Abstract
Alterations in mitochondrial homeostasis have been implicated in the etiology of Parkinson disease (PD) as demonstrated by human tissue studies, cell culture and in vivo genetic and toxin models. Mutations in the genes encoding PTEN-induced kinase 1 (PINK1), Omi/HtrA2 and parkin contribute to rare forms of parkinsonian neurodegeneration. Recently, each of these proteins has been shown to play a normal role in regulating mitochondrial structure, function, fission-fusion dynamics, or turnover (autophagy and biogenesis), promoting neuronal survival. Here, we review the biochemical mechanisms of mitochondrial protection conferred by each of these PD associated gene products in neurons, neuronal cell lines and other cell types. Potential molecular interactions and mitoprotective signaling pathways involving these three PD associated gene products are discussed in the context of mitochondrial quality control, in response to increasing levels of mitochondrial damage. We propose that PINK1, Omi/HtrA2 and parkin participate at different levels in mitochondrial quality control, converging through some overlapping and some distinct steps to maintain a common phenotype of healthy mitochondrial networks.
Keywords: mitochondria, ser/thr kinases, PINK1, Parkin, Omi/HtrA2, neurotoxin, autophagy, ubiquitin, neurodegeneration, oxidative stress, Parkinson’s disease
Introduction
Parkinson disease (PD) is a progressive and incurable disease that affects approximately 1% of the North American population. This devastating neurodegenerative disease is characterized by the progressive loss of dopaminergic neurons of the substantia nigra, with involvement of other neuronal populations resulting in autonomic and cognitive dysfunction. Motor symptoms in PD may not be apparent until the majority of dopaminergic neurons are lost (Betarbet et al. 2002), most likely due to multiple compensatory mechanisms.
PD is a multifactorial disease for which the etiology in most cases remains unknown. While >90% of cases are sporadic, mutations in several nuclear encoded genes have been linked to the development of autosomal recessive and dominant familial parkinsonian syndromes (Bogaerts et al. 2008), enhancing our understanding of biochemical and cellular mechanisms contributing to disease. Cell culture and mouse models that overexpress PD associated mutations or knockout versions of PD-implicated genes implicate alterations in mitochondrial biology, antioxidant defense mechanisms, the ubiquitin-proteasome pathway and autophagolysosome systems.
While human tissue, cybrid models and neurotoxin models of dopaminergic cell injury have long implicated mitochondrial pathobiology as a key mechanism in PD, the discovery of PTEN-induced kinase 1 as the gene mediating the PARK6 autosomal recessive locus added further impetus to this area of research (Valente et al. 2004). PINK1 is the first member of the large canonical serine/threonine (Ser/Thr) protein kinases shown to be regulated by a canonical N-terminal mitochondrial targeting sequence [reviewed in (Mills et al. 2008)]. Interestingly, other PD associated gene encoded proteins such as α-synuclein, LRRK2 and parkin show at least partial localization to mitochondria even though these proteins lack canonical mitochondrial targeting sequences (Darios et al. 2003; Biskup et al. 2006; Devi et al. 2008; Narendra et al. 2008). In the following sections, we will review evidence supporting the concept of impaired mitochondrial quality control as a point of convergence for multiple parkinsonian pathways, indicating a central role in PD pathogenesis.
The role of mitochondrial dysfunction in PD pathogenesis
Mitochondria play a central role in most eukaryotic metabolic processes by serving as the main generators of ATP, acting as calcium sinks for buffering intracellular calcium levels, and integrating multiple metabolic and apoptotic signaling pathways. Mitochondrial are dynamic organelles that exhibit bidirectional movement with the capacity to undergo fragmentation (fission) and elongation (fusion). These processes regulate neuronal survival, stability of the mitochondrial genome, mitochondrial transport, synaptogenesis, and functional refilling of the neurotransmitter vesicle reserve pool (Karbowski and Youle 2003; Li et al. 2004; Verstreken et al. 2005; Lu 2009). Mitochondrial fission is also an important step for the autophagic clearance of depolarized or damaged mitochondria, as overexpression of the fission mediator Drp1 promotes mitophagy (Twig et al. 2008).
