51:117–21. doi: 10.1111/j.1423-0410.1986.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 36.Chilcott J, Lloyd Jones M, Wight J, et al. A review of the clinical effectiveness and cost effectiveness of routine anti-D prophylaxis for pregnant women who are Rhesus (RhD) negativeNational Institute of Clinical Excellence. London; 2002. Available at: http://guidance.nice.org.uk/page.aspx?o=31689 [DOI] [PubMed]
- 37.Bowman JM. Preventing Rh sensitisation during pregnancy. Perinatal Care. 1978;2:24–32. [Google Scholar]
- 38.Hensleigh PA. Preventing Rhesus isoimmunization: antepartum Rh immune globulin prophylaxis versus a sensitive test for risk identification. Am J Obstet Gynecol. 1983;146:749–55. [PubMed] [Google Scholar]
- 39.Tabsh KM, Lebherz TB, Crandall BF. Risks of prophylactic anti-D immunoglobulin after second-trimester amniocentesis. Am J Obstet Gynecol. 1984;150:225–6. doi: 10.1016/0002-9378(84)90202-3. [DOI] [PubMed] [Google Scholar]
- 40.Thornton JG, Page C, Foote G, et al. Efficacy and long term effects of antenatal prophylaxis with anti-D immunoglobulin. BMJ. 1989;298:1671–3. doi: 10.1136/bmj.298.6689.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Contreras M. The prevention of Rh haemolytic disease of the fetus and newborn – general background. Br J Obstet Gynaecol. 1998;105(Suppl 18):7–10. doi: 10.1111/j.1471-0528.1998.tb10285.x. [DOI] [PubMed] [Google Scholar]
- 42.Crowther CA, Middleton P. Anti-D administration in pregnancy for preventing Rhesus alloimmunization. Cochrane Database Syst Rev. 20002:CD000020. doi: 10.1002/14651858.CD000020.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Branch DR, Shabani F, Lund N, Denomme GA. Antenatal administration of Rh-immune globulin causes significant increases in the immunomodulatory cytokines transforming growth factor-â and prostaglandin E2. Transfusion. 2006;46:1316–22. doi: 10.1111/j.1537-2995.2006.00898.x. [DOI] [PubMed] [Google Scholar]
- 44.Kumpel BM. On the mechanism of tolerance to the Rh D antigen mediated by passive anti-D (Rh D prophylaxis) Immunol Lett. 2002;82:67–73. doi: 10.1016/s0165-2478(02)00020-2. [DOI] [PubMed] [Google Scholar]
- 45.Kumpel BM. On the immunological basis of Rh immune globulin (anti-D) prophylaxis [editorial] Transfusion. 2006;46:1652–6. doi: 10.1111/j.1537-2995.2006.00924_1.x. [DOI] [PubMed] [Google Scholar]
- 46.MacKenzie IZ, Roseman F, Findlay J, et al. The kinetics of routine antenatal prophylactic intramuscular injections of polyclonal anti-D immunoglobulin. BJOG. 2006;113:97–101. doi: 10.1111/j.1471-0528.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- 47.MacKenzie IZ, Bichler J, Mason GC, et al. Efficacy and safety of a new, chromatographically purified Rhesus (D) immunoglobulin. Eur J Obstet Gynecol Reprod Biol. 2004;117:154–61. doi: 10.1016/j.ejogrb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Bichler J, Schondorfer G, Pabst G, Andresen I. Pharmacokinetics of anti-D IgG in pregnant RhD-negative women. BJOG. 2003;110:39–45. [PubMed] [Google Scholar]
- 49.Eklund J, Herman M, Kjellman H, Pohja P. Turnover rate of anti-D IgG injected during pregnancy. Br Med J (Clin Res Ed) 1982;284:854–5. doi: 10.1136/bmj.284.6319.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Controlled trial of various anti-D dosages in suppression of Rh sensitisation following pregnancy. Report to the Medical Research Council by the working party on the use of anti-D immunoglobulin for the prevention of isoimmunization of Rh-negative women during pregnancy. Br Med J. 1974;2:75–80. [PMC free article] [PubMed] [Google Scholar]
