Abstract
Background
. The fact that only a small percentage of cord blood units (CBU) stored are actually used for transplantation contributes to raising the already high costs of their processing and cryopreservation. The identification of predictors allowing the early identification of suitable CBU would allow a reduction of costs for the collection, storage and characterisation of CBU with insufficient volume or cell numbers. In our bank we have adopted a cut-off value for using CBU of 8 × 108 nucleated cells and a volume ≥ 60 mL.
Materials and methods
In 365 banked CBU, we evaluated the correlation between neonatal/gestational parameters and laboratory data used to assess their quality.
Results
Biparietal diameter (BPD) and abdominal circumference were significantly and positively correlated with CBU volume (r2=0.12, p=0.0011 and r2=0.092, p=0.0063, respectively). Receiver operating characteristic (ROC) analysis showed that both parameters can be used to identify CBU with insufficient volume (BPD: area under the curve 0.69, 95% CI=0.57–0.82, p=0.004; abdominal circumference: area under the curve 0.67, 95% CI=0.54–0.79, p<0.01). BPD and head circumference, but not abdominal circumference or femoral length, were positively correlated with white blood cell (WBC) count (r2=0.215, p=0.031, and r2=0.299, p=0.015, respectively). Abdominal circumference, but not BPD, head circumference or femoral length, was statistically significantly correlated with the number of CD34+ cells in the CBU. Weight at birth and placental weight were positively correlated with WBC count, blood volume, CD34+ cell count, total colony-forming units and burst-forming units.
Conclusion
. Pre-birth assessment of BPD might allow the selection of donors who would yield CBU of sufficient volume and WBC count and avoid the costs of collecting, transferring, storing and analysing CBU with a high probability of resulting unsuitable for transplantation.
Keywords: cord blood transplantation, cord blood units, predictive factors, CD34+ cell count, biparietal diameter
Introduction
Cord blood has been used as an alternative source of hematopoietic stem cells. In fact, both term and preterm umbilical/placental cord blood contains a high number of early and committed progenitor cells. Gluckman et al. first reported the successful use of human leucocyte antigen (HLA)-matched sibling umbilical cord blood stem cells to reconstitute a child with severe Fanconi's anaemia1. Since 1988, about 20,000 umbilical cord blood transplants from related and unrelated donors have been performed2–5. Paediatric and selected adult recipients have successfully received cord blood allografts6–9 for haematopoietic reconstitution in a variety of disorders such as aggressive malignancies, congenital immunodeficiency states, inborn errors of metabolism, haemoglobinopathies, and marrow failure syndromes10–12. Only 30% of patients in need of allogeneic bone marrow transplantation have a fully HLA-matched sibling donor13.
Cord blood has several advantages over adult haematopoietic stem cell sources; these advantages include ease and safety of procurement, rapid availability, no donor attrition, decreased risk of transmission of infectious viral diseases, unlimited supply, wider ethnic representation, abundance of haematopoietic progenitor cells, enhanced in vitro proliferative and self-renewal capacity, immaturity of T-cell-mediated immunity, reduced probability of causing graft-versus-host disease14–16 and diminished need for HLA matching17.
Nevertheless, stem cells from cord blood have several limitations, including delayed neutrophil and platelet engraftment and immune reconstitution, uncertain graft-versus-tumour activity, and the modest cell dose, which can make them unsuitable for larger recipients9,14,16,18–22.
Cord blood processing and cryopreservation are very costly procedures; in fact cord blood units (CBU) require expensive storage in nitrogen tanks, but only a small percentage of them are used for transplantation. The aim of the present study was to provide a basis for the pre-birth selection of cord blood donors likely to provide CBU of sufficient volume and white blood cell (WBC) count, which would help to reduce the costs of collecting, transferring, storing and analysing CBU subsequently found to be unsuitable for transplantation.
Materials and methods
Umbilical cord blood collection
Three hundred and sixty-five consecutive CBU collected between January 2006 and February 2008 at the Blood Bank of the AOUP-Pisa (Italy) were evaluated in this study.
CBU were collected by gravity, puncturing the umbilical vein and collecting the blood into a labelled collection bag (Fresenius Hemocare, MO, Italy). After clamping the bag, the CBU were sent to Cord Blood Bank and stored for up to 36 hours at 4 ± 2°C before processing. Aliquots of blood were used for routine tests, including cell counts, CD34+ and viability assays, HLA typing, clonogenic and sterility tests. Testing for transmissible diseases (human immunodeficiency viruses 1 and 2, human T-lymphotropic viruses 1 and 2, hepatitis B virus, hepatitis C virus, NAT HIV, HCV, HBV, syphilis, toxoplasma, and cytomegalovirus) was also performed.
