Abstract
Rationale
It is known that dopamine (DA) D1 receptor activation stimulates striatal nitric oxide (NO) synthesis, whereas D2 receptor activation produces the opposite effect. However, the mechanisms involved in the dopaminergic modulation of NO synthase (NOS) are unknown.
Objectives
We hypothesized that the effects of DA on striatal NO signaling are dependent on ongoing glutamatergic activation of NOS. Therefore, the current study examined whether intact NMDA receptor activation is required for the dopaminergic modulation of NOS activity.
Methods
We assessed the impact of pharmacological manipulations of D1, D2 and NMDA receptors on NOS activity in the dorsal striatum and motor cortex using nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d) histochemistry. Drugs were administered systemically to conscious animals and NADPH-d staining was quantified in these regions using ex vivo measurements of tissue optical density.
Results
Administration of the neuronal NOS inhibitor NG-propyl-L-arginine (NPA), the D1 receptor antagonist SCH 23390, and the NMDA receptor antagonist 3-phosphonopropyl-piperazine-2-carboxylic acid (CPP) all attenuated staining selectively in the striatum. Administration of the D2 receptor agonist quinpirole decreased NADPH-d staining in both the striatum and cortex. Striatal NADPH-d staining elicited by administration of the D1 receptor agonist SKF 81297 or the D2 receptor antagonist eticlopride was attenuated by NPA, SCH 23390, and CPP pretreatment. Quinpirole pretreatment also abolished the facilitatory effect of SKF 81297.
Conclusions
These studies show for the first time that ongoing NMDA receptor activation is necessary for modulation of striatal NOS activity by both facilitatory (D1 receptor activation) and inhibitory (D2 receptor activation) dopaminergic signaling mechanisms.
Keywords: dopamine, nitric oxide, nitric oxide synthase, NMDA receptor, striatum, cortex
Nitric oxide (NO) is a key modulator of neuronal activity in the dorsal striatum and is thought to play an important role in complex processes including control of motor function and motivated behavior (Prast and Philippu 2001; West et al. 2002; Del Bel et al. 2005). NO is synthesized within medium-sized aspiny interneurons by type-1/neuronal NO synthase (nNOS). Striatal nNOS expressing interneurons and their processes are readily labeled using nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d) histochemical staining techniques (Hope et al. 1991; Kawaguchi 1993; Kharazia et al. 1994; Vincent and Kimura 1992). Although nNOS interneurons comprise only 1–2% of the neuronal population in the striatal complex (Bredt and Snyder 1991), they give rise to extensive axonal projections. Once synthesized, NO diffuses from nNOS interneurons to neighboring neurons and blood vessels where it activates soluble guanylyl cyclase to produce the second messenger cGMP (Garthwaite 2008). Striatal NO-cGMP signaling has numerous effects on protein kinases and phosphodiesterases (Greengard et al. 1999), which ultimately modulate neuronal membrane excitability and corticostriatal transmission (West and Grace 2004; Calabresi et al. 2007).
Under physiological conditions, nNOS synthesizes NO in a calcium/calmodulin-dependent manner (Marletta 1994). This reaction uses β-nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor and drives the conversion of L-arginine and oxygen to citrulline and NO (Garthwaite 1991). NADPH-d activity arises from the catalytic domain of the nNOS enzyme (Dawson et al. 1991; Hope et al. 1991; Griffith and Stuehr 1995), which converts the substrate nitroblue tetrazolium to a formazan salt in a manner that accurately reflects nNOS enzyme activity in the dorsal striatum (Morris et al. 1997; Sancesario et al. 2004; Fernandez et al. 2005).
Striatal nNOS interneurons receive asymmetric synapses from glutamatergic afferents (Salin et al. 1990; Vuillet et al. 1989) and express N-methyl-D-aspartic acid (NMDA), α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) and metabotrophic glutamate receptors (Gracy and Pickle 1997; Kawaguchi 1997; Nishi et al. 2005). Work in our lab using NO-selective microsensors has shown that striatal NO efflux is robustly increased in vivo by electrical stimulation of corticostriatal afferents via an NMDA receptor- and nNOS-dependent mechanism (Sammut et al. 2007b). Intrastriatal infusion of NMDA has also been shown to activate NO efflux in vivo (Crespi and Rossetti 2004; Iravani et al. 1988; Rossetti and Crespi 2004), indicating that NMDA receptors play a primary role in stimulating nNOS activity.
In addition to glutamatergic inputs, striatal nNOS expressing interneurons are robustly innervated by dopaminergic pathways to the striatum (Kerkerian et al. 1996; Fujiyama and Masuko 1996; Meredith and Totterdell 1999; Hidaka and Totterdell 2001). It is also clear that striatal nNOS interneurons express dopamine (DA) D1 mRNA and D1/5 receptor protein (Le et al. 1991; Centonze et al. 2003; Rivera et al. 2002). Additionally, electrical and chemical stimulation of the substantia nigra and systemic D1 receptor agonist administration were all observed to increase striatal NO efflux via nNOS and D1 receptor-dependent mechanisms (Sammut et al. 2006). Furthermore, the facilitatory effects of D1 receptor agonist on striatal NO synthesis were attenuated by D2 receptor agonist, whereas administration of D2 receptor antagonist augmented NOS activity (Altar et al. 1990; Morris et al. 1997; Di Stefano et al. 2005; Siuciak et al. 2006; Sammut et al. 2007a).
Taken together, the above studies indicate that DA modulates nNOS activity in the dorsal striatum via both facilitatory (D1 receptor activation) and inhibitory (D2 receptor activation) signaling. However, the mechanisms underlying the modulatory influence of D1 and D2 receptor activation on NO synthesis are currently unknown. Interestingly, numerous findings indicate that D1 receptor activation potentiates NMDA-induced responses in striatal neurons (Cepeda and Levine 1998; 2006). Previous reports using NADPH-d histochemistry have shown that both NMDA and D1 receptor antagonists and striatal 6-OHDA lesions decrease NOS activity measured ex vivo (Morris et al. 1997; Sancesario et al. 2004). Thus, it is likely that D1 receptor facilitation of NMDA receptor activity may play a critical role in upregulating striatal NO synthesis. Since D2 heteroreceptors are localized on glutamate terminals (Wang and Pickel 2002) and decrease terminal excitability (Garcia-Munoz et al. 1991) and transmitter release (Maura et al. 1989; Bamford et al. 2004), we hypothesized that the inhibitory effect of D2 receptor activation on NO signaling may be mediated via suppression of glutamatergic activation of nNOS interneurons. As a first step towards testing these hypotheses, the current studies examined whether intact NMDA receptor activation is required for the dopaminergic modulation of striatal nNOS activity.
