Abstract
The atheroprotective effects of HDL are mediated by several mechanisms including its role in reverse cholesterol transport and via its anti-inflammatory properties. However, not all HDL is functionally similar. HDL and apolipoprotein A-I may become dysfunctional or even pro-inflammatory, and thus promote atherosclerosis. ApoAI post-translational modification can have a large impact on its function. Myeloperoxidase modification of apoAI impairs it function as a cholesterol acceptor, and the molecular changes induced by myeloperoxidase have been studied in detail. These studies provide the basis for the development of an oxidant resistant form of apoAI and clinical measures of HDL modification and dysfunction, which may be useful as a treatment criterion.
Keywords: HDL, apolipoprotein A-I, reverse cholesterol transport, myeloperoxidase, anti-inflammatory
HDL-cholesterol (HDL-C) is an independent protective factor for coronary artery disease (CAD), as demonstrated by numerous epidemiological studies, such as the Framingham Heart Study1. In interventional settings, niacin, which effectively raises HDL levels, given as a monotherapy or combined with other drugs has been shown to reduce CAD and cerebrovasuclar events2. There are many proposed pathways by which HDL may protect against CAD. HDL and its major protein constituent apolipoprotein A-I (apoAI) play prime roles in mediating reverse cholesterol transport (RCT), the transfer of cholesterol from peripheral tissues, such as arterial wall cells, to the liver for excretion, and this likely plays a central role in the mechanism of HDL’s atheroprotective effect3,4. RCT involves many steps, including the removal of cholesterol from cells (through ABCA1, which is responsible for the formation of nascent HDL, along with ABCG1, and SR-BI), the maturation of HDL through lecithin cholesterol acyltransferase (LCAT) mediated free cholesterol esterification, and the uptake and excretion of HDL cholesterol by the liver.
HDL cholesterol esters can also be transferred to apoB containing lipoproteins through the action of cholesterol ester transfer protein (CETP), and this cholesterol pool can participate in RCT by delivery to the liver through LDL receptor mediated uptake. Although CETP inhibition raises HDL-C levels, the recent failure of torcetrapib, a CETP inhibitor, in a clinical outcomes trial5 raised the specter that CETP inhibition may lead to the production of dysfunctional HDL. However, due to the off target effects of torcetrapib on blood pressure and the plasma levels of potassium, sodium, bicarbonate, and aldosterone it is impossible to determine if the failure of this drug could be due to the formation of dysfunctional HDL5. In a post hoc analysis of the torcetrapib study by intravascular ultrasound, atheroma regression, rather than progression, was observed in the subjects who achieved the highest levels of HDL-C, suggesting that CETP inhibition does not lead to dysfunctional HDL6.
HDL has many other activities aside from its role in RCT that can also contribute to its protective mechanism. These include the removal and/or detoxification of oxidized sterols and phospholipids, anti-inflammatory activity, antioxidant activity, anti thrombotic activity, and its protective and healing activities on endothelial cells3,7,8. So, HDL-cholesterol is the “good cholesterol”, but there are many unresolved questions: are all HDL particles equally good, does HDL from different subjects perform the same; can HDL become dysfunctional; and, can “good cholesterol” be made into “better cholesterol”? This review describes the evidence for dysfunctional HDL and its underpinnings, as well as the potential use of HDL functionality as a diagnostic tool and therapeutic target.
Evidence for and causes of dysfunctional HDL
Fogelman and colleagues developed an assay to measure the anti-inflammatory activity of HDL by determining the extent to which HDL inhibits monocyte chemotaxis induced by LDL using an in vitro reconstituted artery wall model by the co-culture of smooth muscle cells and endothelial cells9. Other assays for HDL function include cell free anti-oxidant activity10, inhibition of endothelial cell adhesion molecule expression11, and the ability of HDL to act as an acceptor of cellular cholesterol. Navab, Fogeleman, and colleagues using this artery wall model system have demonstrated several conditions in which HDL can lose its anti-inflammatory properties, including during the acute phase response when serum amyloid A partially replaces apoAI in HDL, during influenza A infection, and in apolipoprotein AII transgenic mice12,13,14. In a seminal study, Ansell et al. discovered that HDL from CAD subjects who had high levels of HDL had less anti-inflammatory activity, in this co-culture assay, than HDL derived from healthy control subjects, thus indicating that HDL from CAD subjects is dysfunctional10. In fact, HDL from many CAD patients was actually proinflammatory, thus increasing monocyte chemotaxis in response to LDL, unlike the HDL from healthy controls that reduced monocyte chemotaxis10. There are many possible alterations between this dysfunctional HDL and normal functional HDL.