There is growing consensus that mitochondrial dysfunction is a major contributor to the pathogenesis of PD. Midbrain dopaminergic neurons are particularly highly vulnerable to oxidative stress due to the highly oxidative nature of the dopamine biosynthetic pathways and their low mitochondrial reserve compared to other neuronal populations (Liang et al. 2007). In addition, the observation that exposure to certain mitochondrial complex I inhibitors recapitulates many symptoms of PD in humans and other mammalian species demonstrates a critical role of mitochondrial dysfunction in dopaminergic neuron dysfunction. Our laboratory and others have shown that exposure to parkinsonian neurotoxins lead to generation of mitochondrial superoxide, mitochondrial fission and swelling, mitochondrial dysfunction, activation of mitochondrial pools of mitogen activated protein kinases, mitochondrial autophagy resulting in degradation of outer membrane and inner membrane localized proteins, and nuclear depletion of prosurvival transcription factors (Heikkila and Cohen 1973; Cassarino et al. 1999; Zhu et al. 2002; Chalovich et al. 2006; Kulich et al. 2007; Zhu et al. 2007; Dagda et al. 2008; Gomez-Lazaro et al. 2008). Most of the mitochondrial deficits induced by exposure to neurotoxins are recapitulated in loss-of-function culture models of PINK1, Parkin and human high temperature requirement protein A2 (Omi/HtrA2) (Martins et al. 2004; Mortiboys et al. 2008; Dagda et al. 2009b; Dagda et al. 2009a; Flinn et al. 2009; Lutz et al. 2009), suggesting that PINK1, Parkin and Omi/HtrA2 normally function to promote maintenance of mitochondrial integrity.
Mitochondrial quality control pathways
The major mechanisms of mitochondrial quality control can be conceptualized in three major tiers. The first line of defense involves chaperones and prevention of mitochondrial injury and oxidative stress. The second involves localized degradation of damaged proteins by mitochondrial proteases, and ubiquitin-proteasomal degradation of certain outer membrane proteins (Tatsuta 2009; Tatsuta and Langer 2009). The third pathway is activated by more severe dysfunction and involves fission-based sequestration and autophagic clearance of damaged mitochondria. Each of these quality control steps would also be dependent upon effective biogenesis of new mitochondrial components (Cherra and Chu 2008). Since neurons are so highly dependent upon oxidative phosphorylation, it is likely that key regulators of mitochondrial quality control may regulate more than one of these tiers in order to effectively integrate cellular responses to achieve mitochondrial and neuronal homeostasis.
PD-linked genes: PINK1, HtrA2/Omi and Parkin
PINK1 was initially discovered to be mutated in two large consanguineous families of Spanish and Italian descent that were affected with early onset parkinsonism (Valente et al. 2004). With the exception of the N-terminal mitochondrial targeting region, more than 50 mutations of PINK1 have been mapped throughout the kinase and C-terminal regulatory domains with differential effects on kinase activity and/or protein stability, strongly implicating neuroprotective role for PINK1 [reviewed by (Mills et al. 2008)]. Different in vitro kinase studies have shown that PINK1 contains some degree of ser/thr kinase activity towards casein and myelin basic protein, two commonly used in vitro kinase substrates [reviewed by (Cookson et al. 2007)], although putative biological targets of PINK1 activity remain elusive.
There is a strong cyto-protective role of PINK1 in maintaining mitochondrial homeostasis via different mechanisms. Overexpression of wild-type PINK1 in SH-SY5Y neuroblastoma cells stabilizes respiring mitochondrial networks through various mechanisms that include maintaining mitochondrial membrane potential, reducing basal and neurotoxin-induced ROS, suppression of cytochrome c release, reversal of toxin-induced fission, and suppression of autophagy (Dagda et al. 2009b; Gegg et al. 2009; Sandebring et al. 2009). Adding to the multiplicity of prosurvival functions of PINK1, it has been shown to phosphorylate the mitochondrial molecular chaperone heat shock protein 75 kDa, also known as tumor necrosis factor receptor-associated protein-1 (TRAP1), increasing neuronal survival against oxidative stress or heat shock by preventing the release of cytochrome c (Pridgeon et al. 2007).
