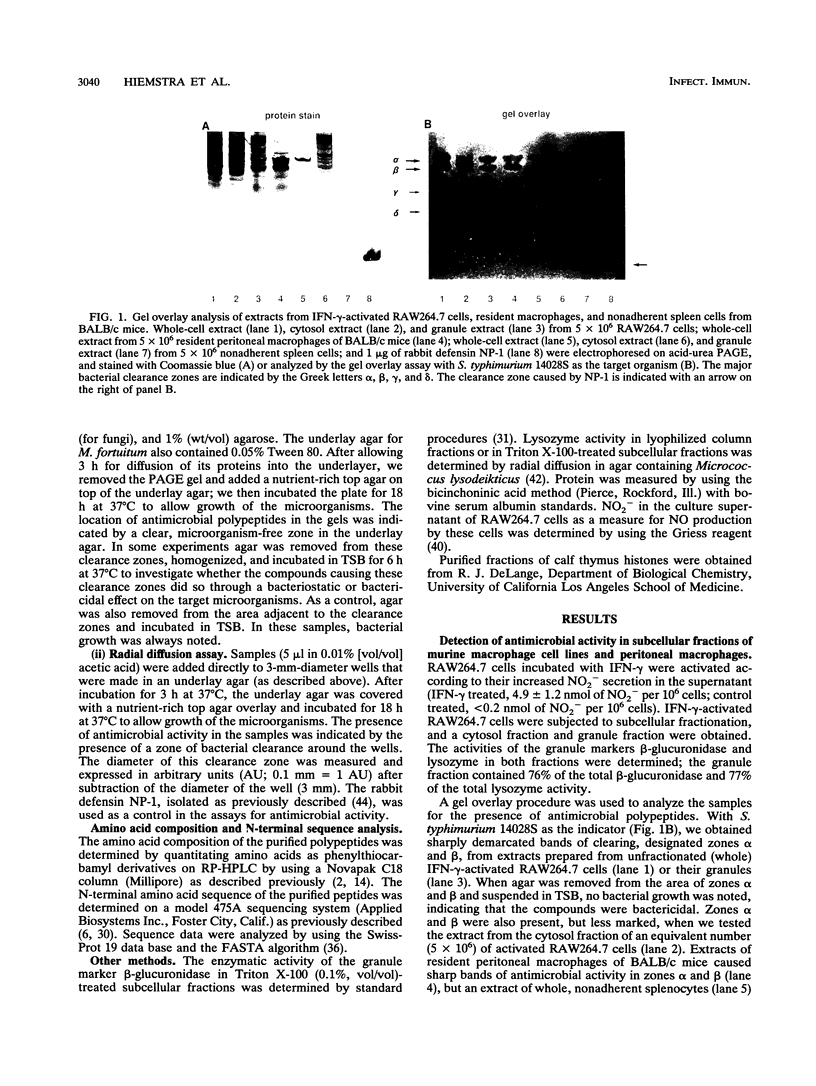
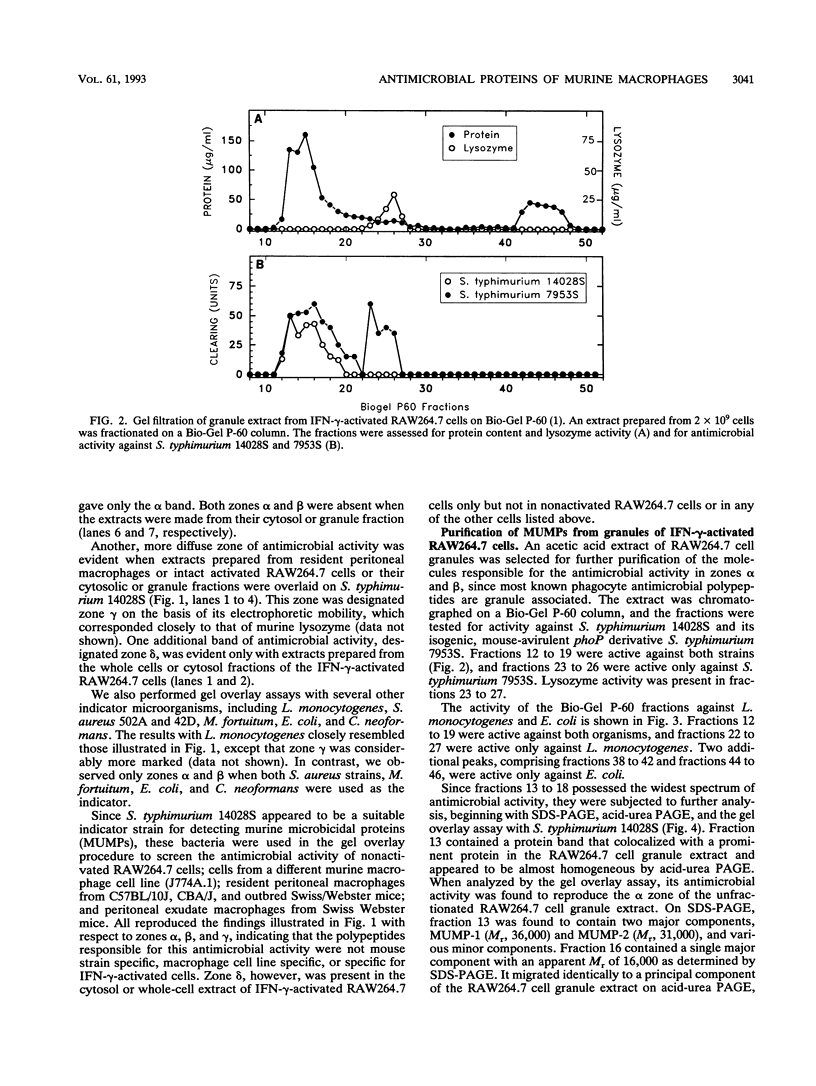
Abstract
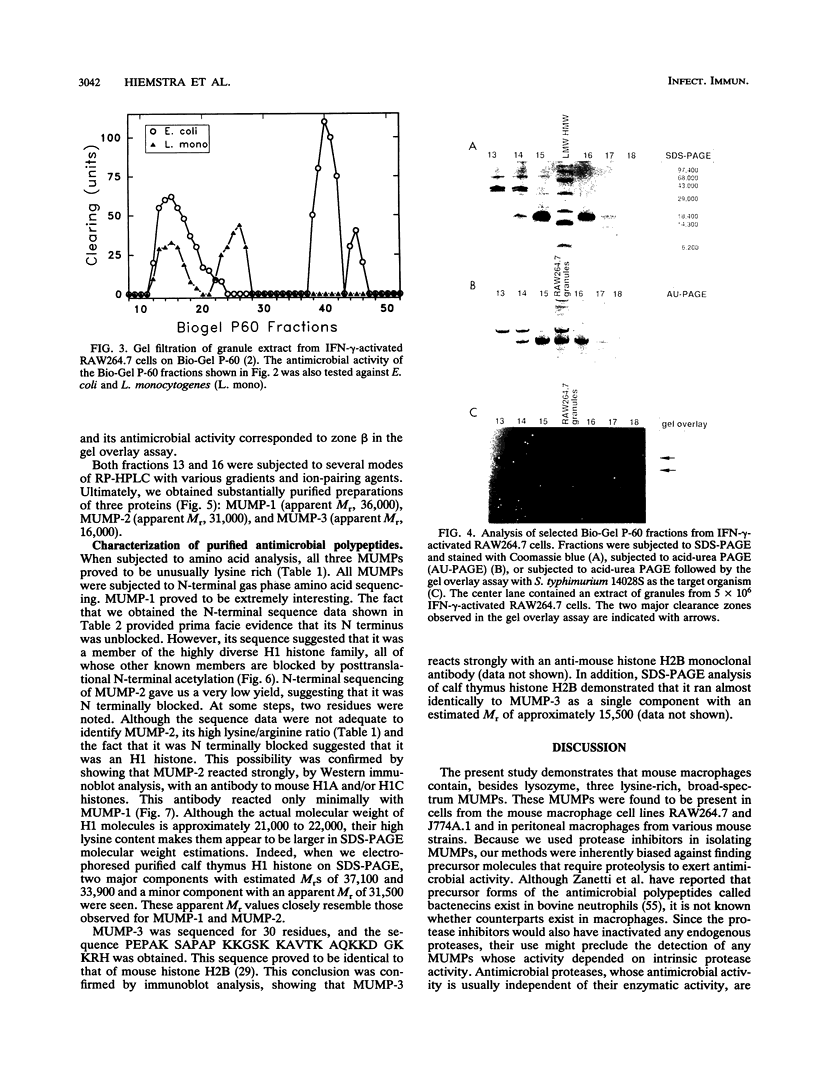
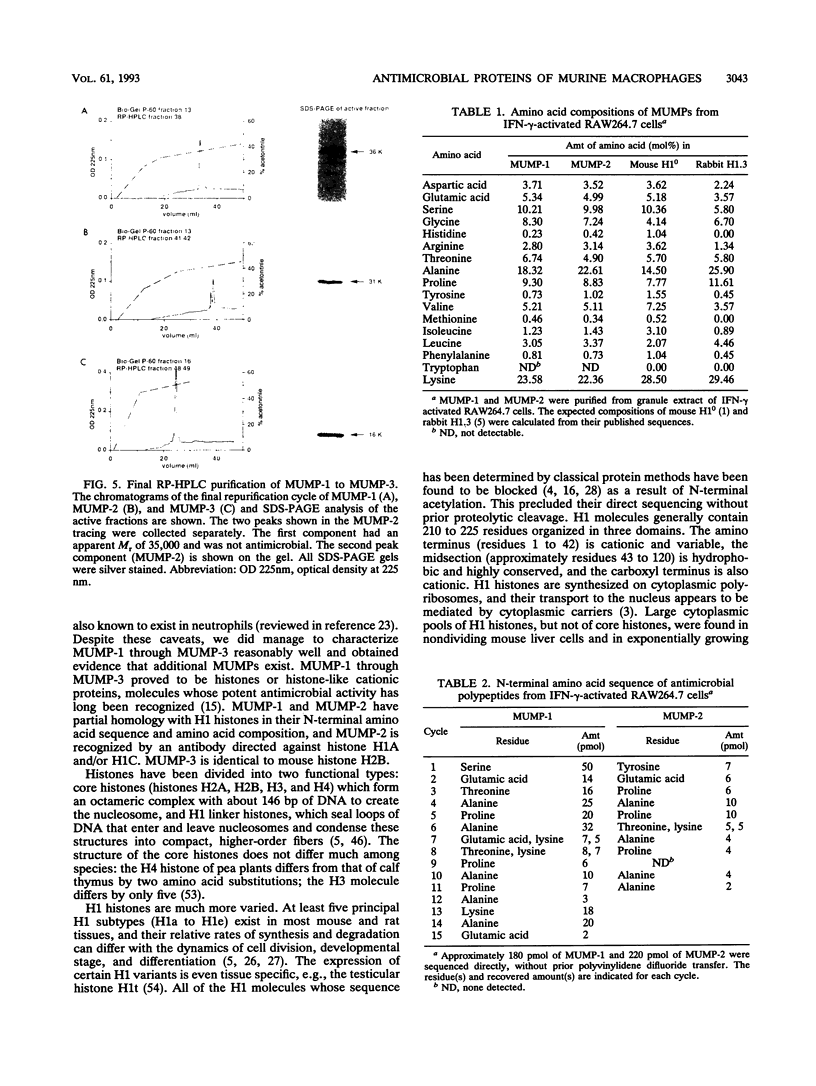
Three murine microbicidal proteins (MUMPs) were purified from cells of the murine macrophage cell line RAW264.7 that had been activated by gamma interferon. Similar proteins were also present in nonactivated RAW264.7 cells, in cells of the murine macrophage cell line J774A.1, and in resident and activated murine peritoneal macrophages. MUMP-1, MUMP-2, and MUMP-3 killed Salmonella typhimurium, Escherichia coli, Staphylococcus aureus, Listeria monocytogenes, Mycobacterium fortuitum, and Cryptococcus neoformans in vitro. MUMP-1 resembled an H1 histone but was unusual because its N-terminal residue (serine) was not N acetylated. Although MUMP-2 was N terminally blocked, its high lysine/arginine ratio and its reactivity with an antibody to H1 histones suggested that it also belonged to the H1 histone family. MUMP-3 was identical to histone H2B in 30 of 30 amino-terminal residues. Although the antimicrobial properties of histones have been recognized for decades, this is the first evidence that such proteins may endow the lysosomal apparatus of macrophages with nonoxidative antimicrobial potential. Other MUMPs, including some with a more restricted antimicrobial spectrum and one that appeared to be induced in RAW264.7 cells after gamma interferon stimulation, were noted but remain to be characterized.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso A., Breuer B., Bouterfa H., Doenecke D. Early increase in histone H1(0) mRNA during differentiation of F9 cells to parietal endoderm. EMBO J. 1988 Oct;7(10):3003–3008. doi: 10.1002/j.1460-2075.1988.tb03163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Breeuwer M., Goldfarb D. S. Facilitated nuclear transport of histone H1 and other small nucleophilic proteins. Cell. 1990 Mar 23;60(6):999–1008. doi: 10.1016/0092-8674(90)90348-i. [DOI] [PubMed] [Google Scholar]