Materials and Methods
Chemicals
Chloral hydrate, the D1 receptor agonist R-(+)-SKF 81297 hydrobromide (SKF 81297), the D1 receptor antagonist R-(+)-SCH 23390 hydrochloric acid (SCH 23390), the D2 receptor agonist (−)-quinpirole hydrochloric acid (QNP), the D2 receptor antagonist S-(−)-eticlopride hydrochloric acid (ETI), and reduced NADPH were purchased from Sigma-RBI (St. Louis, MO). The selective nNOS inhibitor NG-propyl-L-arginine (NPA), the selective NMDA receptor antagonist 3-((±)2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP), and nitroblue tetrazolium were purchased from Tocris (Ellisville, MO) All other reagents were of the highest grade commercially available.
Animals
Histochemical measurements were taken from a total of 92 adult male Sprague-Dawley (Harlan, Indianapolis, IN) rats weighing 252–330 grams. Rats were housed two-per cage under conditions of constant temperature (21–23°C) and maintained on a 12:12 hour light/dark cycle with food and water available ad libitum. All animal procedures were approved by the Rosalind Franklin University of Medicine and Science Institutional Animal Care and Use Committee and adhere to the Guide for the Care and Use of Laboratory Animals published by the USPHS.
Drug Preparation and Administration
All drugs were dissolved in vehicle consisting of physiological saline (SAL, 0.9% NaCl) and administered using intraperitoneal delivery. The selective nNOS inhibitor NPA has been shown to exhibit a relatively low Ki for inhibiting nNOS (57 nM), as well as a superior selectivity factor as compared to other NOS inhibitors (Zhang et al. 1997). In a previous study using NO microsensor recordings we found that systemic administration of NPA (10 mg/kg, i.p.) decreased cortically-evoked NO efflux by approximately 50% (Sammut et al. 2007b). Similar effects were observed using two other NOS inhibitors (Sammut et al. 2007b). Other studies performed in intact animals have used NPA at doses ranging from 1–20 mg/kg (Ma et al. 2003; Atalla and Kuschinsky 2006). Based on outcomes from these reports, the current studies with NPA were performed using a dose of 20 mg/kg.
Studies using reduced preparations have shown that the selective D1/5 receptor antagonist SCH 23390 and the selective D1/5 receptor agonist SKF 81297 both exhibit relatively low (Ki for SCH 23390= 0.2–0.3 nM, Ki for SKF 81297= 1.0 nM) dissociation constants (Seeman and Van Tol 1994). Based on our previous in vivo studies (Sammut et al. 2006; Sammut et al. 2007a) and reports by other laboratories which examined the electrophysiological effects of these drugs on striatal spiny neurons (Gonon 1997; Floresco et al. 2001a;b; Reynolds et al. 2001), the current studies with SCH 23390 and SKF 81297 were performed using a dose of 500μg/kg. The D2 receptor ligands used in these studies are also well characterized. The selective D2-like receptor antagonist ETI and the selective D2-like receptor agonist QNP both exhibit relatively low (Ki for ETI= 0.17 nM, Ki for QNP= 5–30 nM) dissociation constants (Seeman and Van Tol 1994; Hall et al. 1985). We have shown in a previous study that when co-administered at doses of 100μg/kg, ETI blocks the inhibitory effects of QNP on NO efflux elicited via D1/5 receptor activation (Sammut et al. 2007a). Therefore, we used this same dose in the current studies with ETI and QNP. CPP is a non-competitive NMDA receptor antagonist which possesses good selectivity and a relatively high (Ki for CPP=7.3 μM) dissociation constant (Tacconi et al. 1993). In the current study CPP was administered using a dose of 1 mg/kg. Previous studies by Floresco and colleagues have shown that this dose of CPP effectively blocks the facilitatory effects of glutamatergic afferents on spiny neurons recorded in the nucleus accumbens (Floresco et al. 2001a;b).
On the day of drug administration, animals were weighed and allowed to acclimate in the home cage for at least 10 min prior to injection of drug or SAL vehicle. In initial studies rats were administered SKF 81297 or SAL alone to determine if D1 agonism alters striatal NADPH-d staining. In subsequent studies, all rats were administered one of the following injection sequences (1 ml/kg, i.p.). Experiment 1: 1) SAL/SAL, 2) NPA/SAL, 3) SCH 23390/SAL, 4) CPP/SAL, 5) SAL/SKF 81297, 6) NPA/SKF 81297, 7) SCH 23390/SKF 81297, and 8) CPP/SKF 81297. Experiment 2: 1) QNP/SAL, 2) SAL/ETI, 3) NPA/ETI, 4) QNP/ETI, and 5) CPP/ETI. Experiment 3: 1) SAL/SAL, 2) SAL/SKF 81297, 3) QNP/SAL, 4) QNP/SKF 81297. All rats were returned to their home cage immediately after administration of the first vehicle/drug treatment. Twenty min later, rats were given the second injection of drug or vehicle and returned to their home cage. Twenty five min later, rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) and decapitated (5 min delay). Brains were processed as described below. No significant difference in NADPH-d staining was observed between treatment groups receiving SKF 81297 alone or after pretreatment with SAL (data not shown), thus the data were pooled and are represented in all results/figures as a single SAL/SKF 81297 treatment group.
NADPH-diaphorase histochemistry and histology
NADPH-d staining was performed as described previously with minor modifications (Johnson and Ma 1993; Sancesario et al 2004; Sammut et al. 2007b). Brains were extracted and immersed in a 30% sucrose/10% formalin solution for 24 hours, then frozen over dry ice and stored at −80°C. Next, brains were cut on a cryostat to obtain 40μm thick coronal sections. Serial, coronal sections were then mounted on gelatinized slides, rinsed 3 times in 0.1 mol/L phosphate buffered SAL, washed in phosphate buffered SAL containing 0.25% Triton X-100, and incubated at room temperature on a rotary shaker for 5 min, followed by incubation at 37°C for 60 min in phosphate buffered SAL/Triton solution containing 0.2 mg/mL NADPH and 0.25 mg/mL nitroblue tetrazolium. Slides were then rinsed, dehydrated and coverslipped.
In the current study, several steps were taken to minimize variation in stain intensity and maximize the signal to noise ratio of the NADPH-d reaction: 1) All brains were processed in an identical manner (e.g., standardized drug exposure and incubation times for all rats/tissue) and analyzed by a single investigator, 2) all sections from a given brain were sliced and stained on the same day in a random order using identical solutions in an environmentally controlled room, and 3) tissue samples from different animals were yoked so that samples from different treatment groups were always sliced and processed on the same day using the same procedures/solutions. This allowed us to compare outcomes from tissue samples (processed in an identical manner on the same day) derived from animals receiving treatments that were hypothesized to have either facilitatory (e.g., SAL/SKF 81297) or inhibitory (e.g., CPP/SAL) effects (or no effect in the case of vehicle (SAL/SAL)) on NOS activity.