- 51.ACOG practice bulletin. Prevention of Rh D alloimmunization. Number 4, May 1999 (replaces educational bulletin Number 147, October 1990). Clinical management guidelines for obstetrician-gynecologists. American College of Obstetrics and Gynecology. Int J Gynaecol Obstet. 1999;66:63–70. [PubMed] [Google Scholar]
- 52.U.S. Preventive Services Task Force. Recommendation Statement: Screening for Rh (D) incompatibility. Rockville, MD; 2004. Available at: http://www.ahrq.gov/clinic/3rduspstf/rh/rhrs.pdf
- 53.National Institute for Clinical Excellence. Guidance on the use of routine antenatal anti-D prophylaxis for RhD-negative women. Technology Appraisal No. 41. London; 2002. Available at: http://guidance.nice.org.uk/TA41/guidance/pdf/English
- 54.Parker J, Wray J, Gooch A, et al. British Committee for Standards in Haematology. Guidelines for the use of prophylactic anti-D immunoglobulinLondon; 05/12/2006. Available at: http://www.bcshguidelines.com/pdf/Anti-D_070606.pdf
- 55.Lee D, Contreras M, Robson SC, et al. Recommendations for the use of anti-D immunoglobulin for Rh prophylaxis. Transfus Med. 1999;9:93–7. doi: 10.1046/j.1365-3148.1999.009001093.x. [DOI] [PubMed] [Google Scholar]
- 56.National Health and Medical Research Council Guidelines on the prophylactic use of Rh D immunoglobulin (anti-D) in obstetricsCommonwealth of Australia 2003. Available at: http://www.nba.gov.au/PDF/glines_anti_d.pdf
- 57.Royal Australian and New Zealand College of Obstetricians and GynaecologistsGuidelines for the use of Rh (D) immunoglobulin (Anti-D) in obstetrics in Australia. Australia; March 2007. Available at: http://www.ranzcog.edu.au/publications/statements/C-obs6.pdf
- 58.Cortey A, Brossard Y. Prevention of fetomaternal Rhesus-D allo-immunization. Practical aspects. J Gynecol Obstet Biol Reprod (Paris) 2006;35(Suppl 1):S123–30. [PubMed] [Google Scholar]
- 59.Council of Health InsurancesBlood Testing in Pregnancy. Pregnancy Immunization, Hepatitis B and Lues. Kingston, Council of Health Insurances, National Health Inspection;1998
- 60.Muñiz-Diaz E, Oyonarte S, Rodríguez-Villanueva J, et al. Sociedad Española de Transfusión Sanguínea, Sociedad Española de Obstetricia y Ginecología Protocolo de Diagnóstico y Prevención de la Enfermedad Hemolítica del feto y del Recién Nacido(Marzo 2008). Available at: http://www.sets.es/index.php?option=com_content&view=article&id=33:protocolo-de-diagnostico-y-prevencion-de-la-enfermedad-hemolitica-del-feto-y-del-recien-nacido&catid=43:guias&Itemid=41 Last accessed: 11/12/2009
- 61.Hann IM, Gibson BES, Letsky EA. Fetal and Neonatal Haematology. London: Bailliere Tindal; 1991. [Google Scholar]
- 62.Choavaratana R, Uer-Areewong S, Makanantakosol S. Fetomaternal transfusion in normal pregnancy and during delivery. J Med Assoc Thai. 1997;80:96–100. [PubMed] [Google Scholar]
- 63.Bowman JM. Controversies in Rh prophylaxis: who needs Rh immune globulin and when should it be given? Am J Obstet Gynecol. 1985;151:289–94. doi: 10.1016/0002-9378(85)90288-1. [DOI] [PubMed] [Google Scholar]
- 64.Chilcott J, Tappendem, Lloyd Jones M, et al. The economics of routine antenatal anti-D prophylaxis for pregnant women who are Rhesus negative. BJOG. 2004;111:903–7. doi: 10.1111/j.1471-0528.2004.00226.x. [DOI] [PubMed] [Google Scholar]
- 65.MacKenzie IZ, Findlay J, Thompson K, Roseman F. Compliance with routine antenatal Rhesus D prophylaxis and the impact on sensitisations: observations over 14 years. BJOG. 2006;113:839–43. doi: 10.1111/j.1471-0528.2006.00988.x. [DOI] [PubMed] [Google Scholar]