Foetal biometry
Foetal biometric data, assessed by ultrasound scanning, were retrieved from the clinical charts of the donors. The data collected were biparietal diameter (BPD), head circumference, abdominal circumference and femur length in the third trimester, at week 34±7 of gestation. Although ultrasound scanning was a routine analysis during gestation, data were available only for CBU collected in the hospital of Pisa.
White blood cell counts
White blood cell (WBC) counts were determined with an automated cell counter (Cell Dyn 3500R-Abbott, Altameda, CA, USA) employing two technologies: multi-angle polarised scatter separation, which provides the primary WBC count, and reticulocyte analysis and impedance, which provides additional information about fragile lymphocytes and hypotonically resistant red blood cells.
CD34+ cell counts
CD34+ cell counts were determined with the ISHAGE gating strategy and absolute count beads using CD34-phycoerythrin and CD45-fluorescein isothiocyanate monoclonal antibodies (Becton Dickinson); conjugated 7-AAD PerCP (BD Pharmingen) was used to stain dead cells. Samples were analysed using a FACScan flow cytometer and CellQuest Software (Becton Dickinson).
Colony-forming unit assays
Cord blood was resuspended in Iscove's modified Dulbecco's medium (Sigma, St. Louis, MO, USA) containing 2% foetal bovine serum (Euroclone, Milan, Italy). Cells were plated at a concentration of 2×104 cells/mL in methylcellulose medium (Miltenyi Biotec, Bologna, Italy) containing 50 ng/mL stem cell factor, 20 ng/mL granulocyte-macrophage colony-stimulating factor, 20 ng/mL granulocyte colony-stimulating factor, 20 ng/mL interleukin-3, 20 ng/mL interleukin-6 and 3 U/mL erythropoietin. Plates were incubated at 37° C for 14 days after which the granulocyte-macrophage (GM), granulocyte-erythrocyte-macrophage-megakaryocyte (GEMM) and total colony-forming units (CFU) and burst-forming units-erythroid (BFU-E) were counted. A colony was defined as a cluster of at least 40 cells. Plates were read by two independent readers and the result was the mean of the readings.
Statistical analysis
Descriptive statistics are presented for each maternal and neonatal factor. Spearman's rank correlation, Wilcoxon W and Mann-Whitney tests were used for the statistical analyses. The level of statistical significance was set at 0.01 (two-sided) for Spearman's rank correlation and at 0.05 for the Wilcoxon W and Mann-Whitney tests. Receiver operating characteristics (ROC) curves were analysed.
Results
Characterisation of the donors of CBU
Of the 365 donors recruited in this study, (49% males and 51% females), 82% were delivered by the vaginal route. The mean weight at birth of the donors was 3484 ± 389 grams, and their mean gestational age was 40 ±1 weeks. The donors' physiological parameters are presented in table I. The mean volume of the CBU was 118.2 ± 21.8 mL, and the mean total nucleated cell (TNC) content of the CBU was 1.51×109. A full haematological characterisation of the CBU is presented in table II.
Table I.
Antenatal and neonatal characteristics considered in the correlation with suitability of the donated CBU
| Parameters | Mean±SD or percentage | Range |
|---|---|---|
| Duration of gestation (weeks) | 40±1 | 37–42 |
| Placental weight (g) | 606±114.7 | 300–1,120 |
| Birth weight of baby (g) | 3484±389 | 2,780–4,320 |
| Biparietal diameter (mm) | 83.96±4.5 | 74–97 |
| Head circumference (mm) | 298.97±12.4 | 265–331 |
| Abdominal circumference (mm) | 285.47±21.84 | 239–361 |
| Femoral length (mm) | 63.39±4.04 | 53–78 |
| Route of delivery | ||
| vaginal | 82% | |
| abdominal | 18% | |
| Gender of baby | ||
| male | 49% | |
| female | 51% |
Table II.