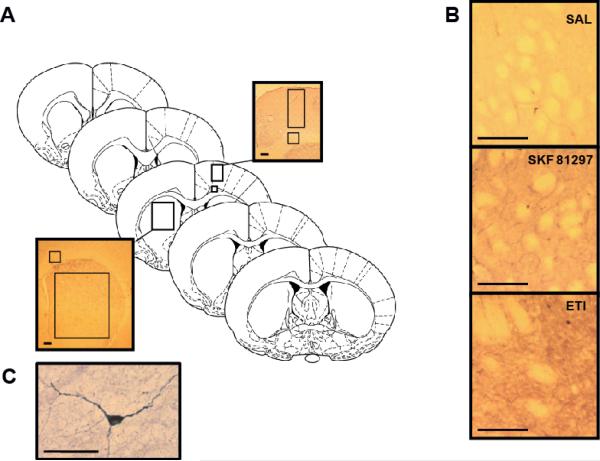
In order to produce accurate and non-biased sampling of striatal subregions, five equidistant coronal sections were chosen from each brain (Fig 1a), which corresponded to stereotaxic coordinates representative of the rostral and central dorsal striatum (+1.7, +1.2, +0.7, +0.2, −0.26 from bregma as determined using the atlas of Paxinos and Watson (1986)). Stained slides were viewed at 2.5 × magnification on a Ziess AxioImager A1 microscope (Zeiss, Oberkochen, Germany) and photographed with a Cannon EOS40D digital camera (Cannon, Tokyo, Japan). All photographs were taken under constant controlled light, with uniform camera and microscope settings.
Figure 1. Quantification of NADPH-d staining in striatum and cortex.
A) For both cortex and striatum, the optical density of the region of interest (boxed area) in the right and left hemispheres of each of the five representative coronal sections was measured. Insets show representative staining in examined regions of cortex (top) and striatum (bottom). The corpus callosum was used as a comparison area for the generation of measures of non-specific staining. Optical Density values measured in the dorsal striatum or motor cortex were subtracted from those measured in the corpus callosum to give a relative optical density value (Diagram is derived from the atlas of Paxinos and Watson, 1986). B) Examples of typical NADPH-d staining observed in SAL/SAL treated animals (top), SAL/SKF 81297 treated animals (middle) and SAL/ETI treated animals (bottom). Images were photographed at 10 × magnification. All scale bars represent 250 μm. C) An example of a typical NADPH-d stained interneuron observed in the striatum of a SAL/SAL treated animal. Scale bar represents 50 μm.
Data Analysis
The optical density of areas containing nNOS cell bodies, dendrites and axons was measured using Image J software (NIH) as described previously (Fernandez et al. 2005) and indicated in Fig 1. Sampled striatal area consisted of a box measuring 2.02 mm by 1.35 mm and sampled corpus callosum area consisted of a box measuring 0.27 mm by 0.27 mm. Optical density of selected subregions was measured on a scale ranging from 0 to 255 (0 representing the darkest labeling). Average background staining was measured in the white matter of the corpus callosum and average striatal and cortical optical density values were subtracted from these background values. Measures were obtained from each subregion (i.e. striatum and cortex) within both the right and left hemispheres of all five coronal sections and averaged to give a value for each subregion (Fig 1a,b). In addition, slides from each treatment group were tested for uniform light transmission through the blank portions of the slide to ensure consistency within measures across groups. The intensity of staining in each subregion was expressed as the mean relative optical density ± SEM. The statistical significance of drug-induced changes in measures of NADPH-d staining and interactions between drug pretreatment and treatment was determined using a two-way ANOVA with an all pair-wise Bonferroni post-hoc test.
Results
Effects of D1 receptor agonist administration on NADPH-d staining: Impact of inhibitor/antagonist pretreatments
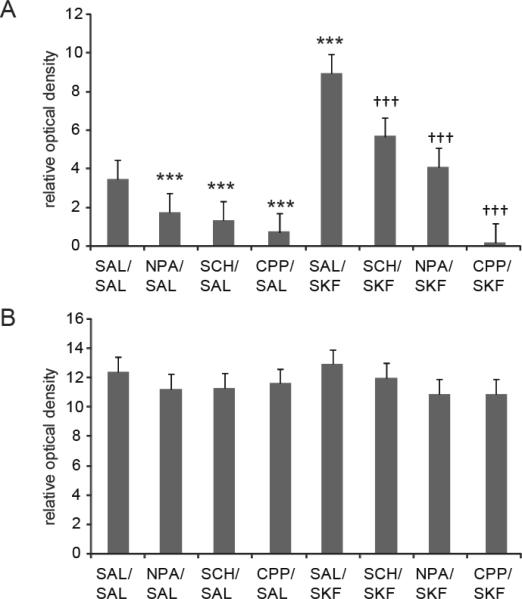
The current study examined the impact of SAL (vehicle) or D1 receptor agonist (SKF 81297) treatment on striatal and cortical NADPH-d staining in animals pretreated with either SAL or drug (i.e., NPA, SCH 23390, or CPP). In studies using striatal tissue, a main effect of SKF 81297 treatment was observed (F (1, 53) = 34.681, p<0.001) indicating that D1 receptor agonism increases NADPH-d staining in this region relative to SAL treated controls (Fig 2a). Significant main effects of drug pretreatment on striatal NADPH-d staining were also observed (F (3, 53) = 29.366, p<0.001). Furthermore, significant interactions were observed in all cases between drug pretreatment and SKF 81297 treatment (F (3, 53) = 8.426, p<0.001). Significant main effects of SKF 81297 treatment or drug pretreatment on NADPH-d staining were not observed in the motor cortex of the same animals (Fig 2b, p>0.05).
Figure 2. Modulation of striatal and cortical NADPH-d staining by NMDA and DA D1 receptor activation.
A) The mean ± SEM of NADPH-d staining in the dorsal striatum was decreased relative to SAL/SAL treated controls following both NPA, SCH 23390 and CPP administration (*** p<0.001 Bonferroni post-hoc test, n=5–10 rats per group). The mean ± SEM of NADPH-d staining in the dorsal striatum was significantly increased in animals treated with SAL/SKF 81297 compared to SAL/SAL treated controls (*** p<0.001 Bonferroni post-hoc test, n=5–17 rats per group). The mean ± SEM increase in NADPH-d staining elicited by SAL/SKF 81297 administration was significantly attenuated following pretreatment with SCH 23390, NPA and CPP (††† p<0.005 Bonferroni post-hoc test, n=5–17 rats per group). B) The mean ± SEM of NADPH-d staining in the cortex was not changed following drug/SAL administration as compared to all treatments (p>0.05, n=5–17 rats per group).