One possibility is a change in the protein composition of HDL. Human HDL particles are quite heterogeneous, encompassing a range of sizes and densities. Each HDL particle carries apoAI and may also carry other apolipoproteins, such as apoAII, apoAIV, apoE, apoCs. In addition, HDL is associated with a panoply of accessory proteins, including LCAT, phospholipid transfer protein (PLTP), paraoxonase 1 (PON1), myeloperoxidase (MPO), serum amyloid A1, (SAA1), and platelet activating factor acetylhydrolase (PAF-AH). The HDL proteome is quite large15, and each HDL particle cannot accommodate all of the accessory proteins; thus, a minority of the HDL particles must be associated with each accessory protein. Changes in HDL protein composition due to infection, inflammation, or diabetes have been shown to be associated with decreased function12,14,16. An HDL proteomic study of HDL3 from different subjects found that the levels of PON1, PON3, and apoL-I correlate with HDL’s antioxidant activity17. Also, mouse models have been used to determine the effects of HDL protein composition changes, for example, HDL isolated from transgenic mice over expressing apoAII or PLPT show reduced function compared to control HDL13,18.
Many studies have been performed to investigate the changes in HDL associated with inflammation. de Beer and colleagues have shown extensive HDL remodeling after treatment of mice with endotoxin, or in humans after surgery, leading to the so-called acute phase HDL, in which SAA and group IIa secretory phospholipase A2 (sPLA2-IIa) are increased and apoAI levels (and CETP levels in humans) are decreased19. Since SAA itself can act as an acceptor for cellular cholesterol via ABCA1, SR-BI, and other pathways20,21,22, the consequences of acute phase HDL on HDL function and RCT must be determined experimentally. Post surgical human serum containing acute phase HDL maintains most of its ABCA1 and ABCG1 dependent cholesterol acceptor activity despite decreased HDL-C and apoAI levels23. The in vitro incubation of HDL with SAA, sPLA2-IIa, and CETP leads to the marked displacement of apoAI from HDL generating lipid free or lipid poor apoAI, expected to undergo more rapid catabolism, but also expected to be a good ABCA1-mediated acceptor of cellular lipids23. The over expression of SAA and SR-BI in mice via adenoviral vectors also leads to the production of small lipid poor apoAI particles which are more rapidly cleared24. Recently, Reilly’s lab demonstrated that LPS treatment in mice decreases RCT, although this was mediated primarily through the inhibition of liver sterol excretion associated with decreased expression of hepatic ABCG5, ABCG8, and ABCB1125.
Another factor that might make HDL dysfunctional is a change in the HDL associated lipids. HDL may acquire oxidized lipids from cells and by exchange with other particles, or HDL lipids may be oxidized in situ. Lipid peroxides may interfere with HDL’s antioxidant, anti-inflammatory, and cholesterol acceptor activities. For example, treatment of HDL with 15-lipoxygenase, an enzyme forming lipid peroxides, reduces HDL cholesterol acceptor and anti-inflammatory activities26,27. HDL carries the bulk of lipid hydroperoxides in plasma28. These lipid hydroperoxides can be converted into less reactive lipid hydroxides through specific methionine residues of apoAI and apoAII, with the concomitant formation of methionine sulfoxide29. Fully functional HDL may promote lipid hydroperoxide metabolism and its uptake and clearance by the liver, which would be protective28. In contrast, dysfunctional HDL might promote the transfer of lipid hydroperoxides to apoB containing lipoproteins and promote VLDL and LDL oxidation30.