Laboratory parameters considered in the correlation with suitability of the donated CBU
| Parameters | Mean±SD | Median | Range |
|---|---|---|---|
| Volume (mL) | 118.2±21.8 | 74.9–201.9 | |
| TNC count (×109) | 1.51±0.96 | 0.64–2.99 | |
| CD34+ count (×106) | 3.1 | 0.211–19.1 | |
| CFU count (×106) | 0.33 | 0.025–11.5 | |
| BFU-E count (×106) | 0.24 | 0.00–7.67 | |
| CFU-GM count (×106) | 0.07 | 0.00–3.81 | |
| WBC/mL (×107) | 1.07±0.44 | 0.034–2.29 | |
| CFU-GEMM count (×106) | 0.01 | 0.00–0.14 |
Effects of neonatal parameters on CBU
Gestation duration. No correlations were found between the duration of gestation and any of the CBU parameters considered (volume, TNC count, CD34+ count, CFU count, BFU-E count, CFU-GM count and CFU-GEMM count) (Table III).
Table III.
Neonatal parameters associated with volume, TNC count, CD34+ count, total CFU count, BFU-E count, CFU-GM count and/or CFU-GEMM count*
| Parameters | Volume | TNC count | CD34+ count | Total CFU count | BFU-E count | CFU-GM count | CFU-GEMM count |
|---|---|---|---|---|---|---|---|
| Duration of gestation | NS n=363 |
NS n=362 |
NS n=362 |
NS n=345 |
NS n=345 |
NS n=345 |
NS n=345 |
| Placental weight | p<0.001 n=358 |
NS n=357 |
p<0.001 n=357 |
p<0.01 n=340 |
p<0.01 n=340 |
NS n=340 |
NS n=340 |
| Birth weight of baby | p<0.001 n=354 |
p<0.01 n=353 |
p<0.01 n=353 |
p<0.01 n=342 |
p<0.01 n=342 |
NS n=342 |
NS n=352 |
| WBC/mL | NS n=365 |
p<0.001 n=364 |
p<0.001 n=364 |
p<0.001 n=347 |
p<0.001 n=347 |
p<0.001 n=347 |
p<0.001 n=347 |
| Route of delivery | NS n=365 |
p<0.05 n=358 |
NS n=365 |
NS n=347 |
NS n=347 |
NS n=347 |
NS n=347 |
| Gender of baby | NS n=361 |
NS n=360 |
NS n=360 |
NS n=343 |
NS n=343 |
NS n=343 |
NS n=343 |
based on Spearman's rank correlation, Wilcoxon W and Mann-Whitney tests.
NS = not significant ; WBC= white blood cells; TNC=total nucleated cells; CD= cluster of differentiation; CFU= colony-forming unit; BFU-E= burst-forming unit - erythroid; CFU-GM=colony-forming unit – granulocyte-macrophage; CFU-GEMM= colony-forming unit - granulocyte-erythrocyte-macrophage megakaryocyte
Placental weight. While significant positive correlations were found between placental weight and volume (p<0.001), CD34+ count (p<0.001), CFU count (p<0.01), and BFU-E count (p<0.01), there were no correlations with TNC count, CFU-GM count or CFU-GEMM count (Table III).
Birth weight. Strong positive correlations were found between the donors' weight at birth and volume of the CBU (p<0.001). Birth weight also correlated with TNC count, CD34+ count, CFU count and BFU-E count (all p<0.01). No correlations were found with CFU-GM count or CFU-GEMM count (Table III).
Delivery. The only correlation found with the route of delivery was for TNC count (p<0.05). No correlations were found with volume, CD34+ count, CFU count, BFU-E count, CFU-GM count or CFU-GEMM count (Table III).
Gender of baby. No correlations were found between the donors' gender and any of the CBU parameters considered (volume, TNC count, CD34+ count, CFU count, BFU-E count, CFU-GM count or CFU-GEMM count) (Table III).
Effects of foetal biometric parameters on CBU
Biparietal diameter. Positive correlations were found between BPD and TNC count, CFU-GM count and CFU-GEMM count (all p<0.05). No correlations were found with CD34+ count, CFU count or BFU-E count (Table IV).
Table IV.
Foetal biometric parameters associated with TNC count, CD34+ count, total CFU count, BFU-E count, CFU-GM count and/or CFU-GEMM count*
| Parameters | TNC count | CD34+ count | Total CFU count | BFU-E count | CFU-GM count | CFU-GEMM count |
|---|---|---|---|---|---|---|
| Biparietal diameter | p<0.05 n=101 |
NS n=102 |
NS n=93 |
NS n=93 |
p<0.05 n=93 |
p<0.05 n=93 |
| Head circumference | p<0.05 n=66 |
NS n=67 |
NS n=61 |
NS n=61 |
p<0.05 n=61 |
NS n=61 |
| Abdominal circumference | NS n=85 |
p<0.01 n=86 |
p<0.01 n=78 |
p<0.05 n=78 |
p<0.01 n=78 |
p<0.01 n=78 |
| Femoral length | NS n=101 |
NS n=102 |
NS n=93 |
NS n=93 |
NS n=93 |
NS n=93 |
based on Spearman's rank correlation
Abbreviations as for Table III.