Pairwise comparisons revealed that NPA administration attenuated NADPH-d staining observed in the dorsal striatum following a SAL challenge (Fig 2a, p<0.001). These findings further validate that NADPH-d staining is a suitable marker for striatal nNOS activity (Morris et al. 1997; Sancesario et al. 2004; Fernandez et al. 2005). The D1 antagonist SCH 23390 was also administered to a separate group of animals prior to delivery of a challenge injection of SAL. SCH 23390 pretreatment attenuated striatal NADPH-d staining observed following SAL challenge (Fig 2a, p<0.001). This observation is consistent with several studies showing that activation of striatal nNOS is attenuated by D1 receptor antagonism (Morris et al. 1997; Sammut et al. 2006). A separate group of animals was injected with the competitive NMDA receptor antagonist CPP prior to receiving a challenge injection of SAL in order to examine the impact of NMDA receptor activation on NADPH-d staining. CPP pretreatment strongly decreased striatal NADPH-d staining observed following a SAL challenge (Fig 2a, p<0.001). Because CPP/SAL pretreatment attenuated striatal NADPH-d staining relative to SAL/SAL treated controls, basal nNOS activation is likely to be dependent on NMDA receptor activation.
Further pairwise comparisons revealed that animals pretreated with NPA prior to SKF 81297 administration exhibited less robust increases in striatal NADPH-d staining relative to the SAL/SKF 81297 treated group (Fig 2a, p<0.001). The specificity of the effect of SKF 81297 for the D1 receptor was assessed further using pretreatment with the D1 antagonist SCH 23390. Animals pretreated with SCH 23390 prior to SKF 81297 administration exhibited less robust increases in staining relative to the SAL/SKF 81297 treated group (Fig 2a, p<0.005). Interestingly, CPP pretreatment blocked the increase in striatal NADPH-d staining elicited by SKF 81297 treatment (Fig 2a, p<0.001), indicating that ongoing NMDA receptor stimulation is necessary for D1 receptor-dependent activation of striatal nNOS.
Effects of D2 receptor antagonist administration on NADPH-d staining: Impact of inhibitor/antagonist pretreatments
The current study also examined the impact of D2 receptor antagonist (ETI) treatment on striatal and cortical NADPH-d staining in animals pretreated with either SAL or drug (i.e., NPA, QNP, or CPP). In studies using striatal tissue, a main effect of ETI treatment was observed (F (1, 40) = 33.704, p<0.001) indicating that D2 receptor antagonism increases NADPH-d staining in this region relative to SAL treated controls (Fig 3a). Significant main effects of drug pretreatment on striatal NADPH-d staining were also observed (F (3, 40) = 39.226, p<0.001). Furthermore, significant interactions were observed in all cases between drug pretreatment and ETI treatment (F (3, 40) = 14.066, p<0.001). Significant main effects of ETI treatment on NADPH-d staining were not observed in the motor cortex of the same animals (Fig 3b, p>0.05). However, significant main effects of drug pretreatment were observed in this region (F (3, 41) = 3.677, p<0.05).
Figure 3. Modulation of striatal and cortical NADPH-d staining by NMDA and DA D2 receptor activation.
A) QNP/SAL administration decreased striatal NADPH-d staining relative to SAL/SAL treated controls (***p<0.001, n=5–10 rats per group). The mean ± SEM of NADPH-d staining in the dorsal striatum was significantly increased in animals treated with SAL/ETI compared to SAL/SAL treated controls (*** p<0.001 Bonferroni post-hoc test, n=5–10 rats per group). The mean ± SEM increase in NADPH-d staining elicited by SAL/ETI administration was significantly attenuated following pretreatment with QNP, NPA and CPP (††† p<0.001 Bonferroni post-hoc test, n=5–10 rats per group). B) QNP/SAL administration decreased cortical NADPH-d staining relative to SAL/SAL treated controls (*p<0.05 Bonferroni post-hoc test, n=5–10 rats per group), an effect which was not observed in QNP/ETI treated rats (p>0.05, n=5–10 rats per group). The mean ± SEM of NADPH-d staining in the cortex was not changed following ETI administration combined with SAL or any of the drug pretreatments (p>0.05, n=5–10 rats per group).
Pairwise comparisons of the effects of NPA and CPP pretreatments on NADPH-d staining observed in the striatum following a SAL challenge are reported above (see also Fig 3a). Additional pairwise comparisons revealed that QNP pretreatment modestly decreased NADPH-d staining observed in both the striatum (Fig 3a, p<0.001) and motor cortex (Fig 3b, p<0.05) following a SAL challenge as compared to SAL/SAL treated controls. These observations are consistent with our previous studies showing that activation of nNOS is attenuated by D2 receptor agonism (Sammut et al. 2007a).
Animals pretreated with NPA prior to ETI administration exhibited less robust increases in striatal NADPH-d staining relative to the SAL/ETI group, indicating that the facilitatory effect of D2 antagonism on staining is at least partially dependent on nNOS activity (Fig 3a, p<0.001). NADPH-d staining in the motor cortex of the same animals was unchanged following NPA/ETI treatment as compared to SAL/SAL or SAL/ETI treated groups (Fig 3b, p>0.05). The specificity of the effect of ETI for the D2 receptor was assessed in further studies in which animals were pretreated with the D2 agonist QNP prior to the systemic injection of D2 antagonist ETI. QNP pretreatment attenuated the increase in NADPH-d staining observed in the dorsal striatum of ETI treated animals (Fig 3a, p<0.001), indicating that the facilitatory effect of ETI was mediated via a D2 receptor-dependent mechanism. Moreover, CPP pretreatment blocked the increase in NADPH-d staining observed in the dorsal striatum following ETI treatment (Fig 3a, p<0.001), indicating that the facilitatory effect of ETI was dependent on NMDA receptor activation.
Effects of D2 receptor agonist pretreatment on the facilitation of striatal NADPH-diaphorase staining by D1 receptor agonist administration
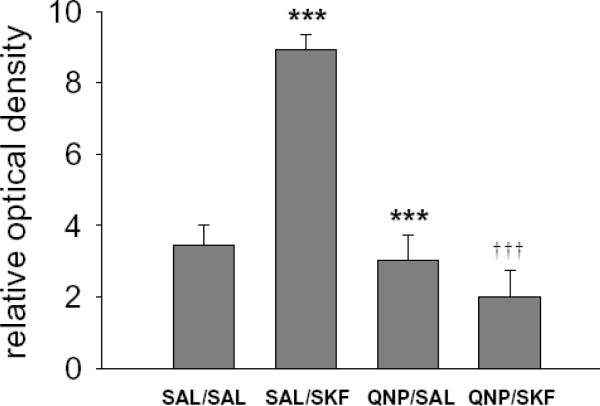
Animals were pretreated with QNP prior to systemic injection of SKF 81297 to examine the potential interaction between D1 and D2 receptor dependent mechanisms in the regulation of striatal NOS activity. Consistent with the above studies, a main effect of SKF 81297 treatment was observed (F (1, 35) = 12.917, p<0.001) further indicating that D1 receptor agonism increases striatal NADPH-d staining (Fig 4). Significant main effects of QNP pretreatment on striatal NADPH-d staining were also observed (F (1, 35) = 34.872, p<0.001). Furthermore, significant interactions were observed between QNP pretreatment and SKF 81297 treatment (F (1, 35) = 27.005, p<0.001). Pairwise comparisons revealed that QNP pretreatment attenuated the increase in NADPH-d staining observed in the striatum of SKF 81297 treated animals (Fig 4, p<0.001), indicating that the facilitatory effect of D1 receptor activation is opposed by a D2 receptor-dependent mechanism.