A prominent factor that can lead to HDL dysfunction is the post-translational modification of apoAI. Copper mediated oxidation of HDL leads to altered HDL migration on an agarose gel, apoAI proteolysis, and decreased ability of HDL to unload cholesterol esters from cholesterol loaded macrophages31. Malondialdehyde modification of HDL leads to similar changes in regard to HDL structure and function, and is associated with the loss of lysine and tryptophan residues, and apoAI polymerization32. HDL from diabetic subjects has evidence of glycated apoAI and apoAII, and this glycated apoAI has altered structure and lipid binding activity33,34. Similarly, HDL incubated with high glucose results in a reduction in paraoxonase activity and decreased anti-inflammatory activity35. Lipid peroxides added to human plasma can covalently modify apoAI36. Also, incubation of HDL with activated neutrophils was shown to render apoAI more negatively charged and decrease its cellular cholesterol acceptor activity37,38.
Myeloperoxidase modification of apoAI
Myeloperoxidase (MPO) is an enzyme found in neutrophils, monocytes, and some macrophages that uses hydrogen peroxide to generate chlorinating and nitrating oxidants, which play an important role in killing microorganisms. However, these same reactive species can also modify host proteins and lipids. MPO is enriched in human atheroma 39, and its presence may promote lesion progression, by increasing LDL oxidation, and block plaque regression, by modifications of apoAI/HDL that impair RCT. Bergt et al. demonstrated that incubation of HDL with MPO, in a chlorinating reaction (MPO/H2O2/Cl−) that produces HOCl (chlorine bleach), or with reagent HOCl (at a very high 100:1 ratio of HOCl:apoAI) leads to a loss of unsaturated fatty acids in phospholipids and cholesterol esters, and the loss of cholesterol acceptor activity40. Bergt et al. also showed that modification of lipid-free apoAI at an HOCl:apoAI ratio of 25:1 led to the loss of apoAI methionine residues40, consistent with the high sensitivity of methionine to MPO/H2O2/Cl−41. Subsequently, it was shown that selective apoAI methionine oxidation does not disrupt its structure, and actually leads to an increase in lipid binding and cellular lipid acceptor activities42; although apoAI Met-148 modification by MPO is associated with loss of LCAT activation43. Bergt et al. also used mass spectroscopy to identify specific alterations caused by reagent or MPO generated HOCl and they confirmed the disappearance of the methionine-containing tryptic peptides with concomitant production of methionine sulfoxide-containing peptides44. The cholesterol acceptor activity of recombinant HDL (rHDL) made by cholate dialysis using apoAI and phosphatidylcholine is much more sensitive to the HOCl treatment, with loss of activity starting at an HOCl:apoAI ratio of 5:140. In addition to decreased lipid acceptor activity, Panzenboeck et al. also showed that MPO or HOCl modified HDL was more susceptible to uptake and degradation by macrophages, thus turning HDL from a lipid accepting lipoprotein to a lipid loading lipoprotein45. Although methionine and cysteine residues (apoAI has no Cys residues) are the most sensitive to MPO generated oxidants, tyrosine, lysine, tryptophan, and other residues are also targets, with 3-chlorotyrosine being used as a fingerprint for MPO oxidants, as HOCl uniquely forms this adduct46,47.
Since 2004, the combined Smith, Hazen, and Kinter labs at the Cleveland Clinic, and the combined Heineke and Oram labs at the University of Washington have each examined the MPO induced molecular alterations in apoAI and their functional significances. Both teams fundamentally agree that MPO modification of apoAI is physiologically relevant by demonstrating that: 1) apoAI in plasma and more so in arterial lesions is a selective target of MPO modification that leads to the nitration and chlorination of specific apoAI tyrosine residues; 2) plasma apoAI nitro-and chloro-tyrosine levels are higher in coronary artery disease patients than in control subjects; and 3) apoAI tyrosine chlorination, whether in endogenous plasma or after in vitro MPO-mediated modification, is associated with the specific loss of ABCA1-mediated cholesterol acceptor activity, such that apoAI cholesterol acceptor activity was inversely correlated with apoAI nitro- and chloro- tyrosine levels48,49,50,51,52,53. Wu et al. demonstrated LCAT binding to apoAI at residues 159–180 in rHDL; and, that Tyr166 is required for LCAT activation, such that MPO induced loss of LCAT activation correlates with loss of the Tyr166 containing parent peptide54. Additionally, Zheng et al. demonstrated direct binding of MPO with apoAI, and identified residues 190–203 in apoAI helix 8 as the region that bind to MPO, thus providing a mechanism for the selective modification of apoAI in plasma48. However, the precise mechanism by which MPO modification renders apoAI dysfunctional remains controversial.