The consequences of stable loss of endogenous PINK1 have been extensively documented in mammalian cell models. Loss of PINK1 leads to severe alterations in mitochondrial homeostasis as evidenced by aberrations in mitochondrial cytoarchitecture (ie., reduced mitochondrial cristate density), mitochondrial dynamics, calcium homeostasis, biosynthetic pathways, and increased mitochondrial ROS inducing a robust increase in mitochondrial autophagy (mitophagy) (Exner et al. 2007; Wood-Kaczmar et al. 2008; Chu 2009; Dagda et al. 2009b; Gegg et al. 2009; Sandebring et al. 2009).
Omi/HtrA2 is a serine protease with an N-terminal mitochondrial targeting sequence (Strauss et al. 2005) that may represent an indirect target of PINK1 activity (Plun-Favreau et al. 2007). Omi/HtrA2, is an intermembrane space localized serine protease that activates proapoptotic proteins upon its release to the cytosol from damaged mitochondria. Pharmacological inhibition of Omi/HtrA2 increases survival of cells against oxidative stress(Hegde et al. 2002; van Loo et al. 2002). However, the view that Omi/HtrA2 is a proapoptotic protein has been challenged by recent experimental evidence showing that targeted disruption of Omi/HtrA2 leads to dopaminergic degeneration and motor function impairment in mice (Martins et al. 2004). Several protease inactivating mutations in Omi/HtrA2 have been identified as high risk factors for developing PD (Strauss et al. 2005). In other words, Omi/HtrA2 may act as a double-edged sword with both pro-survival and pro- death functions depending upon localization.
Lastly, Parkin is an E3 ubiquitin ligase and Parkin is the most common gene mutated in autosomal recessive familial parkinsonism. Parkin’s neuroprotective effects extend beyond its ability to mediate the ubiquitin mediated sequestration and degradation of protein aggregates (Olzmann and Chin 2008), as it is targeted to mitochondria promote autophagy of damaged mitochondria (Narendra et al. 2008), also functioning to increase mitochondrial biogenesis (Kuroda et al. 2006) and suppress mitochondrial oxidative stress through unknown mechanisms (Jiang et al. 2004).
The PINK1-Omi/HtrA2pathway
Recent experimental evidence supports an interaction of PINK1 and Omi/HtrA2 at the mitochondria. First, Omi/HtrA2 is indirectly phosphorylated and physically interacts with PINK1 in relation to a signaling pathway that involves p38 in mammalian cell models. PINK1 dependent phosphorylation of Omi/HtrA2 enhances its protease activity leading to enhanced survival against oxidative stress (Plun-Favreau et al. 2007). Secondly, Drosophila models have shown a genetic interaction of PINK1 and Omi/HtrA2, suggesting the possibility of a common prosurvival pathway (Whitworth et al. 2008; Tain et al. 2009). Overexpression of Omi/HtrA2can reverse phenotypic effects attributed to loss of PINK1 function while PINK1 cannot reverse degeneration in Omi/HtrA2 (Yun et al. 2008; Tain et al. 2009). Other genetic interaction studies carried out in Drosophila have placed Omi/HtrA2 downstream of PINK1, but in an independent pathway from the E3 ubiquitin ligase Parkin (Whitworth et al. 2008). It is worth noting that Omi/HtrA2 is not essential for all the protective functions of PINK1 in Drosophila (Yun et al. 2008; Tain et al. 2009), and recent experimental data suggest a non-linear pathway for PINK1-parkin as well (Chu 2009). Another mitochondrial protease rhomboid-7 has been implicated in post-translational regulation of both PINK1 and Omi/HtrA2 (Whitworth et al. 2008). We hypothesize that, in conjunction with chaperones and ATP dependent proteases, PINK1 may function in the first line of mitochondrial quality control, monitoring respiratory chain function and triggering the localized degradation of improperly folded or assembled mitochondrial proteins.
The PINK1-Parkin pathway
There is ample evidence establishing direct or indirect interactions of PINK1 with Parkin in promoting mitochondrial homeostasis. The pathology attributed to loss of PINK1 function can be reversed in mammalian and Drosophila models by ectopic expression of Parkin (Exner et al. 2007; Deng et al. 2008; Poole et al. 2008; Whitworth et al. 2008; Dagda et al. 2009b). Furthermore, direct interaction of Parkin with PINK1 has been documented by several groups (Kim et al. 2008; Xiong et al. 2009). Parkin can be phosphorylated by PINK1 in its RING finger domain during in vitro kinase reactions, which promotes translocation of Parkin to mitochondria (Kim et al. 2008). Furthermore, Parkin has been reported to facilitate the selective clearance of depolarized mitochondria via autophagy (Narendra et al. 2008).