Figure 4. Striatal NADPH-d staining elicited via D1 receptor agonist administration is blocked by D2 receptor agonism.
The mean ± SEM of NADPH-d staining in the dorsal striatum was significantly increased in animals treated with SAL/SKF 81297 compared to SAL/SAL treated controls (*** p<0.001 Bonferroni post-hoc test, n=5–17 rats per group). The mean ± SEM increase in NADPH-d staining elicited by SAL/SKF 81297 administration was significantly blocked following pretreatment with QNP (†††p<0.001 Bonferroni post-hoc test, n=10–17 rats per group).
Discussion
Glutamatergic activation of NMDA receptors stimulates nNOS activity and NO production (Garthwaite 2008) in a manner which is likely to be potently modulated by DA (West et al. 2002). In the current study we have provided strong evidence for this modulatory role of DA, as striatal nNOS activity was found to be up-regulated by D1 receptor activation and down-regulated by D2 receptor activation in a manner that was dependent on ongoing NMDA receptor activation. D2 receptor activation was also shown to block the facilitatory effects of D1 receptor activation on nNOS activity. Importantly, in all cases significant interactions were observed between drug pretreatments (NPA, SCH 23390, QNP, CPP) and D1 agonist/D2 antagonist treatments, indicating that the drug pretreatments were acting to specifically attenuate the effect of the DA modulation, and not by simply lowering overall basal levels of NADPH-d activity in a manner independent of a DA receptor-mediated mechanism.
Technical Considerations
Reports from several laboratories indicate that NADPH-d staining is a reliable marker of striatal nNOS interneurons (Dawson et al. 1991; Hope et al. 1991; Vincent and Kimura 1992; Kharazia et al. 1994). Given that the catalytic activity of the nNOS enzyme is responsible for producing NADPH-d staining in striatal interneurons (Dawson et al.1991; Hope et al. 1991), optical density measurements of staining accurately reflect enzyme activity (Kuo et al. 1994; Morris et al. 1997; Sanceserio et al. 2004; Fernandez et al. 2005). The current study and others indicate that striatal NADPH-d staining is highly sensitive to changes in DA innervation and receptor occupation (Morris et al. 1997; Sanceserio et al. 2004). However, with the exception of QNP, none of the drugs tested herein (including NPA and CPP) affected cortical measures of NADPH-d staining. This is not surprising given that NOS does not fully account for NADPH-d activity in cortex as it does in the dorsal striatum (Kharazia et al. 1994).
We examined the impact of drug manipulations on a large area of the dorsal striatum which contained numerous NADPH-d positive cell bodies, dendrites and axons. We chose to quantify the relative optical density of the neuropil rather than cell bodies, as previous studies have shown that striatal nNOS protein is predominantly expressed in nerve fibers (Bredt et al. 1991). Importantly, outcomes observed in the current study using SCH 23390 and ETI administration were highly consistent with an earlier study that restricted the analysis of NADPH-d staining to the cell bodies and proximal dendrites (Morris et al. 1997).
In line with our previous studies using electrochemical measures of NO efflux (Sammut et al. 2006; Sammut et al. 2007a), the increase in NADPH-d staining observed following systemic administration of the D1/5 receptor agonist SKF 81297 was attenuated by pretreatment with either a D1/5 antagonist or nNOS inhibitor. The effects of both NPA and SCH 23390 were relatively modest compared to those observed following similar pretreatment with the NMDA receptor antagonist CPP. Doses of NPA and SCH 23390 were selected based on our previous studies and the work of other laboratories (see Materials and Methods section). However, the observed outcomes suggest that we may have used sub-optimal doses of inhibitor/antagonist in our studies. Consistent with the current work, our previous studies using a substantial dose of the nNOS inhibitor NPA (10 mg/kg, i.p.) found that only modest maximal decreases in NO synthesis (~50%) are observed following systemic administration of this drug (Sammut et al. 2007b). Similar observations have been reported in studies using other selective and non-selective nNOS inhibitors (Kalisch et al. 1996; Sammut et al. 2006; Sammut et al. 2007b; Ondracek et al. 2008), indicating that currently available NOS inhibitors are only moderately effective when given systemically. Thus, the modest efficacy associated with systemic NOS inhibitor administration may result from poor brain penetration or the existence of a drug-resistant isoform of striatal NOS which may contribute to outcomes measured in these studies.
Glutamatergic stimulation of nNOS activity
The current studies found that striatal NADPH-d staining was reduced relative to SAL treated controls following NMDA antagonist pretreatment. These findings are consistent with previous histochemical studies which reported a decrease in NADPH-d staining in the somata of striatal interneurons in animals treated with the NMDA receptor antagonist MK-801 (Morris et al. 1997). These observations presumably are due to the direct effects of the antagonist on nNOS interneurons as anatomical studies have shown that these neurons express NMDA receptor mRNA (Price et al. 1993) and protein (Gracy and Pickle 1997). Furthermore, striatal glutamatergic tone is likely to be derived largely from cortical inputs. Thus, electrophysiological, electrochemical, and molecular studies indicate that nNOS interneurons are potently activated by stimulation of corticostriatal afferents (Berretta et al. 1997; Kawaguchi 1993; Sammut et al. 2007b; Ondracek et al. 2008). Electrical stimulation of the parafascicular thalamus was also shown to increase striatal cGMP efflux via nNOS and NMDA receptor-dependent mechanisms (Consolo et al. 1999). Taken together, these studies indicate that activation of both corticostriatal and thalamostriatal afferents stimulate NO production via NMDA receptor-dependent glutamatergic transmission.
Dopaminergic modulation of nNOS activity
Similar to previous studies (Morris et al. 1997), pretreatment with SCH 23390 alone selectively attenuated striatal NADPH-d staining, indicating that DA transmission maintains striatal nNOS activity via D1 receptor activation. Other studies have shown that D1 receptor activation increased striatal tissue levels of cGMP (Altar et al. 1990; Di Stefano et al. 2005; Siuiak et al. 2006), whereas D1 antagonism produced the opposite effect (Altar et al. 1990; Di Stefano et al. 2005). Striatal nNOS interneurons are thought to express D1-like (probably D5) receptors (Le et al. 1991; Centonze et al. 2003; Rivera et al. 2002). In support of this, bath application of D1 receptor agonist produced a strong depolarization of nNOS interneurons recorded in striatal brain slices during tetrodotoxin co-perfusion (Centonze et al. 2002; 2003). This indicates that D1 agonist induced activation of striatal nNOS activity is most likely a direct effect on the nNOS interneuron.