- Cole K. D., York R. G., Kistler W. S. Sequence of the amino terminal half of rat testis-specific histone variant H1t. Biochim Biophys Acta. 1986 Feb 14;869(3):223–229. doi: 10.1016/0167-4838(86)90062-2. [DOI] [PubMed] [Google Scholar]
- Cole R. D. Microheterogeneity in H1 histones and its consequences. Int J Pept Protein Res. 1987 Oct;30(4):433–449. doi: 10.1111/j.1399-3011.1987.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Couto M. A., Harwig S. S., Cullor J. S., Hughes J. P., Lehrer R. I. Identification of eNAP-1, an antimicrobial peptide from equine neutrophils. Infect Immun. 1992 Aug;60(8):3065–3071. doi: 10.1128/iai.60.8.3065-3071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Eisenhauer P. B., Harwig S. S., Lehrer R. I. Cryptdins: antimicrobial defensins of the murine small intestine. Infect Immun. 1992 Sep;60(9):3556–3565. doi: 10.1128/iai.60.9.3556-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer P. B., Lehrer R. I. Mouse neutrophils lack defensins. Infect Immun. 1992 Aug;60(8):3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Schwamberger G., Kaufmann S. H. Fungicidal activity of IFN-gamma-activated macrophages. Extracellular killing of Cryptococcus neoformans. J Immunol. 1989 May 1;142(9):3219–3224. [PubMed] [Google Scholar]
- Ganz T., Sherman M. P., Selsted M. E., Lehrer R. I. Newborn rabbit alveolar macrophages are deficient in two microbicidal cationic peptides, MCP-1 and MCP-2. Am Rev Respir Dis. 1985 Oct;132(4):901–904. doi: 10.1164/arrd.1985.132.4.901. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G. Bactericidal action of histone. J Exp Med. 1958 Dec 1;108(6):925–944. doi: 10.1084/jem.108.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Jones G. M., Rall S. C., Cole R. D. Extension of the amino acid sequence of a lysine-rich histone. J Biol Chem. 1974 Apr 25;249(8):2548–2553. [PubMed] [Google Scholar]