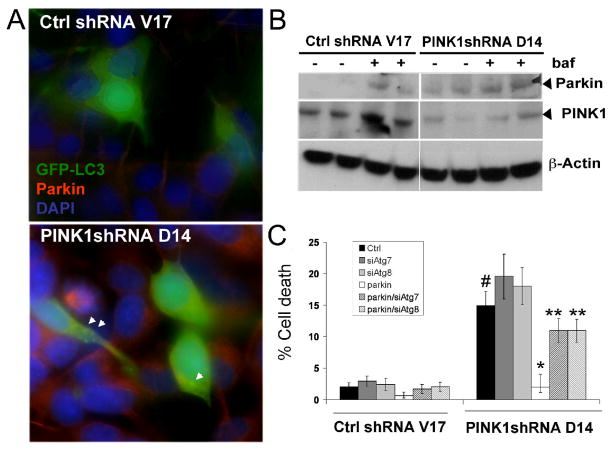
Hypothetically, compensations for PINK1 loss may include increased expression of TRAP1, HtrA2, Parkin, chaperones, antioxidants or enhanced autophagolysosomal efficiency. The spontaneous autophagic response observed in PINK1 shRNA lines could be associated with increased Parkin levels, as endogenous Parkin expression is slightly increased in some PINK1 deficient lines (Fig. 1AB). The degree of endogenous Parkin upregulation and mitophagy induction in clonal SH-SY5Y lines is insufficient, however, to completely prevent the enhanced cell death exhibited by PINK1 shRNA lines (Dagda et al. 2009b). Transient overexpression of Parkin further augments mitochondrial autophagy in PINK1 deficient neuroblastoma cells, resulting in cytoprotection and restoration of interconnected mitochondrial networks (Dagda et al. 2009b). In this model system, the protective effects of overexpressed Parkin are predominantly mediated through autophagy, as RNAi knockdown of either Atg7 or Atg8, two specific components of the autophagy machinery, significantly abrogates Parkin’s ability to compensate for PINK1 deficiency (Fig. 1C).
Figure 1. Autophagy contributes a key role to Parkin neuroprotection in PINK1-deficient SH-SY5Y cells, which exhibit compensatory upregulation of endogenous Parkin.
A. SH-SY5Y clonal lines transiently transfected with GFP-LC3 two days prior were fixed in paraformaldehyde, immunostained for endogenous Parkin (1:500 dilution; Covance) and counterstained for nuclei using DAPI (1.25μg/ml; blue channel). All conditions were photographed using an Olympus IDX71 epifluorescence microscope using the same settings. Note the increased expression of endogenous Parkin in the PINK1 knockdown cell line (bottom panel, red channel) compared to the control cell line (top panel, red channel). All cells in the bottom panel express PINK1 shRNA and nearly all show increased endogenous parkin, but only a subset of cells have been transfected with GFP-LC3 (green channel), revealing increased puncta indicative of autophagosomes (arrowheads). See also print version for individual channels in greyscale.
B. Stable vector control SH-SY5Y cell line and a PINK1 knockdown stable cell line were treated with DMSO or with 20nM bafilomycin to arrest autophagic degradation for 4 hrs. Cell lysates were resolved on a 5–15% Ammediol-buffered gel and immunoblotted for PINK1 (1:2000, C8830) or Parkin (1:1000 dilution; Covance), then stripped and re-probed for β-actin as loading control. Note that both Parkin and PINK1 levels within the respective cell lines are increased by bafilomycin, implicating lysosomal turnover. Moreover, stable knockdown of PINK1 leads to increased Parkin levels, which are nearly undetectable in control cells unless autolysosomal degradation is inhibited.
C. Stable vector control shRNA or PINK1shRNA clonal cell lines were transiently co-transfected with GFP and control vector or HA-Parkin plasmids and with either scrambled siRNA control or siRNA directed against the autophagy proteins Atg7 or Atg8. Small siRNAs are introduced with >93% efficiency into GFP-co-expressing SH-SY5Y cells (Plowey et al. 2008). Three days after transient transfection, the percentage of transfected GFP-positive cells with apoptotic nuclei was determined as a measure of cell death. (#:p<0.0001 vs. Ctrl shRNA/Ctrl vector; *:p<0.0001 vs. PINK1shRNA/Ctrl vector; **:p<0.001 vs. PINK1shRNA/Parkin; means ± S.E, n=7–10 random fields containing 20–40 transfected cells quantified per condition).