Our results also confirm the involvement nNOS and D2 receptors in the ETI effect observed in previous studies (Morris et al., 1997) since both NPA and QNP blocked the ETI-mediated increase in NADPH-d staining. Furthermore, treatment with QNP alone decreased NADPH-d staining in the striatum and cortex. The findings of the current study are consistent with numerous studies showing that basal D2 receptor stimulation down-regulates striatal nNOS activity by an unknown inhibitory mechanism (Altar et al. 1990; Morris et al. 1997; Lau et al. 2003; Di Stefano et al. 2005; Siuciak et al. 2006; Sammut et al. 2007a). Initial work by Altar and colleagues showed that the D2 receptor antagonists haloperidol and sulpiride both increased tissue levels of cGMP (Altar et al. 1990). More recent studies have shown that chronic haloperidol treatment increases measures of NOS activity, nNOS protein levels, and mRNA expression in rats (Lau et al. 2003). Moreover, similar to our observations in NPA/ETI treated rats, the facilitatory effect of haloperidol on striatal cGMP levels was completely blocked in nNOS −/− mice (Siuciak et al. 2006). Using electrochemical methods we have shown that D2 receptor antagonism increases striatal NO efflux evoked via electrical stimulation of the substantia nigra. Conversely, D2 receptor stimulation decreases electrochemical measures of evoked NO efflux (Sammut et al. 2007a). Thus, under non-stimulated conditions, endogenous DA suppresses basal nNOS activity via D2 receptor activation. When nNOS activity is robustly stimulated by excitatory inputs, further D2 receptor stimulation is likely to oppose this excitation.
Dopamine and glutamate interactions and the regulation of striatal nNOS activity
The impact of DA-glutamate interactions on nNOS activity is likely to be complex given the multitude of transmitter receptors involved and the fact that these transmitter systems converge both at the level of the nNOS interneurons (Fujiyama and Masuko 1996; Hidaka and Totterdell 2001) and the principle spiny neurons (Hidaka and Totterdell 2001; Sancesario et al. 2000). Evidence that the D1-like receptor-dependent effect observed in the current study requires NMDA receptor activation is consistent with numerous studies demonstrating reciprocal functional interactions between D1 and NMDA receptors in a variety of neurons in the CNS (Cepeda and Levine 2006). These receptor interactions are thought to occur via direct physical associations and following activation of second messengers (Cepeda and Levine 1998; 2006). Given the above, it is likely that reciprocal D1-NMDA receptor interactions play a critical role in regulating striatal nNOS interneuron activity.
To our knowledge, D2-like receptors have not been shown to colocalize with any of the various markers for striatal nNOS interneurons (e.g., somatostatin, neuropeptide Y, nNOS, and NADPH-d staining), suggesting that the D2 receptor-mediated effects observed herein are of an indirect nature. Moreover, bath application of D2 agonist in the presence of tetrodotoxin does not affect the membrane activity of electrophysiologically-identified NOS interneurons (Centonze et al. 2002). Given this, it is likely that D2 receptor-dependent suppression of nNOS activity occurs via modulation of mono- or trans-synaptic excitatory inputs to striatal nNOS interneurons.
Interestingly, the present studies demonstrate that D2 receptor agonism can block the D1 receptor-mediated facilitation of nNOS activity. This observation is consistent with electrochemical findings from our lab showing that the increase in striatal NO efflux observed with D1 agonist administration is attenuated by pretreatment with a D2 receptor agonist (Sammut et al. 2007a). It is plausible that this inhibitory effect occurs through an indirect mechanism in which stimulation of presynaptic D2 receptors on glutamatergic terminals (Wang and Pickel 2002; Bamford et al. 2004) decreases direct glutamatergic excitation of postsynaptic NMDA receptors on nNOS interneurons, thus blocking D1 receptor-dependent stimulation. The D2 mediated effect may also modulate nNOS activity via actions on GABAergic terminals arising from intrinsic and extrinsic sources such as the globus pallidus (Bevan et al. 1998; Fernandez et al. 2005). Additional studies are needed to clarify these issues.
Functional Implications
Our previous studies have shown that robust activation of glutamatergic inputs to the striatum activates a powerful NO-mediated feed-forward excitation of corticostriatal transmission (Sammut et al. 2007b; Ondracek et al. 2008). This feed-forward influence of NO was also shown to attenuate concurrent D2 receptor-mediated short-term inhibitory influences on striatal neuron activity. Given the results of the current study, it is likely that this D2 receptor-dependent suppression arises from at least two independent mechanisms: 1) suppression of D1-NMDA receptor-dependent activation of NO synthesis, and 2) attenuation of the excitatory postsynaptic effects of NO signaling on spiny neuron activity (Ondracek et al. 2008). Indeed, intact DAergic modulation of NO signaling is likely to be critical for normal motor behavior as systemic and intrastriatal administration of NOS inhibitors decreases locomotion and induces catalepsy (reviewed in Del Bel et al. 2005). Thus, a better understanding of how pathophysiological and pharmacological disruption of D1 and D2 receptor activation affects the regulation of NO signaling cascades may lead to promising new approaches for treating disorders of the basal ganglia that involve dysfunctional glutamatergic and/or dopaminergic transmission.
Acknowledgements
The authors thank Dr. Gloria E. Meredith for expert advice on performing and quantifying the NADPH-d histochemistry and for insightful comments on data interpretation and preparation of this manuscript. We are also grateful to Dr. Kuei Tseng for insightful comments on data interpretation. We would like to thank Alexander Dec and Soleil Leilabadi for their assistance with the histology. This work was supported by the Chicago Medical School and United States Public Health grant NS 047452 (ARW).