- Lambert L. E., Paulnock D. M. Differential induction of activation markers in macrophage cell lines by interferon-gamma. Cell Immunol. 1989 May;120(2):401–418. doi: 10.1016/0008-8749(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Barton A., Ganz T. Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J Immunol Methods. 1988 Apr 6;108(1-2):153–158. doi: 10.1016/0022-1759(88)90414-0. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T. Antimicrobial polypeptides of human neutrophils. Blood. 1990 Dec 1;76(11):2169–2181. [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Szklarek D., Selsted M. E. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J Clin Invest. 1988 Jun;81(6):1829–1835. doi: 10.1172/JCI113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Rosenman M., Harwig S. S., Jackson R., Eisenhauer P. Ultrasensitive assays for endogenous antimicrobial polypeptides. J Immunol Methods. 1991 Mar 21;137(2):167–173. doi: 10.1016/0022-1759(91)90021-7. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox R. W., Cohen L. H. The histone H1 complements of dividing and nondividing cells of the mouse. J Biol Chem. 1983 Jan 10;258(1):262–268. [PubMed] [Google Scholar]
- Lennox R. W. Differences in evolutionary stability among mammalian H1 subtypes. Implications for the roles of H1 subtypes in chromatin. J Biol Chem. 1984 Jan 10;259(1):669–672. [PubMed] [Google Scholar]
- Liao L. W., Cole R. D. The amino acid sequence of residues 1-104 of CTL-1, a bovine H1 histone. J Biol Chem. 1981 Mar 25;256(6):3024–3029. [PubMed] [Google Scholar]
- Liu T. J., Liu L., Marzluff W. F. Mouse histone H2A and H2B genes: four functional genes and a pseudogene undergoing gene conversion with a closely linked functional gene. Nucleic Acids Res. 1987 Apr 10;15(7):3023–3039. doi: 10.1093/nar/15.7.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs G. L., Rotstein O. D., Grinstein S. Phagosomal acidification is mediated by a vacuolar-type H(+)-ATPase in murine macrophages. J Biol Chem. 1990 Dec 5;265(34):21099–21107. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Multigner L., Gagnon J., Van Dorsselaer A., Job D. Stabilization of sea urchin flagellar microtubules by histone H1. Nature. 1992 Nov 5;360(6399):33–39. doi: 10.1038/360033a0. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Mechanisms of macrophage antimicrobial activity. Trans R Soc Trop Med Hyg. 1983;77(5):620–630. doi: 10.1016/0035-9203(83)90190-6. [DOI] [PubMed] [Google Scholar]
