Abstract
Background
In 2006 in Italy 2,404,267 donations of blood components were made by 1,539,454 donors; approximately 55% of the donations were collected directly by Transfusion Structures (TS), while about 45% were collected in Donation Centres managed by Associations and Federations of Donors. The aim of the READ (Rilevamento Eventi Avversi alla Donazione) project is to create a network of TS to test a standardised system for monitoring adverse events (AE) related to blood donations.
Materials and methods
Shared, standardised data collection forms, compatible with the forms produced by the ISBT-EHN, were prepared. Two types of form were used: (i) a form to collect data on single events (READ 1), to be used at the individual collection sites; (ii) a form for processing the data collected by each TS (READ 2).
Results
Between February and August 2008 six TS collected data related to the donation of 89,332 units of blood. Overall, 523 AE were recorded. The AE occurred in 0.59% of the donations. The mean duration of the symptoms was 17 minutes. Fifteen percent of the symptoms were related to the venipuncture (mainly haematomas) and 77% to vasovagal AE. The AE were defined severe (grade C) in 47 cases. The donations in which AE were recorded were completed in 81% of the cases; 59% of the AE did not require treatment. Three donors were monitored briefly (for less than 4 hours) in hospital.
Conclusions
The use of standardised forms enabled the collection of data that could be analysed. Some problems related to the performance of the haemovigilance programme did, however, emerge: (i) organisational problems, (ii) limited sensitivity, (iii) inadequate training, and (iv) poorly defined responsibilities. These problems must be resolved at various levels: local, regional and national.
Keywords: blood donation, adverse events to donation, Italian multicentre study
Background
The transfusion world has concentrated almost exclusively on the recording and management of unforeseen and unexpected adverse events (AE) related to the transfusion of blood components, from the transmission of viral diseases (HIV, HCV, HBV) to immediate and delayed transfusion reactions. There are numerous guidelines on the management of such AE, including those issued by the World Health Organisation. Furthermore, in Italy, particular consideration has been given to the prevention and management of transfusion reactions caused by ABO incompatibility; sentinel event number 5, for which we have some data at a regional level1, is part of the more general haemovigilance system
However, “haemovigilance also concerns donors and donations of blood and must enable the collection of information on reactions occurring during the donation of blood, the selection of donors (including the frequency and causes of exclusion), and epidemiological data on donors confirmed to be positive following screening for markers of infectious diseases”2.
In 2006 in Italy 2,404,267 donations of blood components were made by 1,539,454 donors; approximately 55% of the donations were collected directly in Transfusion Structures (TS), while about 45% were collected in centres managed by various Associations and Federations3. Despite the large number of donations, there are only few and anecdotal data on the incidence of AE occurring in donors during or following donations and these have almost always been reported by individual centres4–7.
Relevant legislation and definitions
The current national and European legislation requires that AE related to donations, both of homologous and autologous blood, are recorded8–11.
As said, various TS are already organised in order to collect and analyse such data, but in the absence of a standardised form used throughout the country, there is wide variability and, consequently, great difficulty in processing and managing data which often cannot be compared. For this reason the National Blood Centre endorsed a project that, by involving some transfusion facilities in Italy, could enable data on AE related to donations to be collected using a standard form. It is hoped that the experience gained with this study could be useful to the National Blood Centre to spread a national recording system for these events through the Informatics System of Transfusion Services (SISTRA)12.
“Serious adverse events” and “serious adverse reactions” are defined as follows10:
a “serious adverse event”: any untoward occurrence associated with the collection […] that might […] lead to disability or incapacity of the donor or patient or results in or prolongs hospitalisation or morbidity;
a “serious adverse reaction”: an unintended response in a donor or patient, connected to the collection or transfusion of blood or blood components, which is fatal, life-threatening, incapacitating or disabling, or results in or prolongs hospitalisation or morbidity.
Serious adverse events and serious adverse reactions must always be notified.
The Standards of the Italian Society of Transfusion Medicine and Immunohaematology also set out that such AE must be recorded13.
Aim
The aim of the READ project is to create a network of TS involving a sufficient number of Donor Centres managed directly by the TS, by Associations of Donors, or jointly by the TS and Associations in order to be able to test and implement a standardised system for monitoring AE.
The experimental model, which could be used, perhaps after validation by the National Blood Centre, at a national level, consisted of standardised forms for collecting data.