To summarize, while loss of either PINK1 or Parkin leads to DRP1-dependent fragmentation of mitochondria (Dagda et al. 2009b; Lutz et al. 2009; Sandebring et al. 2009), the mechanisms by which wild type PINK1 and Parkin promote interconnected mitochondrial networks may involve different steps in mitochondrial quality control, with autophagy being harnessed by Parkin to compensate for PINK1 deficiency.
Non-linear and redundant pathways to mitochondrial health
The observation that loss of function of these three different PD-implicated proteins lead to similar mitochondrial alterations and degenerative phenotypes in Drosophila and in mammalian models has raised the possibility that Parkin, PINK1 and Omi/HtrA2 could participate in a common mitoprotective signaling pathway. However, a growing body of literature indicates that the interaction among these proteins is a more complex scenario than initially conceived (Yun et al. 2008; Chu 2009), particularly as the important cellular process of mitochondrial quality control involves many layers and probable redundancies (Tatsuta 2009; Tatsuta and Langer 2009).
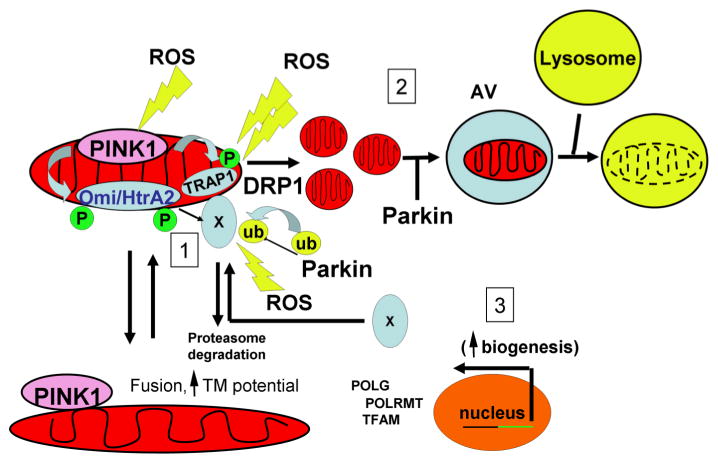
Based upon existing information discussed above, including the ability of PINK1 to regulate phosphorylation of TRAP1, HtrA2 and Parkin, we propose the following as a framework for future testing. Localized or mild mitochondrial injury, as may be encountered as a side effect of oxidative phosphorylation or transient heat shock, may be countered by phosphorylation of the chaperone TRAP1 or by triggering localized degradation of intermembrane space and matrix proteins by proteases such as HtrA2/Omi. However, a greater degree of mitochondrial injury may require organelle-level responses including Drp1-dependent fission and Parkin-facilitated mitochondrial autophagy (Figure 2). Mitochondrial biogenesis, which may also be regulated by PINK1 or Parkin, would be essential for success of these degradative quality control pathways, and thus, excessive rates of mitochondrial autophagy that exceed regenerative capacity may ultimately prove harmful (Cherra and Chu 2008). Finally, severe mitochondrial injury causing release of Omi/HtrA2 into the cytoplasm could convert this protein into a death mediator, in analogy to cytochrome c, as a response to irreparable cellular damage. Future experimental work would be needed to validate aspects of this proposed framework, filling in conceptual gaps towards a better understanding of the mitochondria protective roles play by these PD-associated gene products.
Figure 2. Schematic diagram highlighting a proposed framework for the roles of PINK1, Parkin and Omi/HtrA2 in mediating mitochondrial homeostasis.
PINK1 directly or indirectly promotes phosphorylation of TRAP1, HtrA2 and parkin, resulting in activation of both localized and organelle level responses to promote neuronal cell survival.