Head circumference. Positive correlations were found with TNC count and CFU-GM count (p<0.05). There were no correlations between head circumference and any of the other CBU parameters considered (Table IV).
Abdominal circumference. Positive correlations were found between abdominal circumference and CD34+ count, CFU count, CFU-GM count, and CFU-GEMM count (all p<0.01) as well as with BFU-E count (p<0.05). There was, however, no correlation with TNC count (Table IV).
Femoral length. No correlation was found with any of the CBU parameters considered (Table IV).
Correlations between foetal biometric parameters and CBU suitability
When BPD, head circumference, abdominal circumference and femoral length were considered as criteria for screening CBU to be stored in view of their clinical utilisation, only BPD and abdominal circumference were found to allow pre-birth identification of suitable CBU.
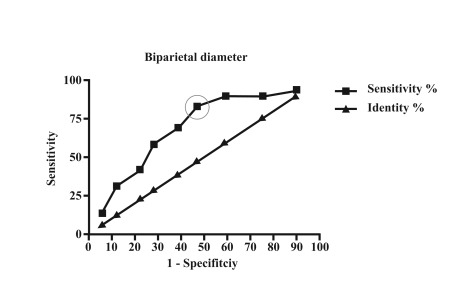
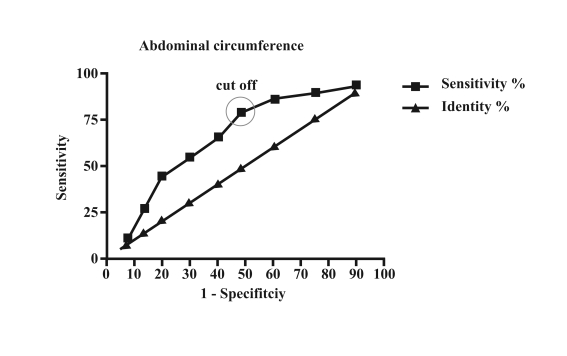
ROC analysis of BPD showed an area under the curve of 0.69 (95% CI=0.57–0.82, p=0.004) (Figure 1) and that of abdominal circumference showed an area under the curve of 0.67 (95% CI=0.54–0.79, p<0.01) (Figure 2).
Figure 1.
ROC analysis of biparietal diameter with an area under the curve of 0.69, 95% CI=0.57–0.82, p=0.004. The cut-off shown has a sensitivity of 82.76% and a specificity of 53.06%
Figure 2.
ROC analysis of abdominal circumference with an area under the curve of 0.67, 95% CI 0.54–0.79, p<0.01. The cut-off has a sensitivity of 79.31% and a specificity of 51.02%
A cut-off of 83.8 mm for BPD showed a sensitivity of 82.76% and specificity of 53.06% for determining whether CBU were suitable for use, while a cut-off of 281 mm for abdominal circumference showed a sensitivity of 79.31% and specificity of 51.02%.
Discussion
Cord blood is an effective alternative source of stem cells for related and unrelated allogeneic stem cell transplantation. The dose of TNC/Kg has been shown to be strongly correlated with engraftment and graft survival in recipients of cord blood transplants from unrelated donors. Based on a Eurocord study, Rocha et al. hypothesised that a minimum dose of 2×107 TNC/kg (in the pre-cryopreservation sample) is necessary for successful transplantation23.
For these reasons a small volume of cord blood and a low number of cells in the unit represent the main drawbacks of placental blood as an alternative source to bone marrow for haematopoietic stem cell transplantation. The expansion of cord blood cells is being studied as a method of increasing the number of progenitor cells, but conclusive data on this strategy are not currently available24; thus, at the moment, improvements in the methods of collecting and selecting CBU are the directions being taken to increase the efficacy and reduce the costs of cord blood transplantation.
Several studies have been carried out with the aim of identifying parameters that may affect good placental blood collection.
In our analysis we considered neonatal and biometric parameters and found that the birth weight correlates positively with CBU volume, CD34+ number, TNC count, CFU count and BFU-E count. These data are in partial agreement with those of Ballen24 and Mancinelli25. Moreover we found positive correlations between placental weight and CBU volume, CD34+ number, total CFU count and BFU-E count. In previous studies Askari26 and Solves27 reported that placental weight seemed to be the determining factor for increased TNC number.