While waiting for adequate computerised support, it was considered necessary to prepare printed paper forms, with an electronic version, in Word® or Excel® format, which enabled processing of the data collected. The data in these forms are processed in the single reference TS. The processed, summarised data are then sent to a co-ordinating centre in order that the relative statistics can be extracted.
Materials and methods
Data collection forms, integrated and standardised with the forms already available in the various TS involved and with those produced by European organisations (ISBT-EHN), were prepared (Figure 1).
Figure 1.
Project READ
The plan was to collect data relative to about 90,000 donations, in 6 months; six TS were involved (Table I). The number of units of blood components collected was equally distributed between TS that collect donations directly and those that collect the donations indirectly via Donor Associations.
Table I.
Characteristics of the Transfusion Structures involved in the study: data updated to 31.12.2007
| Centre | Donors (n.) | Male (%) | Female (%) | Total units collected (n.) | Units/day | Donor Association | Collection |
|---|---|---|---|---|---|---|---|
| Bergamo | 31,296 | 71.2 | 28.8 | 37,299 | 104 | AVIS | 100% Association |
| Bologna | 22,928 | 73.8 | 26.2 | 40,229 | 114 | AVIS | 100% TS |
| Lucca | 6,729 | 66.3 | 33.7 | 11,246 | 32 | Fratres-CRI - Croce Verde | 100% TS |
| Ragusa | 9,160 | 62.8 | 37.2 | 14,182 | 44 | AVIS | 100% TS+Association |
| Ravenna | 18,429 | 69.8 | 30.2 | 35,816 | 116 | AVIS-ADVS | 44% TS, 56% TS+Association |
| Verona | 12,087 | 74.5 | 24.5 | 35,651 | 125 | AVIS - FIDAS | 100% TS |
| Total | 100,629 | 69.7 | 30.3 | 174,423 | 88 |
Given the different requirement for recording and processing the data, two forms were prepared:
-
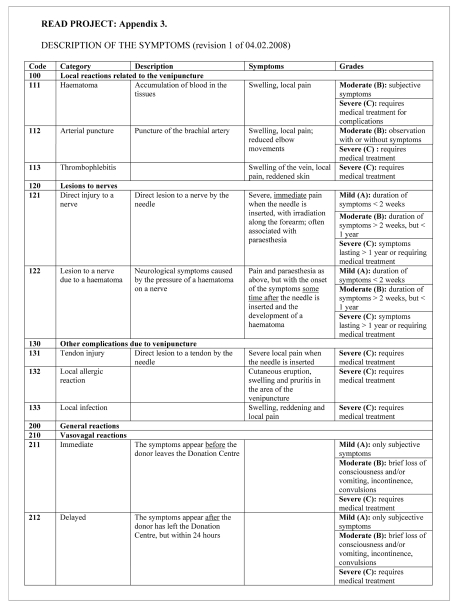
READ 1, a form to record the single events, to be used in the individual donor centres, and a related Excel® spreadsheet for the entry and processing of data (Figure 1). The READ 1 form is a refinement of the various forms already in use in the TS and the EHN-ISBT form. In particular it allows for the distinction between various types of donations made, as well as the number of donations given before the recorded event: this enables an estimate of the incidence of AE in relation to different types of donation and of the incidence in first-time donors. An evaluation of the “grade” of the AE (mild, moderate, severe) is made, according to the definitions reported in Figure 3, allowing standardisation of the data.
The READ 1 form, which requires a very detailed report of the events, can and should be considered as a work instrument for continuous improvement of the quality systems in use at the individual TS independently of their type of management (public or by Associations), enabling an evaluation of the training credits of the staff involved, and for improvement of the performances of the TS and of the Associations and Federations of Donors that collect the donations. Finally, this form also provides for a distinction between donors of homologous blood and donors of autologous blood, thus also allowing its use in TS in which self-donation is practised.
-
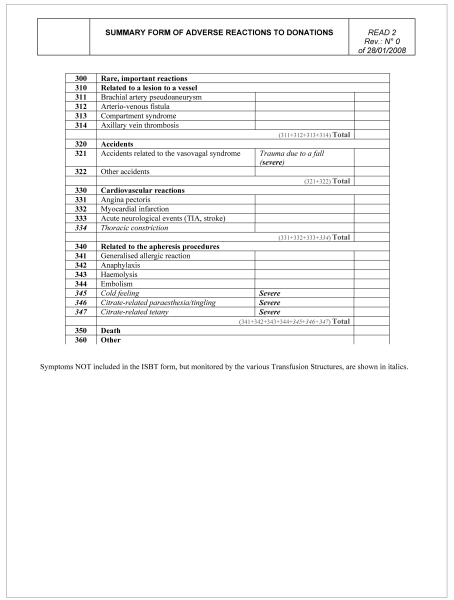
READ 2, a form for processing the data arriving from the individual TS and managed by the coordinating centre. This form (Figure 2) includes the general, summarised data, as planned by the EHN-ISBT, managed by the various co-ordinating centres, TS which receive data from Donor Centres, Regional Blood Centres, and the National Blood Centre, in order to compare Italian data with European data. Only the general data related to the AE, divided according to degree of severity, are reported in this form.