References
- Altar CA, Boyar WC, Kim HS. Discriminatory roles for D1 and D2 dopamine receptor subtypes in the in vivo control of neostriatal cyclic GMP. Eur J Pharmacol. 1990;181:17–21. doi: 10.1016/0014-2999(90)90240-7. [DOI] [PubMed] [Google Scholar]
- Atalla A, Kuschinsky K. Effects of blockade of glutamate NMDA receptors or of NO synthase on the development or the expression of associative or non-associative sensitization to locomotor activation by morphine. J Neural Transm. 2006;113:1–10. doi: 10.1007/s00702-005-0298-0. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14(1):21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Berretta S, Parthasarathy HB, Graybiel AM. Local release of GABAergic inhibition in the motor cortex induces immediate-early gene expression in indirect pathway neurons of the striatum. J Neurosci. 1997;17:4752–4763. doi: 10.1523/JNEUROSCI.17-12-04752.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Booth PA, Eaton SA, Bolam JP. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. J Neurosci. 1998;22:9438–52. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di FM. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Centonze D, Bracci E, Pisani A, Gubellini P, Bernardi G, Calabresi P. Activation of dopamine D1-like receptors excites LTS interneurons of the striatum. Eur J Neurosci. 2002;15:2049–2052. doi: 10.1046/j.1460-9568.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavon N, Pisani A, Bernardi G, Moratalla R, Calabresi P. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci. 2003;23:8506–8512. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Where do you think you are going? The NMDA-D1 receptor trap. Sci STKE. 2006;2006:e20. doi: 10.1126/stke.3332006pe20. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Dopamine and N-methyl-D-aspartate receptor interactions in the neostriatum. Dev Neurosci. 1998;20:1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- Consolo S, Cassetti A, Uboldi MC. The parafascicular thalamic nucleus but not the prefrontal cortex facilitates the nitric oxide/cyclic GMP pathway in rat striatum. Neuroscience. 1999;91:51–58. doi: 10.1016/s0306-4522(98)00601-0. [DOI] [PubMed] [Google Scholar]
- Crespi F, Rossetti ZL. Pulse of nitric oxide release in response to activation of N-methyl-D-aspartate receptors in the rat striatum: rapid desensitization, inhibition by receptor antagonists, and potentiation by glycine. J Pharmacol Exp Ther. 2004;309:462–468. doi: 10.1124/jpet.103.061069. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci USA. 1991;88:7797–801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bel EA, Guimarãúes FS, Bermúdez-Echeverry M, Gomes MZ, Schiaveto-de-Souza A, Padovan-Neto FE, Tumas V, Barion-Cavalcanti AP, Lazzarini M, Nucci-da-Silva LP, de P. Role of nitric oxide on motor behavior. Cell Mol Neurobiol. 2005;25:371–392. doi: 10.1007/s10571-005-3065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano A, Sozio P, Cacciatore I, Cocco A, Giorgioni G, Costa B, Montali M, Lucacchini A, Martini C, Spoto G, Di Pietrantonio F, Di Matteo E, Pinnen F. Preparation and pharmacological characterization of trans-2-amino-5(6)-fluoro-6(5)-hydroxy-1-phenyl-2,3-dihydro-1H-indenes as D2-like dopamine receptor agonists. J Med Chem. 2005;48:2646–2654. doi: 10.1021/jm040889k. [DOI] [PubMed] [Google Scholar]
- Fernandez ML, Wright AK, Arbuthnott GW. The Basal Ganglia VIII. Volume 56. Springer; US: 2005. Activation of NOS interneurons in striatum after excitotoxic lesions of rat globus pallidus; pp. 485–491. [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci. 2001a;21:2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. J Neurosci. 2001b;21:6370–6. doi: 10.1523/JNEUROSCI.21-16-06370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Masuko S. Association of dopaminergic terminals and neurons releasing nitric oxide in the rat striatum: An electron microscopic study using NADPH-diaphorase histochemistry and tyrosine hydroxylase immunohistochemistry. Brain Res Bull. 1996;40:121–127. doi: 10.1016/0361-9230(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Garcia-Munoz M, Young SJ, Groves PM. Terminal excitability of the corticostriatal pathway. I. Regulation by dopamine receptor stimulation. Brain Res. 1991;551:195–206. doi: 10.1016/0006-8993(91)90933-m. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991;14:60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Gonon F. Prolonged and Extrasynaptic Excitatory Action of Dopamine Mediated by D1 Receptors in the Rat Striatum In Vivo. J Neurosci. 1997;17:5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracy KN, Pickel VM. Ultrastructural localization and comparative distribution of nitric oxide synthase and N-methyl-D-aspartate receptors in the shell of the rat nucleus accumbens. Brain Res. 1997;747:259–272. doi: 10.1016/s0006-8993(96)01249-8. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23(3):435–47. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–36. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- Hall H, Kohler C, Gawell L. Some in vitro binding properties of 3[H]eticlopride, a novel substituted benzamide, selective for dopamine-D2 receptors in the rat brain. Eur J Pharmacol. 1985;111(2):191–199. doi: 10.1016/0014-2999(85)90756-3. [DOI] [PubMed] [Google Scholar]
- Hidaka S, Totterdell S. Ultrastructural features of the nitric oxide synthase-containing interneurons in the nucleus accumbens and their relationship with tyrosine hydroxylase-containing terminals. J Comp Neurol. 2001;431:139–154. doi: 10.1002/1096-9861(20010305)431:2<139::aid-cne1061>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci USA. 1991;88:2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani MM, Millar J, Kruk ZL. Differential release of dopamine by nitric oxide in subregions of rat caudate putamen slices. J Neurochem. 1998;71:1969–1977. doi: 10.1046/j.1471-4159.1998.71051969.x. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Ma PM. Localization of NADPH diaphorase activity in monoaminergic neurons of the rat brain. J Comp Neurol. 1993;22(3324):391–406. doi: 10.1002/cne.903320402. [DOI] [PubMed] [Google Scholar]
- Kalisch BE, Connop BP, Jhamandas K, Beninger RJ, Boegman RJ. Differential action of 7-nitro indazole on rat brain nitric oxide synthase. Neurosci Lett. 1996;219:75–78. doi: 10.1016/s0304-3940(96)13194-3. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neurosci Res. 1997;27:1–8. doi: 10.1016/s0168-0102(96)01134-0. [DOI] [PubMed] [Google Scholar]
- Kerkerian L, Bosler O, Pelletier G, Nieoullon A. Striatal neuropeptide Y neurons are under the influence of nigrostriatal dopaminergic pathway: immunohistochemical evidence. Neurosci Lett. 1986;66:106–112. doi: 10.1016/0304-3940(86)90174-6. [DOI] [PubMed] [Google Scholar]
- Kharazia VN, Schmidt HH, Weinberg RJ. Type I nitric oxide synthase fully accounts for NADPH-diaphorase in rat striatum, but not cortex. Neuroscience. 1994;62:983–987. doi: 10.1016/0306-4522(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Kuo H, Grant S, Muth N, Hengemihle J, Ingram DK. The correlation between neuron counts and optical density of NADPH-diaphorase histochemistry in the rat striatum: a quantitative study. Brain Res. 1994;660:57–65. doi: 10.1016/0006-8993(94)90838-9. [DOI] [PubMed] [Google Scholar]