Both groups using mass spectroscopy studies identified apoAI Tyr192, within helix 8, as the most sensitive site to MPO mediated chlorination 51,52. Furthermore, Bergt et al. demonstrated, using short synthetic peptides, that tyrosine chlorination is maximally stimulated when the tyrosine is three residues away from a lysine residue, which is the case for the sensitive Tyr192 53. The University of Washington group claimed that MPO impairment of apoAI’s ABCA1-mediated cholesterol acceptor activity is through methionine oxidation and site-specific chlorination of Tyr192, as treatment of MPO modified apoAI with the enzyme methionine sulfide reductase could partially restore apoAI’s cholesterol acceptor activity, particularly in a recombinant apoAI (r-apoAI) isoform with Try192 replaced by Phe43. On the other hand, the Cleveland Clinic group made r-apoAI with all seven Tyr residues replaced by Phe (7YF isoform), and found that it is equally susceptible to MPO or HOCl loss of cholesterol acceptor activity as the wild type isoform55. Furthermore, replacement of the three Met residues in apoAI with Val residues actually increased susceptibility to low doses of H2O2 in the complete MPO chlorination system, showing that apoAI Met residues can absorb HOCl oxidation without loss of function, hence protecting the other residues56, and in agreement with the prior finding42. Thus, the site directed substitution and cholesterol efflux data indicate that neither methionine nor tyrosine serve as the oxidant sensitive residue involved in MPO-induced apoAI inactivation. Thus, the levels of plasma apoAI nitro-and chloro-tyrosine may serve as physiological useful and specific markers of MPO activity in vivo, even though tyrosine modification does not appear to be responsible for turning the “good” HDL-cholesterol “bad”.
Discovery of MPO-resistant apoAI
The Cleveland group extended and confirmed several prior observations on the effects of MPO or HOCl on apoAI structure, specifically the loss of alpha helix content, and the loss of tryptophan and lysine residues. MPO treatment of apoAI leads to the transient conversion of apoAI lysine residues to lysine ε-chloramine that spontaneously decompose to α-aminoadipic acid, a product that is enriched in apoAI recovered from atheroma vs. plasma55,56. However, apoAI with lysine residues modified and rendered less reactive by reductive methylation is not protected from MPO induced loss of function57,56. Thus, lysine modification by MPO is not likely to be the initiating event in formation of dysfunctional HDL. The Cleveland group used mass spectrometry to identify mono- and di-hydroxytryptophan at all four Trp positions in apoAI isolated from human atheroma56. Thus, Peng et al. created r-apoAI with all four Trp residues replaced. The replacement by four Leu residues leads to an inactive 4WL isoform, while the replacement by four Phe residues creates an active 4WF isoform56. Thus, apoAI activity appears to require both hydrophobic and aromatic residues in the Trp positions. When the 4WF and wild type apoAI isoforms were treated with increasing oxidant stress from MPO or HOCl, the 4WF isoform is markedly resistant to loss of cholesterol acceptor activity56. Thus, the 4WF apoAI isoform is MPO resistant, and may be a better therapeutic reagent to promote the regression of atherosclerotic plaques, an environment where MPO and MPO-derived oxidants are abundant. If this strategy is successful, then “good” cholesterol can be made “better”.