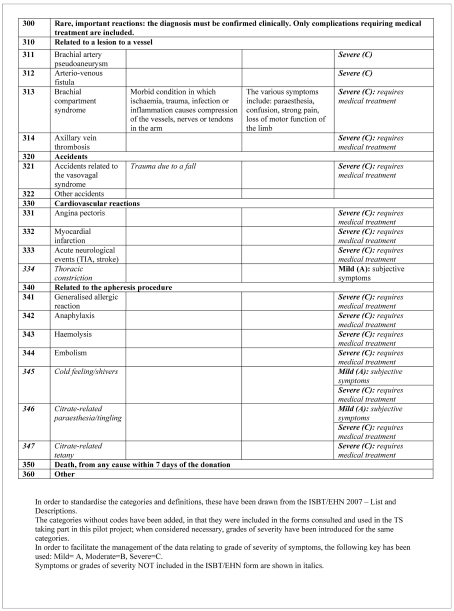
In order to improve the understanding of the data and to standardise reporting, a document listing the complications of donations, as well as their description and severity, was prepared (Figure 3)14.
Figure 3.
Project READ
Figure 2.
Project READ
Results
Between February and August 2008 six TS collected information related to the donation of 89,332 units of blood components: 68,968 units of whole blood, 17,449 plasmapheresis, 60 plateletpheresis, 1,886 plasma plateletpheresis, 735 erythro-plasmapheresis, 214 erythro-plateletpheresis, and 20 double red cell apheresis.
Overall 523 AE were recorded, of which 51% occurred in men and 49% among women: the percentage of male donors in the TS involved was 69,7%, while the percentage of female donors was 30,3%. The distribution of the AE by age was as follows: 22% in the age range from 18–25 years old, 24% in the group 26–35 years, 30% in the group 36–45 years, 15% in the group 46–55 years, and 9% in the group 56–65 years old.
Seventeen percent of the AE occurred in first-time donors, 30% in donors of 2–5 previous donations, 17% in those who had made 6–10 previous donations, 8% in those who had made 11–15 previous donations and 27% in those who had made more than 15 previous donations.
The AE rates according to the various types of donation were 0.41% for whole blood, 0.84% for plasmapheresis, 17% for plateletapheresis, 3.55% for plasma-plateletapheresis, 1.63% for erythro-plasmapheresis, 0.93% for erythrom-plateletpheresis, and 5% for double red cell apheresis, for an overall mean rate of 0.59%.
The total number of symptoms recorded was 978. The symptoms lasted between 1 minute and 5 hours, with a mean duration of 17 minutes. One hundred and forty-seven symptoms (15%) were related to venipuncture: of these, 145 were haematomas and 103 required local therapy, one was an arterial puncture and one was a mild lesion to a nerve. There were no cases of thrombophlebitis. There were 749 vasovagal symptoms, accounting for 77% of all symptoms: 703 occurred within the donation centre and 46 after leaving the centre, but within 24 hours.
The symptoms were defined as severe (grade C) in 47 cases; 14 of these symptoms were recorded in relation to apheresis procedures (cold feeling: 3, citrate-related paraesthesia: 11); two AE were not among those listed in the form, but both were classified as mild. No important rare event was notified.
Of the 978 symptoms, 164 were considered to be grade C: 103 of these 164 symptoms were related to haematomas; the remaining 61 events included the paraesthesia and cold feeling (treated with oral or intravenous calcium, but easily resolved). In conclusion, only 47 of the 978 symptoms recorded were actually serious enough to be considered severe.
The donations in which AE were recorded were completed in 81% of the cases and suspended in the other 19% of cases. Fifty-nine percent of the donors who had an AE did not require therapy, whereas the other 41% did. Three donors needed a brief period of observation (less than 4 hours) in hospital (in the Accident and Emergency Department).
Discussion
To the best of our knowledge, our project is the first multicentre nationwide study on AE in donors of blood components.