- 66.Ben-David G, Sheiner E, Levy A, et al. An increased risk for non allo-immunization related intrauterine fetal death in RhD-negative patients. J Matern Fetal Neonatal Med. 2008;21:255–9. doi: 10.1080/14767050801928804. [DOI] [PubMed] [Google Scholar]
- 67.Burnouf T. Modern plasma fractionation. Transfus Med Rev. 2007;21:101–17. doi: 10.1016/j.tmrv.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daniels G, Finning K, Martin P, Summers J. Fetal blood group genotyping: present and future. Ann N Y Acad Sci. 2006;1075:88–95. doi: 10.1196/annals.1368.011. [DOI] [PubMed] [Google Scholar]
- 69.Westhoff CM. Molecular testing for transfusion medicine. Curr Opin Hematol. 2006;13:471–5. doi: 10.1097/01.moh.0000245695.77758.3d. [DOI] [PubMed] [Google Scholar]
- 70.Flegel WA. Blood group genotyping in Germany. Transfusion. 2007;47:47S–53S. doi: 10.1111/j.1537-2995.2007.01310.x. [DOI] [PubMed] [Google Scholar]
- 71.Storry JR. New technologies to replace current blood typing reagents. Curr Opin Hematol. 2007;14:677–81. doi: 10.1097/MOH.0b013e3282ef1838. [DOI] [PubMed] [Google Scholar]
- 72.Hillyer CD, Shaz BH, Winkler AM, Reid M. Integrating molecular technologies for red blood cell typing and compatibility testing into blood centers and transfusion services. Transfus Med Rev. 2008;22:117–32. doi: 10.1016/j.tmrv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Avent ND. RHD genotyping from maternal plasma: guidelines and technical challenges. Methods Mol Biol. 2008;444:185–201. doi: 10.1007/978-1-59745-066-9_14. [DOI] [PubMed] [Google Scholar]
- 74.Müller SP, Bartels I, Stein W, et al. The determination of the fetal D status from maternal plasma for decision making on Rh prophylaxis is feasible. Transfusion. 2008;48:2292–301. doi: 10.1111/j.1537-2995.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- 75.Kumpel BM. Efficacy of RhD monoclonal antibodies in clinical trials as replacement therapy for prophylactic anti-D immunoglobulin: more questions than answers. Vox Sang. 2007;93:99–111. doi: 10.1111/j.1423-0410.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 76.Thomson A, Contreras M, Gorick B, et al. Clearance of Rh D-positive red cells with monoclonal anti-D. Lancet. 1990;336:1147–50. doi: 10.1016/0140-6736(90)92767-c. [DOI] [PubMed] [Google Scholar]
- 77.Kumpel BM. Monoclonal anti-D for prophylaxis of RhD haemolytic disease of the newborn. Transfus Clin Biol. 1997;4:351–6. doi: 10.1016/s1246-7820(97)80040-7. [DOI] [PubMed] [Google Scholar]
- 78.Kumpel BM. Monoclonal anti-D development programme. Transpl Immunol. 2002;10:199–204. doi: 10.1016/s0966-3274(02)00066-7. [DOI] [PubMed] [Google Scholar]
- 79.Béliard R. Monoclonal anti-D antibodies to prevent alloimmunization: lessons from clinical trials. Transfus Clin Biol. 2006;13:58–64. doi: 10.1016/j.tracli.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 80.Kumpel BM. Lessons learnt from many years of experience using anti-D in humans for prevention of RhD immunization and haemolytic disease of the fetus and newborn. Clin Exp Immunol. 2008;154:1–5. doi: 10.1111/j.1365-2249.2008.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harkness M, Freer Y, Prescott RJ, Warner P. Implementation of NICE recommendation for a policy of routine antenatal anti-D prophylaxis: a survey of UK maternity units. Transfus Med. 2008;18:292–5. doi: 10.1111/j.1365-3148.2008.00882.x. [DOI] [PubMed] [Google Scholar]