- Ouellette A. J., Greco R. M., James M., Frederick D., Naftilan J., Fallon J. T. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989 May;108(5):1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polet H. Endocytosed non-histone proteins translocate in part to the nucleus in lymphocytes. Exp Cell Res. 1986 Oct;166(2):455–464. doi: 10.1016/0014-4827(86)90490-8. [DOI] [PubMed] [Google Scholar]
- Polet H. The effects of lysosomotropic amines on protein degradation, migration of nonhistone proteins to the nucleus, and cathepsin D in lymphocytes. J Cell Physiol. 1985 Mar;122(3):415–423. doi: 10.1002/jcp.1041220312. [DOI] [PubMed] [Google Scholar]
- Pollack C., Straley S. C., Klempner M. S. Probing the phagolysosomal environment of human macrophages with a Ca2+-responsive operon fusion in Yersinia pestis. 1986 Aug 28-Sep 3Nature. 322(6082):834–836. doi: 10.1038/322834a0. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Miller S. I., Henschen A. H., Ouellette A. J. Enteric defensins: antibiotic peptide components of intestinal host defense. J Cell Biol. 1992 Aug;118(4):929–936. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Szklarek D., Lehrer R. I. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984 Jul;45(1):150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarek M. D., Swank R. T. Turnover of two lysosomal enzymes in macrophages. J Biol Chem. 1981 Oct 10;256(19):10137–10144. [PubMed] [Google Scholar]
- Smith M. M. Histone structure and function. Curr Opin Cell Biol. 1991 Jun;3(3):429–437. doi: 10.1016/0955-0674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Sohnle P. G., Collins-Lech C., Wiessner J. H. Antimicrobial activity of an abundant calcium-binding protein in the cytoplasm of human neutrophils. J Infect Dis. 1991 Jan;163(1):187–192. doi: 10.1093/infdis/163.1.187. [DOI] [PubMed] [Google Scholar]