In accordance with Sparrow28 we observed that TNC count was higher in cord blood collected from babies delivered spontaneously than from those delivered by Caesarean section. This could be due to a foetal response to the stress of labour. The positive correlations between placenta and neonatal weights and an increased number of CD34+ cells is probably due to placental ageing, which could lead to progressive foetal hypoxia and consequently to the generation of defence mechanisms tending to increase haematopoietic cells and circulating blood volume. In our study the numbers of total CFU and BFU-E were significantly correlated with placental and neonatal weight and WBC count, while the numbers of CFU-GM and CFU-GEMM were positively correlated with WBC count. Our data demonstrate that neonatal and placental weight affect BFU-E counts but not the numbers of CFU-GM and CFU-GEMM. In contrast, Ballen and co-workers24 found that CFU-GM increased with high neonatal weight.
Biometric parameters (BPD, head circumference, abdominal circumference and femoral length) evaluated by ultrasound at the end of the gestational period also correlated with the above-mentioned laboratory parameters. In particular, WBC count correlated positively with head circumference and BPD but not with abdominal circumference or femoral length; CD34+ cell count showed a statistically significant correlation with abdominal circumference, but not with BPD, head circumference or femoral length.
This positive correlation is probably due to the dimensions of the liver and its residual haematopoietic activity. In fact, during the course of ontogeny, the homing site for haematopoietic stem cells shifts with a certain predictability from the yolk sac to the liver/spleen and then to the marrow. Zanjani29 has shown that before the development of the bone marrow, transplanted haematopoietic stem cells (whether derived from the liver or marrow) home exclusively to the liver or spleen. Despite a change in homing, followed by the expansion of the marrow compartment of the haematopoietic stem cells, these cells do not participate actively in blood cell formation during most of the prenatal period. Liver and spleen remain major haematopoietic organs throughout gestation. It is only during the perinatal period that the marrow becomes the principal site of haematopoietic function. However, the molecular basis underlying the termination of embryonic liver haematopoiesis is poorly understood, and the anatomical origin of haematopoietic stem cells at birth is not fully resolved.
ROC analysis was conducted in order to establish numerical cut-off values for each biometric parameter. Of all the CBU discarded because of insufficient volume, 69% had been collected from donors with a BPD under the cut-off value and 76% from donors with an abdominal circumference under the cut-off value; these CBU would not have been collected had these parameters been applied.
These observations suggest that pre-birth parameters might be useful for identifying and selecting the potential best collections. This would reduce the costs of history taking, collection and processing of CBU, optimise the collection procedure and minimise the number of rejected CBU.
References
- 1.Gluckman E, Broxmeyer H, Auerbach A, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–8. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 2.Rebulla P. Cord blood banking 2002: 112,010 of 7,914,773 chances. Transfusion. 2002;42:1246–8. doi: 10.1046/j.1537-2995.2002.00256.x. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman E. Hematopoietic stem-cell transplants using umbilical-cord blood. N Engl J Med. 2001;344:1860–1. doi: 10.1056/NEJM200106143442410. [DOI] [PubMed] [Google Scholar]
- 4.Hows JM. Status of umbilical UCB transplantation in the year 2001. J Clin Pathol. 2001;54:428–34. doi: 10.1136/jcp.54.6.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gluckman E, Rocha V. Cord blood transplantation: state of the art. Haematologica. 2009;94:536–41. doi: 10.3324/haematol.2009.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocha V, Comish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukaemia. Blood. 2001;97:2962–71. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman E, Rocha V, Chevret S. Results of unrelated umbilical cord blood hematopoietic stem cell transplant. Transfus Clin Biol. 2001;8:146–54. doi: 10.1016/s1246-7820(01)00132-x. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman E, Rocha V, Boyer-Chemmard A, et al. Outcome of cord blood transplantation from related and unrelated donors. N Engl J Med. 1997;337:373–81. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 9.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental blood transplants from unrelated donors N Engl J Med. 1998;339:1565–77. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 10.Thomas ED, Clift RA, Fefer A, et al. Marrow transplantation for the treatment of chronic myelogenous leukaemia. Ann Intern Med. 1986;104:155–60. doi: 10.7326/0003-4819-104-2-155. [DOI] [PubMed] [Google Scholar]