After having designed the data registration forms, these were used in the various TS from February 2008. Controls of the data registration were conducted in order to make changes to the forms if necessary; however, the forms did not require revision.
The data recorded included, for the first time as an AE, the occurrence of haematomas. No data on haematomas have previously been reported in the Italian scientific literature. Indeed, we did think that considering haematomas as an AE would lead to difficulties in managing the data, particularly with regards to “severe” cases, given that even the application of cutaneous medication led to the event being notified as “severe, grade C”, according to a rigid compliance with the definition. However, we considered it obvious that the appearance of a haematoma could lead to a possible “worsening” of the condition with subsequent development of other, related AE [for example: arterial puncture (only one case reported), thrombophlebitis (no reports), haematoma-induced lesion to a nerve (no reports), pseudo-aneurysm of the brachial artery (no reports), arterio-venous fistula (no reports), compartment syndrome (no reports), axially vein thrombosis (no reports)] and that only in these cases should they be notified and considered grade C, or “severe”. In any case, even considering problems of compensation, which we believe to be the main reason for the inclusion of this AE in the ISBT-EHN list to which we referred15, recently updated Italian legislation provides for insurance cover16. On the other hand, the authors of the ISBT-EHN list planned that “haematomas” would be recorded as AE only in cases that they were present for a prolonged period15.
Another difficulty that we encountered was the limited data for some types of donation, for example plateletpheresis (only carried out in one TS), double red cell apheresis and double units from plateletpheresis: the small number of units obviously prevented data and percentage of AE from being significant. Further, specific studies are, therefore, necessary for these types of donations.
As far as concerns the relation between AE and type of donation, it was found that the incidence of AE was about 5 times higher with multicomponent donations than with donations of whole blood (2.02 vs 0.41 every 100 donations), and 2 times higher than that with donations of single products by apheresis; however, these findings could also be affected by the limited amount of data.
One finding that we believe is important to highlight is a gender-related difference in the incidence of AE, with the incidence being higher among women than among men, since 49% of the AE occurred in women, who accounted for only about 30% of the donors in the six TS involved in the project. In this regard, revision 1 of the READ 1 form included a field related to the weight of the donor, since this parameter appears to be involved in the occurrence of AE17–18.
The mean age of the donors who developed AE was 37 years. It was not possible to calculate the incidence of AE in first-time donors since there was no way of correlating the number of AE that occurred with the total number of first-time donors.
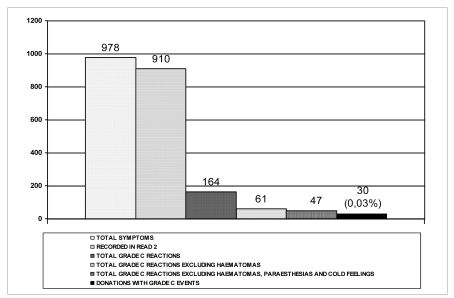
Overall, 978 symptoms were recorded, with some donors having more than one; of these only 910 were recorded on the READ 2 forms, since this passage was not required for 68 of the symptoms. Figure 4 shows the number of AE with their degree of severity: only 47 symptoms were designated as severe AE, complicating 0.03% of all the donations monitored.
Figure 4.
Total symptoms recorded: 978. The number of real, severe AE was 30, for an incidence of 0.03% among all the donations monitored
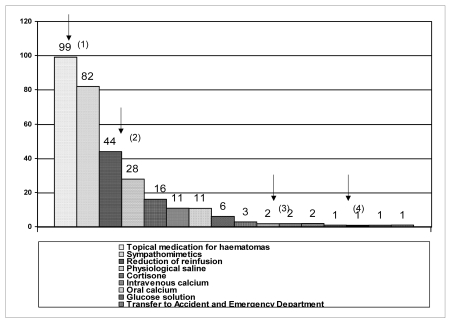
Of the donors developing an AE, 41% needed treatment (Figure 5); three donors required brief monitoring in hospital (in the Accident and Emergency Department). In two cases oxygen therapy was inappropriately administered (personal communication).
Figure 5.
Therapy used to manage the 310 AE that were treated; the arrows indicate: (1): therapy for haematomas, (2): reduction of reinfusion, (3): transfer to the Accident and Emergency Department, (4): oxygen therapy
Conclusions
Besides the fact that the data collected represent only a small part of the Italian reality and cannot, therefore, be readily compared with national data collected in much more substantial surveys19–21, the following problems related to the performance of the haemovigilance programme were noted: (i) organisational problems, (ii) limited sensitivity, (iii) inadequate training, and (iv) poorly defined responsibilities.