Diagnostic use of dysfunctional HDL
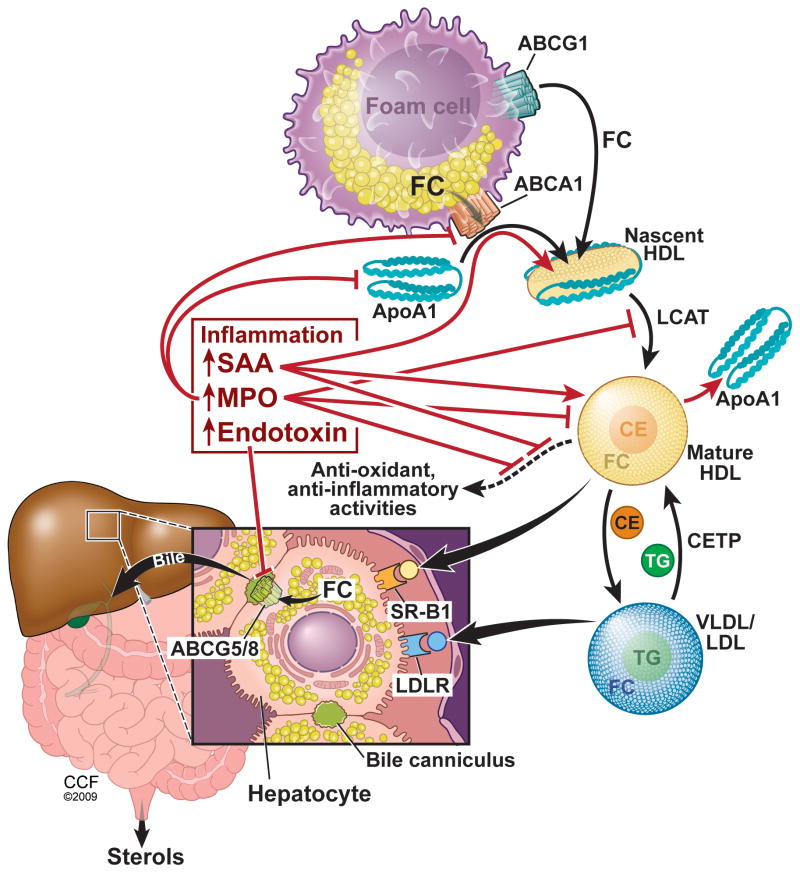
Figure 1 summarizes the central role that inflammation plays in creating dysfunctional HDL and reduced RCT. Biomarkers associated with dysfunctional HDL may be useful for identifying subjects at most risk for a coronary event. Hazen and colleagues reported that plasma MPO levels are positively associated with CAD and the risk of a subsequent major adverse cardiac event 58,59. MPO levels are predictive of events, but are not a specific marker of dysfunctional HDL. In a mixed cohort of cardiovascular disease subjects and controls, Zheng et al., using mass spectrometry, demonstrated that the level of apoAI chlorotyrosine was a better predictor of cardiovascular disease than the levels of total plasma chloro-or nitro-tyrosine 48. This type of assay is more specific for dysfunctional HDL, but is too laborious for routine clinical use. The identification of specific modified residues in apoAI, such as chlorotyrosine and hydroxytryptophan make it possible to develop novel and scalable immunoassays specific for oxidized/dysfunctional HDL. Through the development these new assays, it will be possible to perform observational and interventional studies to determine the association of dysfunctional HDL with CAD, cerebrovascular events, and mortality. Just as the recent JUPITER trial demonstrated the benefit of statin treatment for subjects with elevated CRP and normal LDL levels60, in the future, dysfunctional HDL levels may be used as a criterion for treatment to prevent CAD.
Figure 1.
Central role of inflammation in creating dysfunctional HDL and disrupting RCT. Cholesterol/sterol trafficking from the foam cell to their excretion is shown in black arrows. The effects of inflammation are shown in red. FC, free cholesterol; CE, cholesterol ester; TG, triglycerides.
Acknowledgments
Source of Funding: A portion of the work described in this review was supported by the NIH (HL066082).