- 11.Krivit W, Whitley CB. Bone marrow transplantation for genetic disease. New Engl J Med. 1987;316:1085–90. doi: 10.1056/NEJM198704233161710. [DOI] [PubMed] [Google Scholar]
- 12.Lucarelli G, Galimberti M, Polchi P, et al. Marrow transplantation in patients with advanced thalassemia. New Engl J Med. 1987;316:1050–7. doi: 10.1056/NEJM198704233161703. [DOI] [PubMed] [Google Scholar]
- 13.Anasetti C, Amos D, Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukaemia or lymphoma. New Engl J Med. 1987;197:320–5. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 14.Cairo MS, Wagner JE. Placental and/or umbilical cord blood: an alternative source of hematopoietic stem cells for transplantation. Blood. 1997;90:4665–78. [PubMed] [Google Scholar]
- 15.Gluckman E. Current status of umbilical cord blood hematopoietic stem cell transplantation. Exp Hematol. 2000;28:1197–205. doi: 10.1016/s0301-472x(00)00540-3. [DOI] [PubMed] [Google Scholar]
- 16.Wadlow RC, Porter DL. Umbilical cord blood transplantation: where do we stand? Biol Blood Marrow Transplant. 2002;8:637–47. doi: 10.1053/bbmt.2002.v8.abbmt080637. [DOI] [PubMed] [Google Scholar]
- 17.Wagner JE, Rosenthal J, Sweetman R, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88:795–802. [PubMed] [Google Scholar]
- 18.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical cord blood from unrelated donors. N. Engl J Med. 2001;344:1815–22. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Ghosh A, Goldman S, Slone V, et al. Immunological reconstitution and correlation of circulating serum inflammatory mediators/cytokines with the incidence of acute graft-versus-host disease during the first 100 days following unrelated umbilical cord blood transplantation. Bone Marrow Transplant. 1999;24:535–44. doi: 10.1038/sj.bmt.1701921. [DOI] [PubMed] [Google Scholar]
- 20.Migliaccio AR, Adamson JW, Stevens CE, et al. Cell dose and speed of engraftment in placental/umbilical cord blood transplantation: graft progenitor cell content is a better predictor than nucleated cell quantity. Blood. 2000;96:2717–22. [PubMed] [Google Scholar]
- 21.Moretta A, Maccario R, Fagioli F, et al. Analysis of immune reconstitution in children undergoing cord blood transplantation. Exp Hematol. 2001;29:371–9. doi: 10.1016/s0301-472x(00)00667-6. [DOI] [PubMed] [Google Scholar]
- 22.Thomson BG, Robertson KA, Gowan D, et al. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000;96:2703–11. [PubMed] [Google Scholar]
- 23.Rocha V, Chastang CL, Souillet G, et al. Related cord blood transplants: the Eurocord experience from 78 transplants. Bone Marrow Transplant. 1998;21:s59–62. [PubMed] [Google Scholar]
- 24.Ballen KK, Wilson M, Wuu J, et al. Bigger is better: maternal and neonatal predictors of hematopoietic potential of umbilical cord blood units. Bone Marrow Transplant. 2001;27:7–14. doi: 10.1038/sj.bmt.1702729. [DOI] [PubMed] [Google Scholar]
- 25.Mancinelli F, Tamburini A, Spagnoli A, et al. Optimizing umbilical cord blood collection: impact of obstetric factors versus quality of cord blood units. Transplant Proc. 2006;38:1174–6. doi: 10.1016/j.transproceed.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 26.Askari S, Miller J, Chrysler G, et al. Impact of donor-and collection-related variables on product quality in ex utero cord blood banking. Transfusion. 2005;45:189–94. doi: 10.1111/j.1537-2995.2004.04117.x. [DOI] [PubMed] [Google Scholar]
- 27.Solves P, Perales A, Moraga R, et al. Maternal, neonatal and collection factors influencing the haematopoietic content of cord blood units. Acta Haematol. 2005;113:241–6. doi: 10.1159/000084677. [DOI] [PubMed] [Google Scholar]
- 28.Sparrow RL, Cauchi JA, Ramadi LT, et al. Influence of mode of birth and collection on WBC yields of umbilical cord blood units. Transfusion. 2002;42:210–5. doi: 10.1046/j.1537-2995.2002.00028.x. [DOI] [PubMed] [Google Scholar]
- 29.Zanjani ED, Ascensao LJ, Tavassoli M. Liver-derived fetal hematopoietic stem cells selectively and preferentially home to the fetal bone marrow. Blood. 1993;81:399–404. [PubMed] [Google Scholar]