(1) A mild to modest localized stress to mitochondrial proteins (one yellow bolt) activates the PINK1-TRAP1 and PINK1-Omi/HtrA2 pathways, stabilizing the mitochondrial cytoarchitecture in response to oxidative stress and heat shock. Another response to localized outer membrane damage may involve tagging of damaged proteins for proteasomal degradation by ubiquitin ligases including Parkin, Mitochondrial fusion may act to stabilize membrane potential, diluting damaged DNA or other components.
(2) More extensive damage from persistent mitochondrial ROS (two yellow bolts) may be caused by loss of PINK1 function and/or environmental toxins, leading to compensatory increases in Parkin expression and mitochondrial fission. Parkin participates in a parallel pro-survival pathway, compensating for damaged mitochondrial by driving selective autophagy of depolarized segments isolated by mitochondrial fission. This may also serve to limit release of apoptogenic intermembrane space proteins.
(3) Successful neuronal adaptation to stress employing either of these parallel pathways would require effective transcriptional responses and reassembly of functional mitochondrial components. Nuclear encoded proteins are essential for both mitochondrial DNA replication (POLG) and transcription of mitochondrially encoded genes (POLRMT, TFAM, etc), along with coordinated synthesis, import and processing or assembly of nuclear encoded mitochondrial proteins.
Under conditions of PINK1 loss of function, alternative means to activate mitochondrial quality control pathways may serve to compensate for increased mitochondrial injury, although likely at a greater bioenergetic cost.
Acknowledgments
The authors’ research is supported in part by funding from the National Institutes of Health (AG026389; AG026389-03S1). CTC is a Julie Martin Mid-Career Awardee in Aging Research supported by The Ellison Medical Foundation and AFAR. RKD was supported in part by F32 AG030821.
References
- 1.Betarbet R, Sherer TB, Di Monte DA, Greenamyre JT. Mechanistic approaches to Parkinson’s disease pathogenesis. Brain Pathol. 2002;12:499–510. doi: 10.1111/j.1750-3639.2002.tb00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 3.Bogaerts V, Theuns J, van Broeckhoven C. Genetic findings in Parkinson’s disease and translation into treatment: a leading role for mitochondria? Genes Brain Behav. 2008;7:129–151. doi: 10.1111/j.1601-183X.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassarino DS, Parks JK, Parker WD, Jr, Bennett JP., Jr The parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim Biophys Acta. 1999;1453:49–62. doi: 10.1016/s0925-4439(98)00083-0. [DOI] [PubMed] [Google Scholar]
- 5.Chalovich EM, Zhu JH, Caltagarone J, Bowser R, Chu CT. Functional repression of cAMP response element in 6-hydroxydopamine-treated neuronal cells. J Biol Chem. 2006;281:17870–17881. doi: 10.1074/jbc.M602632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherra SJ, Chu CT. Autophagy in neuroprotection and neurodegeneration: A question of balance. Future Neurol. 2008;3:309–323. doi: 10.2217/14796708.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu CT. Tickled PINK1: Mitochondrial homeostasis and autophagy in recessive Parkinsonism. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbadis.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cookson MR, Dauer W, Dawson T, Fon EA, Guo M, Shen J. The roles of kinases in familial Parkinson’s disease. J Neurosci. 2007;27:11865–11868. doi: 10.1523/JNEUROSCI.3695-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dagda RK, Zhu J, Chu CT. Mitochondrial kinases in Parkinson’s disease: converging insights from neurotoxin and genetic models. Mitochondrion. 2009a;9:289–298. doi: 10.1016/j.mito.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dagda RK, Zhu J, Kulich SM, Chu CT. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: implications for Parkinson’s disease. Autophagy. 2008;4:770–782. doi: 10.4161/auto.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagda RK, Cherra SJ, 3rd, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009b;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darios F, Corti O, Lucking CB, Hampe C, Muriel MP, Abbas N, Gu WJ, Hirsch EC, Rooney T, Ruberg M, Brice A. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum Mol Genet. 2003;12:517–526. doi: 10.1093/hmg/ddg044. [DOI] [PubMed] [Google Scholar]