References
- 1.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DJ, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Brown BG, Zhao XQ. Nicotinic acid, alone and in combinations, for reduction of cardiovascular risk. Am J Cardiol. 2008;101:58B–62B. doi: 10.1016/j.amjcard.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 3.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008;263:256–273. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 4.Feig JE, Shamir R, Fisher EA. Atheroprotective effects of HDL: beyond reverse cholesterol transport. Curr Drug Targets. 2008;9:196–203. doi: 10.2174/138945008783755557. [DOI] [PubMed] [Google Scholar]
- 5.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 6.Nicholls SJ, Tuzcu EM, Brennan DM, Tardif JC, Nissen SE. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation) Circulation. 2008;118:2506–2514. doi: 10.1161/CIRCULATIONAHA.108.790733. [DOI] [PubMed] [Google Scholar]
- 7.Florentin M, Liberopoulos EN, Wierzbicki AS, Mikhailidis DP. Multiple actions of high-density lipoprotein. Curr Opin Cardiol. 2008;23:370–378. doi: 10.1097/HCO.0b013e3283043806. [DOI] [PubMed] [Google Scholar]
- 8.Sviridov D, Mukhamedova N, Remaley AT, Chin-Dusting J, Nestel P. Antiatherogenic functionality of high density lipoprotein: how much versus how good. J Atheroscler Thromb. 2008;15:52–62. doi: 10.5551/jat.e571. [DOI] [PubMed] [Google Scholar]
- 9.Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, Reddy ST, Sevanian A, Fonarow GC, Fogelman AM. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000;41:1495–1508. [PubMed] [Google Scholar]
- 10.Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G, Rahmani S, Mottahedeh R, Dave R, Reddy ST, Fogelman AM. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108:2751–2756. doi: 10.1161/01.CIR.0000103624.14436.4B. [DOI] [PubMed] [Google Scholar]
- 11.Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:1987–1994. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 12.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza a infection. Circulation. 2001;103:2283–2288. doi: 10.1161/01.cir.103.18.2283. [DOI] [PubMed] [Google Scholar]
- 13.Castellani LW, Navab M, Van Lenten BJ, Hedrick CC, Hama SY, Goto AM, Fogelman AM, Lusis AJ. Overexpression of apolipoprotein AII in transgenic mice converts high density lipoproteins to proinflammatory particles. J Clin Invest. 1997;100:464–474. doi: 10.1172/JCI119554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758–2767. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kontush A, Chapman MJ. Why is HDL functionally deficient in type 2 diabetes? Curr Diab Rep. 2008;8:51–59. doi: 10.1007/s11892-008-0010-5. [DOI] [PubMed] [Google Scholar]
- 17.Davidson WS, Silva RA, Chantepie S, Lagor WR, Chapman MJ, Kontush A. Proteomic Analysis of Defined HDL Subpopulations Reveals Particle-Specific Protein Clusters. Relevance to Antioxidative Function. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.109.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moerland M, Samyn H, van GT, Jauhiainen M, Metso J, van HR, Grosveld F, van TA, de CR. Atherogenic, enlarged, and dysfunctional HDL in human PLTP/apoA-I double transgenic mice. J Lipid Res. 2007;48:2622–2631. doi: 10.1194/jlr.M700020-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.van der Westhuyzen DR, de Beer FC, Webb NR. HDL cholesterol transport during inflammation. Curr Opin Lipidol. 2007;18:147–151. doi: 10.1097/MOL.0b013e328051b4fe. [DOI] [PubMed] [Google Scholar]
- 20.Stonik JA, Remaley AT, Demosky SJ, Neufeld EB, Bocharov A, Brewer HB. Serum amyloid A promotes ABCA1-dependent and ABCA1-independent lipid efflux from cells. Biochem Biophys Res Commun. 2004;321:936–941. doi: 10.1016/j.bbrc.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 21.van der Westhuyzen DR, Cai L, de Beer MC, de Beer FC. Serum amyloid A promotes cholesterol efflux mediated by scavenger receptor B-I. J Biol Chem. 2005;280:35890–35895. doi: 10.1074/jbc.M505685200. [DOI] [PubMed] [Google Scholar]