- Lau YS, Petroske E, Meredith GE, Wang JQ. Elevated neuronal nitric oxide synthase expression in chronic haloperidol-treated rats. Neuropharmacology. 2003;45:986–994. doi: 10.1016/s0028-3908(03)00314-9. [DOI] [PubMed] [Google Scholar]
- Le MC, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci USA. 1991;88:4205–4209. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SX, Ji A, Pandjaitan M, Ojije G. Enhanced nitric oxide release/synthesis in the posterior hypothalamus during nitroglycerin tolerance in rats. Eur J Pharmacol. 2003;472:179–187. doi: 10.1016/s0014-2999(03)01937-x. [DOI] [PubMed] [Google Scholar]
- Marletta MA. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994;78(6):927–30. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Maura G, Carbone R, Raiteri MJ. Aspartate-releasing nerve terminals in rat striatum possesses D-2 dopamine receptors mediating inhibition of release. Pharmacol Exp Ther. 1989;251:1142–6. [PubMed] [Google Scholar]
- Meredith GE, Totterdell S. Microcircuits in nucleus accumbens' shell and core involved in cognition and reward. Psychobiology. 1999;27(2):165–186. [Google Scholar]
- Morris BJ, Simpson CS, Mundell S, Maceachern K, Johnston HM, Nolan AM. Dynamic changes in NADPH-diaphorase staining reflect activity of nitric oxide synthase: evidence for a dopaminergic regulation of striatal nitric oxide release. Neuropharmacology. 1997;36:1589–1599. doi: 10.1016/s0028-3908(97)00159-7. [DOI] [PubMed] [Google Scholar]
- Nishi A, Watanabe Y, Higashi H, Tanaka M, Nairn AC, Greengard P. Glutamate regulation of DARPP-32 phosphorylation in neostriatal neurons involves activation of multiple signaling cascades. Proc Natl Acad Sci USA. 2005;102:1199–1204. doi: 10.1073/pnas.0409138102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondracek JM, Dec A, Hoque KE, Lim SA, Rasouli G, Indorkar RP, Linardakis J, Klika B, Mukherji SJ, Burnazi M, Threlfell S, Sammut S, West AR. Feed-forward excitation of striatal neuron activity by frontal cortical activation of nitric oxide signaling in vivo. Eur J Neurosci. 2008;27:1739–1754. doi: 10.1111/j.1460-9568.2008.06157.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. [Google Scholar]
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Price RH, Jr, Mayer B, Beitz AJ. Nitric oxide synthase neurons in rat brain express more NMDA receptor mRNA than non-NOS neurons. Neuroreport. 1993;4:807–10. doi: 10.1097/00001756-199306000-00053. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Rivera A, Alberti I, Martin AB, Narvaez JA, de la CA, Moratalla R. Molecular phenotype of rat striatal neurons expressing the dopamine D5 receptor subtype. Eur J Neurosci. 2002;16:2049–2058. doi: 10.1046/j.1460-9568.2002.02280.x. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Crespi F. Inhibition of nitric oxide release in vivo by ethanol. Alcohol Clin Exp Res. 2004;28:1746–1751. doi: 10.1097/01.alc.0000145755.72834.f1. [DOI] [PubMed] [Google Scholar]
- Salin P, Kerkerian-Le GL, Heidet V, Epelbaum J, Nieoullon A. Somatostatin-immunoreactive neurons in the rat striatum: effects of corticostriatal and nigrostriatal dopaminergic lesions. Brain Res. 1990;521:23–32. doi: 10.1016/0006-8993(90)91520-q. [DOI] [PubMed] [Google Scholar]
- Sammut S, Dec A, Mitchell D, Linardakis J, Ortiguela M, West AR. Phasic dopaminergic transmission increases NO efflux in the rat dorsal striatum via a neuronal NOS and a dopamine D(1/5) receptor-dependent mechanism. Neuropsychopharmacology. 2006;31:493–505. doi: 10.1038/sj.npp.1300826. [DOI] [PubMed] [Google Scholar]
- Sammut S, Bray KE, West AR. Dopamine D(2) receptor-dependent modulation of striatal NO synthase activity. Psychopharmacology (Berl) 2007a doi: 10.1007/s00213-006-0681-z. DOI: 10.1007/s00213-006-0681-z. [DOI] [PubMed] [Google Scholar]
- Sammut S, Park DJ, West AR. Frontal cortical afferents facilitate striatal nitric oxide transmission in vivo via a NMDA receptor and neuronal NOS-dependent mechanism. J Neurochem. 2007b;103:1145–1156. doi: 10.1111/j.1471-4159.2007.04811.x. [DOI] [PubMed] [Google Scholar]
- Sancesario G, Morello M, Reiner A, Giacomini P, Massa R, Schoen S, Bernardi G. Nitrergic neurons make synapses on dual-input dendritic spines of neurons in the cerebral cortex and the striatum of the rat: implication for a postsynaptic action of nitric oxide. Neuroscience. 2000;99:627–642. doi: 10.1016/s0306-4522(00)00227-x. [DOI] [PubMed] [Google Scholar]
- Sancesario G, Giorgi M, D'Angelo V, Modica A, Martorana A, Morello M, Bengtson CP, Bernardi G. Down-regulation of nitrergic transmission in the rat striatum after chronic nigrostriatal deafferentation. Eur J Neurosci. 2004;20:989–1000. doi: 10.1111/j.1460-9568.2004.03566.x. [DOI] [PubMed] [Google Scholar]
- Seeman P, Van Tol HNM. Dopamine receptor pharmacology. Trends in Pharmacol Sci. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Fujiwara RA, James LC, Williams RD, Stock JL, McNeish JD, Strick CA, Menniti FS, Schmidt CJ. Genetic deletion of the striatum-enriched phosphodiesterase PDE10A: Evidence for altered striatal function. Neuropharmacology. 2006;51:374–385. doi: 10.1016/j.neuropharm.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Stefani A, Fedele E, Galati S, Pepicelli O, Frasca S, Pierantozzi M, Peppe A, Brusa L, Orlacchio A, Hainsworth AH, Gattoni G, Stanzione P, Bernardi G, Raiteri M, Mazzone P. Subthalamic stimulation activates internal pallidus: evidence from cGMP microdialysis in PD patients. Ann Neurol. 2005;57:448–452. doi: 10.1002/ana.20402. [DOI] [PubMed] [Google Scholar]
- Tacconi IS, Ratti E, Marien MR, Gaviraghi G, Bowery NG. Inhibition of [3H]-(+)-MK 801 binding to rat brain sections by CPP and 7-chlorokynurenic acid: an autoradiographic analysis. Br J Pharmacol. 1993;108:577–582. doi: 10.1111/j.1476-5381.1993.tb12845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SR, Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46(4):755–84. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- Vuillet J, Kerkerian L, Kachidian P, Bosler O, Nieoullon A. Ultrastructural correlates of functional relationships between nigral dopaminergic or cortical afferent fibers and neuropeptide Y-containing neurons in the rat striatum. Neurosci Lett. 1989;100:99–104. doi: 10.1016/0304-3940(89)90667-8. [DOI] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. J Comp Neurol. 2002;442:392–404. doi: 10.1002/cne.10086. [DOI] [PubMed] [Google Scholar]
- West AR, Galloway MP, Grace AA. Regulation of striatal dopamine neurotransmission by nitric oxide: effector pathways and signaling mechanisms. Synapse. 2002;44:227–245. doi: 10.1002/syn.10076. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. The nitric oxide-guanylyl cyclase signaling pathway modulates membrane activity states and electrophysiological properties of striatal medium spiny neurons recorded in vivo. J Neurosci. 2004;24:1924–1935. doi: 10.1523/JNEUROSCI.4470-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HQ, Fast W, Marletta MA, Martasek P, Silverman RB. Potent and selective inhibition of neuronal nitric oxide synthase by NG-propyl-L-arginine. J Med Chem. 1997;40:3869–3870. doi: 10.1021/jm970550g. [DOI] [PubMed] [Google Scholar]