- 13.Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Exner N, Treske B, Paquet D, Holmstrom K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D, Hoepken HH, Gasser T, Kruger R, Winklhofer KF, Vogel F, Reichert AS, Auburger G, Kahle PJ, Schmid B, Haass C. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flinn L, Mortiboys H, Volkmann K, Koster RW, Ingham PW, Bandmann O. Complex I deficiency and dopaminergic neuronal cell loss in parkin-deficient zebrafish (Danio rerio) Brain. 2009;132:1613–1623. doi: 10.1093/brain/awp108. [DOI] [PubMed] [Google Scholar]
- 17.Gegg ME, Cooper JM, Schapira AH, Taanman JW. Silencing of PINK1 expression affects mitochondrial DNA and oxidative phosphorylation in dopaminergic cells. PLoS One. 2009;4:e4756. doi: 10.1371/journal.pone.0004756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez-Lazaro M, Bonekamp NA, Galindo MF, Jordan J, Schrader M. 6-Hydroxydopamine (6-OHDA) induces Drp1-dependent mitochondrial fragmentation in SH-SY5Y cells. Free Radic Biol Med. 2008;44:1960–1969. doi: 10.1016/j.freeradbiomed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Hegde R, Srinivasula SM, Zhang Z, Wassell R, Mukattash R, Cilenti L, DuBois G, Lazebnik Y, Zervos AS, Fernandes-Alnemri T, Alnemri ES. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J Biol Chem. 2002;277:432–438. doi: 10.1074/jbc.M109721200. [DOI] [PubMed] [Google Scholar]
- 20.Heikkila RE, Cohen G. 6-Hydroxydopamine: evidence for superoxide radical as an oxidative intermediate. Science (New York, NY) 1973;181:456–457. doi: 10.1126/science.181.4098.456. [DOI] [PubMed] [Google Scholar]
- 21.Jiang H, Ren Y, Zhao J, Feng J. Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum Mol Genet. 2004;13:1745–1754. doi: 10.1093/hmg/ddh180. [DOI] [PubMed] [Google Scholar]
- 22.Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell death and differentiation. 2003;10:870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Park J, Kim S, Song S, Kwon SK, Lee SH, Kitada T, Kim JM, Chung J. PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem Biophys Res Commun. 2008;377:975–980. doi: 10.1016/j.bbrc.2008.10.104. [DOI] [PubMed] [Google Scholar]
- 24.Kulich SM, Horbinski C, Patel M, Chu CT. 6-Hydroxydopamine induces mitochondrial ERK activation. Free Radic Biol Med. 2007;43:372–383. doi: 10.1016/j.freeradbiomed.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuroda Y, Mitsui T, Kunishige M, Shono M, Akaike M, Azuma H, Matsumoto T. Parkin enhances mitochondrial biogenesis in proliferating cells. Hum Mol Genet. 2006;15:883–895. doi: 10.1093/hmg/ddl006. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Liang CL, Wang TT, Luby-Phelps K, German DC. Mitochondria mass is low in mouse substantia nigra dopamine neurons: implications for Parkinson’s disease. Exp Neurol. 2007;203:370–380. doi: 10.1016/j.expneurol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Lu B. Mitochondrial dynamics and neurodegeneration. Curr Neurol Neurosci Rep. 2009;9:212–219. doi: 10.1007/s11910-009-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lammermann K, Brunner B, Kurz-Drexler A, Vogel F, Reichert AS, Bouman L, Vogt-Weisenhorn D, Wurst W, Tatzelt J, Haass C, Winklhofer KF. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem. 2009;284:22938–22951. doi: 10.1074/jbc.M109.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martins LM, Morrison A, Klupsch K, Fedele V, Moisoi N, Teismann P, Abuin A, Grau E, Geppert M, Livi GP, Creasy CL, Martin A, Hargreaves I, Heales SJ, Okada H, Brandner S, Schulz JB, Mak T, Downward J. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol. 2004;24:9848–9862. doi: 10.1128/MCB.24.22.9848-9862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills RD, Sim CH, Mok SS, Mulhern TD, Culvenor JG, Cheng HC. Biochemical aspects of the neuroprotective mechanism of PTEN-induced kinase-1 (PINK1) J Neurochem. 2008;105:18–33. doi: 10.1111/j.1471-4159.2008.05249.x. [DOI] [PubMed] [Google Scholar]