- 22.Abe-Dohmae S, Kato KH, Kumon Y, Hu W, Ishigami H, Iwamoto N, Okazaki M, Wu CA, Tsujita M, Ueda K, Yokoyama S. Serum amyloid A generates high density lipoprotein with cellular lipid in an A. J Lipid Res. 2006;47:1542–1550. doi: 10.1194/jlr.M600145-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Jahangiri A, de Beer MC, Noffsinger V, Tannock LR, Ramaiah C, Webb NR, van der Westhuyzen DR, de Beer FC. HDL remodeling during the acute phase response. Arterioscler Thromb Vasc Biol. 2009;29:261–267. doi: 10.1161/ATVBAHA.108.178681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Beer MC, Webb NR, Whitaker NL, Wroblewski JM, Jahangiri A, van der Westhuyzen DR, de Beer FC. SR-BI Selective Lipid Uptake. Subsequent Metabolism of Acute Phase HDL. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.109.186502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGillicuddy FC, de la Llera MM, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pirillo A, Uboldi P, Bolego C, Kuhn H, Catapano AL. The 15-lipoxygenase-modified high density lipoproteins 3 fail to inhibit the TNF-alpha-induced inflammatory response in human endothelial cells. J Immunol. 2008;181:2821–2830. doi: 10.4049/jimmunol.181.4.2821. [DOI] [PubMed] [Google Scholar]
- 27.Pirillo A, Uboldi P, Kuhn H, Catapano AL. 15-Lipoxygenase-mediated modification of high-density lipoproteins impairs SR-BI- and ABCA1-dependent cholesterol efflux from macrophages. Biochim Biophys Acta. 2006;1761:292–300. doi: 10.1016/j.bbalip.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Bowry VW, Stanley KK, Stocker R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc Natl Acad Sci U S A. 1992;89:10316–10320. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garner B, Waldeck AR, Witting PK, Rye KA, Stocker R. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J Biol Chem. 1998;273:6088–6095. doi: 10.1074/jbc.273.11.6088. [DOI] [PubMed] [Google Scholar]
- 30.McPherson PA, Young IS, McKibben B, McEneny J. High density lipoprotein subfractions: isolation, composition, and their duplicitous role in oxidation. J Lipid Res. 2007;48:86–95. doi: 10.1194/jlr.M600094-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Nagano Y, Arai H, Kita T. High density lipoprotein loses its effect to stimulate efflux of cholesterol from foam cells after oxidative modification. Proc Natl Acad Sci U S A. 1991;88:6457–6461. doi: 10.1073/pnas.88.15.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salmon S, Maziere C, Auclair M, Theron L, Santus R, Maziere JC. Malondialdehyde modification and copper-induced autooxidation of high-density lipoprotein decrease cholesterol efflux from human cultured fibroblasts. Biochim Biophys Acta. 1992;1125:230–235. doi: 10.1016/0005-2760(92)90050-6. [DOI] [PubMed] [Google Scholar]
- 33.Calvo C, Talussot C, Ponsin G, Berthezene F. Non enzymatic glycation of apolipoprotein A-I. Effects on its self-association and lipid binding properties. Biochem Biophys Res Commun. 1988;153:1060–1067. doi: 10.1016/s0006-291x(88)81336-6. [DOI] [PubMed] [Google Scholar]
- 34.Calvo C, Ponsin G, Berthezene F. Characterization of the non enzymatic glycation of high density lipoprotein in diabetic patients. Diabete Metab. 1988;14:264–269. [PubMed] [Google Scholar]
- 35.Hedrick CC, Thorpe SR, Fu MX, Harper CM, Yoo J, Kim SM, Wong H, Peters AL. Glycation impairs high-density lipoprotein function. Diabetologia. 2000;43:312–320. doi: 10.1007/s001250050049. [DOI] [PubMed] [Google Scholar]
- 36.Szapacs ME, Kim HY, Porter NA, Liebler DC. Identification of proteins adducted by lipid peroxidation products in plasma and modifications of apolipoprotein A1 with a novel biotinylated phospholipid probe. J Proteome Res. 2008;7:4237–4246. doi: 10.1021/pr8001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cogny A, Atger V, Paul JL, Soni T, Moatti N. High-density lipoprotein 3 physicochemical modifications induced by interaction with human polymorphonuclear leucocytes affect their ability to remove cholesterol from cells. Biochem J. 1996;314 (Pt 1):285–292. doi: 10.1042/bj3140285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cogny A, Paul JL, Atger V, Soni T, Moatti N. Structural changes of high-density-lipoprotein apolipoproteins following incubation with human polymorphonuclear cells. Eur J Biochem. 1994;222:965–973. doi: 10.1111/j.1432-1033.1994.tb18947.x. [DOI] [PubMed] [Google Scholar]
- 39.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergt C, Reicher H, Malle E, Sattler W. Hypochlorite modification of high density lipoprotein: effects on cholesterol efflux from J774 macrophages. FEBS Lett. 1999;452:295–300. doi: 10.1016/s0014-5793(99)00677-8. [DOI] [PubMed] [Google Scholar]