These problems must be resolved at various levels: local, regional and national, as proposed in other nations22. As far as regards local interventions, it is essential that periodic meetings are conducted to train and educate the staff, both of the TS and the Donor Associations, and to spread the AE recording forms. Furthermore, a local representative (a nurse or doctor) must be identified. This person should be responsible for collecting the data for the READ 1 forms, processing them and spreading them locally in order to promote continuous improvement of the individual Donation Centres: the unpleasant experience of an AE undoubtedly causes greater reluctance to make another donation23–25.
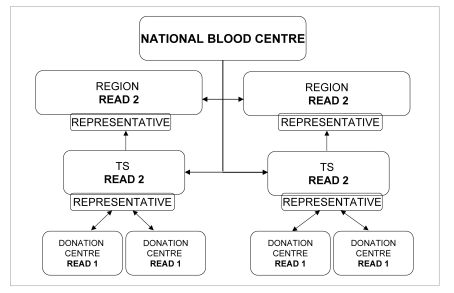
At the level of TS, a person responsible for coordinating the Donation Centres and processing the data must be identified. The data must be made available to all the facilities referring to the TS, enabling direct comparisons and general improvement. Furthermore, the TS representative must send the summarised data (READ 2 form, with grade C AE) to the appropriate regional bodies, where another representative must be identified to coordinate all the TS at a regional level and then send the data to the National Blood Centre (Figure 6). The approach that we propose, which can certainly be improved, represents the basis for a useful and profitable involvement of the various transfusion facilities.
Figure 6.
Flow chart of information
Table II.
Numbers of the different types of donation
| Type of donation | Number |
|---|---|
| Whole blood | 68,968 |
| Plasmapheresis (P) | 17,449 |
| Plateletpheresis (PLT) | 59 |
| Double plateletpheresis (2PLT) | 1 |
| Subtotal - apheresis (P+PLT+2PLT) | 17,509 |
| Plasma-plateletpheresis (P-PLT) | 1,886 |
| Erythro-plasmapheresis (R-P) | 735 |
| Erythro-plateletpheresis (R-PLT) | 214 |
| Double red cell apheresis (2R) | 20 |
| Subtotal - multicomponent (P-PLT+R-P+2R) | 2,641 |
| Total | 89,332 |
References
- 1.Facco G. Haemovigilance at regional level: the experience of the region of Piedmont. Blood Transfus. 2009;7(Suppl 1):LE04. [Google Scholar]
- 2.Council of Europe, January 2007: Guide to the preparation, use and quality assurance of blood components. 13^ eds, Recommendation n° R(95) 15.
- 3.Catalano L, Abbonizio F, Giampaolo A, et al. Istituto Superiore di Sanità: Registro Nazionale e Regionale del Sangue e del Plasma, rapporto 2006, Rapporti ISTISAN 07/46.
- 4.Fagiani P, Abraha TH, Cirillo D, et al. La sicurezza del donatore: prevenzione e assistenza dei malesseri, ABS081, XXXVIII Convegno Nazionale di Studi di Medicina Trasfusionale. Blood Transfus. 2008;6(Suppl. 1):S111–2. [Google Scholar]
- 5.Misso S, Feola B, Nasti F, et al. Analisi degli eventi avversi nei donatori di sangue intero, ABS289, XXXVIII Convegno Nazionale di Studi di Medicina Trasfusionale. Blood Transfus. 2008;6(Suppl. 1):S103–4. [Google Scholar]
- 6.Randi V, Caltavuturo G, Venturi R, et al. Il monitoraggio delle non conformità in sala prelievi come strumento per il miglioramento continuo, ABS291, XXXVIII Convegno Nazionale di Studi di Medicina Trasfusionale. Blood Transfus. 2008;6(Suppl. 1):S215. [Google Scholar]
- 7.Crocco I, Franchini M, Garozzo G, et al. Adverse reactions in blood and apheresis donors: experience from two Italian transfusion centres. Blood Transfus. 2009;7:35–8. doi: 10.2450/2008.0018-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law n. 219 of 21 October, 2005. Nuova disciplina delle attività trasfusionali e della produzione nazionale di emoderivati.
- 9.Legislative Decree n. 207 of 9 November, 2007. Attuazione della direttiva 2005/61/CE che applica la direttiva 2002/98/CE per quanto riguarda la prescrizione in tema di rintracciabilità del sangue e degli emocomponenti destinati a trasfusioni e la notifica di effetti indesiderati ed incidenti gravi.