- 32.Mortiboys H, Thomas KJ, Koopman WJ, Klaffke S, Abou-Sleiman P, Olpin S, Wood NW, Willems PH, Smeitink JA, Cookson MR, Bandmann O. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann Neurol. 2008;64:555–565. doi: 10.1002/ana.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olzmann JA, Chin LS. Parkin-mediated K63-linked polyubiquitination: a signal for targeting misfolded proteins to the aggresome-autophagy pathway. Autophagy. 2008;4:85–87. doi: 10.4161/auto.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plun-Favreau H, Klupsch K, Moisoi N, Gandhi S, Kjaer S, Frith D, Harvey K, Deas E, Harvey RJ, McDonald N, Wood NW, Martins LM, Downward J. The mitochondrial protease HtrA2 is regulated by Parkinson’s disease-associated kinase PINK1. Nat Cell Biol. 2007;9:1243–1252. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
- 36.Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 Protects against Oxidative Stress by Phosphorylating Mitochondrial Chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandebring A, Thomas KJ, Beilina A, van der Brug M, Cleland MM, Ahmad R, Miller DW, Zambrano I, Cowburn RF, Behbahani H, Cedazo-Minguez A, Cookson MR. Mitochondrial alterations in PINK1 deficient cells are influenced by calcineurin-dependent dephosphorylation of dynamin-related protein 1. PLoS One. 2009;4:e5701. doi: 10.1371/journal.pone.0005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, Gasser T, Wszolek Z, Muller T, Bornemann A, Wolburg H, Downward J, Riess O, Schulz JB, Kruger R. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum Mol Genet. 2005;14:2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- 40.Tain LS, Chowdhury RB, Tao RN, Plun-Favreau H, Moisoi N, Martins LM, Downward J, Whitworth AJ, Tapon N. Drosophila HtrA2 is dispensable for apoptosis but acts downstream of PINK1 independently from Parkin. Cell death and differentiation. 2009;16:1118–1125. doi: 10.1038/cdd.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tatsuta T. Protein quality control in mitochondria. J Biochem. 2009;146:455–461. doi: 10.1093/jb/mvp122. [DOI] [PubMed] [Google Scholar]
- 42.Tatsuta T, Langer T. AAA proteases in mitochondria: diverse functions of membrane-bound proteolytic machines. Res Microbiol. 2009 doi: 10.1016/j.resmic.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science (New York, NY) 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 45.van Loo G, van Gurp M, Depuydt B, Srinivasula SM, Rodriguez I, Alnemri ES, Gevaert K, Vandekerckhove J, Declercq W, Vandenabeele P. The serine protease Omi/HtrA2 is released from mitochondria during apoptosis. Omi interacts with caspase-inhibitor XIAP and induces enhanced caspase activity. Cell death and differentiation. 2002;9:20–26. doi: 10.1038/sj.cdd.4400970. [DOI] [PubMed] [Google Scholar]
- 46.Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 47.Whitworth AJ, Lee JR, Ho VM, Flick R, Chowdhury R, McQuibban GA. Rhomboid-7 and HtrA2/Omi act in a common pathway with the Parkinson’s disease factors Pink1 and Parkin. Dis Model Mech. 2008;1:168–174. doi: 10.1242/dmm.000109. discussion 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood-Kaczmar A, Gandhi S, Yao Z, Abramov AS, Miljan EA, Keen G, Stanyer L, Hargreaves I, Klupsch K, Deas E, Downward J, Mansfield L, Jat P, Taylor J, Heales S, Duchen MR, Latchman D, Tabrizi SJ, Wood NW. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE. 2008;3:e2455. doi: 10.1371/journal.pone.0002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong H, Wang D, Chen L, Choo YS, Ma H, Tang C, Xia K, Jiang W, Ronai Z, Zhuang X, Zhang Z. Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J Clin Invest. 2009;119:650–660. doi: 10.1172/JCI37617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yun J, Cao JH, Dodson MW, Clark IE, Kapahi P, Chowdhury RB, Guo M. Loss-of-function analysis suggests that Omi/HtrA2 is not an essential component of the PINK1/PARKIN pathway in vivo. J Neurosci. 2008;28:14500–14510. doi: 10.1523/JNEUROSCI.5141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu J-H, Kulich SM, Oury TD, Chu CT. Cytoplasmic aggregates of phosphorylated extracellular signal-regulated kinase in Lewy body diseases. The American journal of pathology. 2002;161:2087–2098. doi: 10.1016/S0002-9440(10)64487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. The American journal of pathology. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]