- 41.Winterbourn CC. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta. 1985;840:204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- 42.Panzenbock U, Kritharides L, Raftery M, Rye KA, Stocker R. Oxidation of methionine residues to methionine sulfoxides does not decrease potential antiatherogenic properties of apolipoprotein A-I. J Biol Chem. 2000;275:19536–19544. doi: 10.1074/jbc.M000458200. [DOI] [PubMed] [Google Scholar]
- 43.Shao B, Cavigiolio G, Brot N, Oda MN, Heinecke JW. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc Natl Acad Sci U S A. 2008;105:12224–12229. doi: 10.1073/pnas.0802025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergt C, Oettl K, Keller W, Andreae F, Leis HJ, Malle E, Sattler W. Reagent or myeloperoxidase-generated hypochlorite affects discrete regions in lipid-free and lipid-associated human apolipoprotein A-I. Biochem J. 2000;346(Pt 2):345–354. [PMC free article] [PubMed] [Google Scholar]
- 45.Panzenboeck U, Raitmayer S, Reicher H, Lindner H, Glatter O, Malle E, Sattler W. Effects of reagent and enzymatically generated hypochlorite on physicochemical and metabolic properties of high density lipoproteins. J Biol Chem. 1997;272:29711–29720. doi: 10.1074/jbc.272.47.29711. [DOI] [PubMed] [Google Scholar]
- 46.Nightingale ZD, Lancha AH, Jr, Handelman SK, Dolnikowski GG, Busse SC, Dratz EA, Blumberg JB, Handelman GJ. Relative reactivity of lysine and other peptide-bound amino acids to oxidation by hypochlorite. Free Radic Biol Med. 2000;29:425–433. doi: 10.1016/s0891-5849(00)00262-8. [DOI] [PubMed] [Google Scholar]
- 47.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergt C, Pennathur S, Fu X, Byun J, O’brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, Green PS, McDonald TO, Brunzell J, Chait A, Oram JF, O’brien K, Geary RL, Heinecke JW. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem. 2004;279:42977–42983. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- 51.Zheng L, Settle M, Brubaker G, Schmitt D, Hazen SL, Smith JD, Kinter M. Localization of nitration and chlorination sites on apolipoprotein A-I catalyzed by myeloperoxidase in human atheroma and associated oxidative impairment in ABCA1-dependent cholesterol efflux from macrophages. J Biol Chem. 2005;280:38–47. doi: 10.1074/jbc.M407019200. [DOI] [PubMed] [Google Scholar]
- 52.Shao B, Bergt C, Fu X, Green P, Voss JC, Oda MN, Oram JF, Heinecke JW. Tyrosine 192 in Apolipoprotein A-I Is the Major Site of Nitration and Chlorination by Myeloperoxidase, but Only Chlorination Markedly Impairs ABCA1-dependent Cholesterol Transport. J Biol Chem. 2005;280:5983–5993. doi: 10.1074/jbc.M411484200. [DOI] [PubMed] [Google Scholar]
- 53.Bergt C, Fu X, Huq NP, Kao J, Heinecke JW. Lysine residues direct the chlorination of tyrosines in YXXK motifs of apolipoprotein A-I when hypochlorous acid oxidizes high density lipoprotein. J Biol Chem. 2004;279:7856–7866. doi: 10.1074/jbc.M309046200. [DOI] [PubMed] [Google Scholar]
- 54.Wu Z, Wagner MA, Zheng L, Parks JS, Shy JM, III, Smith JD, Gogonea V, Hazen SL. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol. 2007;14:861–868. doi: 10.1038/nsmb1284. [DOI] [PubMed] [Google Scholar]
- 55.Peng DQ, Wu Z, Brubaker G, Zheng L, Settle M, Gross E, Kinter M, Hazen SL, Smith JD. Tyrosine modification is not required for myeloperoxidase-induced loss of apolipoprotein A-I functional activities. J Biol Chem. 2005;280:33775–33784. doi: 10.1074/jbc.M504092200. [DOI] [PubMed] [Google Scholar]
- 56.Peng DQ, Brubaker G, Wu Z, Zheng L, Willard B, Kinter M, Hazen SL, Smith JD. Apolipoprotein A-I Tryptophan Substitution Leads to Resistance to Myeloperoxidase-Mediated Loss of Function. Arterioscler Thromb Vasc Biol. 2008;28:2063–2070. doi: 10.1161/ATVBAHA.108.173815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brubaker G, Peng DQ, Somerlot B, Abdollahian DJ, Smith JD. Apolipoprotein A-I lysine modification: effects on helical content, lipid binding and cholesterol acceptor activity. Biochim Biophys Acta. 2006;1761:64–72. doi: 10.1016/j.bbalip.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 59.Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 60.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]