- Steinbakk M., Naess-Andresen C. F., Lingaas E., Dale I., Brandtzaeg P., Fagerhol M. K. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990 Sep 29;336(8718):763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Kagaya K., Yamada T., Fukazawa Y. Mechanism for candidacidal activity in macrophages activated by recombinant gamma interferon. Infect Immun. 1991 Feb;59(2):521–528. doi: 10.1128/iai.59.2.521-528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. S., Panusz H. T., Hatch C. L., Bonner W. M. Histones and their modifications. CRC Crit Rev Biochem. 1986;20(2):201–263. doi: 10.3109/10409238609083735. [DOI] [PubMed] [Google Scholar]
- Yang Y. S., Brown D. T., Wellman S. E., Sittman D. B. Isolation and characterization of a mouse fully replication-dependent H1 gene within a genomic cluster of core histone genes. J Biol Chem. 1987 Dec 15;262(35):17118–17125. [PubMed] [Google Scholar]
- Zanetti M., Litteri L., Griffiths G., Gennaro R., Romeo D. Stimulus-induced maturation of probactenecins, precursors of neutrophil antimicrobial polypeptides. J Immunol. 1991 Jun 15;146(12):4295–4300. [PubMed] [Google Scholar]
- Zlatanova J. S., Srebreva L. N., Banchev T. B., Tasheva B. T., Tsanev R. G. Cytoplasmic pool of histone H1 in mammalian cells. J Cell Sci. 1990 Jul;96(Pt 3):461–468. doi: 10.1242/jcs.96.3.461. [DOI] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemert F. J., van Dam A. P., Jonk L. J., Destrée O. H., Smeenk R. J. Monoclonal autoantibodies recognizing histone variants. Immunol Invest. 1988 May;17(3):195–215. doi: 10.3109/08820138809052960. [DOI] [PubMed] [Google Scholar]
- van der Meide P. H., Dubbeld M., Vijverberg K., Kos T., Schellekens H. The purification and characterization of rat gamma interferon by use of two monoclonal antibodies. J Gen Virol. 1986 Jun;67(Pt 6):1059–1071. doi: 10.1099/0022-1317-67-6-1059. [DOI] [PubMed] [Google Scholar]