- 10.Ministerial Decree of 3 March, 2005. Caratteristiche e modalità per la raccolta di sangue e di emocomponenti” e successive modificazioni.
- 11.Legislative Decree n. 261 of 20 December, 2007. Revisione del decreto legislativo 19 agosto 2005, n. 191, recante attuazione della direttiva 2002/98/CE che stabilisce norme di qualità e di sicurezza per la raccolta, il controllo, la lavorazione, la conservazione e la distribuzione del sangue umano e dei suoi componenti.
- 12.Ministerial Decree of 21 December, 2008. Istituzione del sistema informativo dei servizi trasfusionali
- 13.Grazzini G, Alfano G, Gandini G, et al. Standard di Medicina Trasfusionale, sezione B, Edizioni SIMTI, 1st editionSeptember2007
- 14.ISBT/EHN: list of adverse events Version 2007, available at www.ehn.gedireg.com, last consulted: 12.02.2009
- 15.Sorensen BS, Johnsen SP, Jorgensen J. Complications related to blood donation: a population-based study. Vox Sang. 2008;94:132–7. doi: 10.1111/j.1423-0410.2007.01000.x. [DOI] [PubMed] [Google Scholar]
- 16.Accordo tra Stato-Regioni e province autonome sul documento recante la “Definizione dello schema tipo per la stipula di convenzioni tra Regioni, Province Autonome e Associazioni e Federazioni dei Donatori di Sangue”, 20 March 2008.
- 17.Newman BH, Satz SL, Janowicz NM, Siegfried BA. Donor reactions in high-school donors: the effects of sex, weight, and collection volume. Transfusion. 2006;46:284–8. doi: 10.1111/j.1537-2995.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- 18.Trouern-Trend JJ, Cable RG, Badon SJ, et al. A case-controlled multicenter study of vasovagal reactions in blood donors: influence of sex, age, donation status, weight, blood pressure, and pulse. Transfusion. 1999;39:316–20. doi: 10.1046/j.1537-2995.1999.39399219291.x. [DOI] [PubMed] [Google Scholar]
- 19.Rebibo D, Hauser L, Simonet M, Jbilou S. pour les équipes de prélèvements et les correspondants d’hémovigilance de l’EFS. Surveillance de 2 764 663 dons de sang en France: quels sont les risques du don et qui sont les donneurs les plus à risques ? Transfusion Clinique et Biologique 15 (2008) n° 5, Edition congrès: VIII Congrès National d’hémovigilance et de securité transfusionnelle, [abstract] page 329.
- 20.Eder AF, Dy BA, Kennedy JM, et al. The American Red Cross donor hemovigilance program: complications of blood donation reported in 2006. Transfusion. 2008;48:1809–19. doi: 10.1111/j.1537-2995.2008.01811.x. [DOI] [PubMed] [Google Scholar]
- 21.Ministry of Health & Social Solidarity, Hellenic centre for disease control & prevention (KEELPNO), Hellenic coordinating haemovigilance centre (SKAE). Summary report, Epidemiological surveillance of transfusion transmitted infections (TTIs) (1996–2007), Surveillance of adverse reactions (ARs-TR) and adverse events (AEs-TR) associated with blood transfusion (1997–2007) Surveillance of adverse reactions (ARs-DN) and adverse events (AEs-DN) during or after donation (2003–2007), Athens, November 2008.
- 22.Rebibo D, Hauser L, Slimani A, Andreu G, pour le comité d’Hemovigilance de l’EFS. Mise en place de la declaration des effets indesiderables graves survenus chez les donneurs de sang- 1er Janvier-30 Juin 2006: VII Congrès National d’hèmovigilance et Securité Transfusionnelle, 2006, [abstract] page 163.
- 23.Newman BH, Newman DT, Ahmad R, Roth AJ. The effect of whole-blood donor adverse events on blood donor return rates. Transfusion. 2006;46:1374–9. doi: 10.1111/j.1537-2995.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen DD, DeVita DA, Hirschler NV, et al. Blood donor satisfaction and intention of future donation. Transfusion. 2008;48:742–8. doi: 10.1111/j.1537-2995.2007.01600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson Sojka B, Sojka P. The blood donation experience: self-reported motives and obstacles for donating blood. Vox Sang. 2008;94:56–63. doi: 10.1111/j.1423-0410.2007.00990.x. [DOI] [PubMed] [Google Scholar]