Abstract
Chemokine (C-C motif) receptor-2 (CCR2) regulates arteriogenesis and angiogenesis, facilitating the MCP-1-dependent recruitment of growth factor-secreting bone marrow-derived cells (BMCs). Here, we tested the hypothesis that the BMC-specific expression of CCR2 is also required for new arteriole formation via capillary arterialization. Following non-ischemic saphenous artery occlusion, we measured the following in gracilis muscles: monocyte chemotactic protein-1 (MCP-1) in wild-type (WT) C57Bl/6J mice by ELISA, and capillary arterialization in WT–WT and CCR2−/−–WT (donor–host) bone marrow chimeric mice, as well as BMC transdifferentiation in EGFP+–WT mice, by smooth muscle (SM) α-actin immunochemistry. MCP-1 levels were significantly elevated 1 day after occlusion in WT mice. In WT–WT mice at day 7, compared to sham controls, arterial occlusion induced a 34% increase in arteriole length density, a 46% increase in SM α-actin+ vessels, and a 45% increase in the fraction of vessels coated with SM α-actin, indicating significant capillary arterialization. However, in CCR2−/−–WT mice, no differences were observed between arterial occlusion and sham surgery. In EGFP+–WT mice, EGFP and SM α-actin never colocalized. We conclude that BMC-specific CCR2 expression is required for skeletal muscle capillary arterialization following arterial occlusion; however, BMCs do not transdifferentiate into smooth muscle.
Keywords: Capillary arterialization, Chemokine (C-C motif) receptor-2, Bone marrow-derived cells
Introduction
Adjustments to microvascular network structure occur in several ways. New capillaries are formed by sprouting from pre-existing microvessels through angiogenesis, while arterioles grow into larger diameter channels through arteriogenesis. Recently, considerable attention has been given to the role of bone marrow-derived cells (BMCs) in angiogenesis and arteriogenesis, particularly with regard to their potential to transdifferentiate into endothelium and/or smooth muscle. Within the context of chronic arterial occlusion models, it has been reported that ex-vivo enriched and re-injected BMCs transdifferentiate into endothelial and/or smooth muscle cells in the heart [1–5] and skeletal muscle [6, 7]. In contrast, endogenous BMCs do not trans-differentiate into endothelium and/or smooth muscle during arteriogenesis during hindlimb ischemia [8, 9].
Monocytes, in particular, facilitate arteriogenesis and angiogenesis as growth factor sources [10–13]. Monocytes are recruited by chemokines, such as monocyte chemotactic protein-1 (MCP-1), and perfusion restoration [14, 15] and angiogenesis [16] are abrogated in MCP-1 deficient mice, while MCP-1 application [17–20] amplifies angiogenesis and/or arteriogenesis. The major receptor for MCP-1 is the seven transmembrane domain C-C chemokine receptor-2 (CCR2). CCR2−/− mice have normal numbers of circulating monocytes and tissue resident macrophages; however, they show marked deficits in monocyte recruitment to sites of inflammation or injury [21, 22]. Interestingly, the impact of CCR2 deletion on arteriogenesis depends on genetic background [23, 24].
Another critical component of microvascular remodeling is new arteriole formation, which occurs when pre-existing capillaries become invested with smooth muscle [25–29] and is called “capillary arterialization” [27] or “arteriolargenesis” [25]. Capillary arterialization occurs in response to many stimuli, including normal growth and maturation [27, 28], skeletal muscle stretch [26], hemodynamic changes [29, 30], electrical stimulation [31], exercise training [32, 33], and growth factor gene [25, 34] and protein [35] delivery, and it appears that mechanical forces play a significant role [36–38]. Many endothelial/smooth muscle growth factor-receptor systems regulate vessel maturation (i.e., PDGF-B, TGF-β, ANG-1, and S1P), and it is likely they are also involved in capillary arterialization [39].
Interestingly, monocytes/macrophages also secrete many of these “vessel maturation-associated” growth factors. Therefore, we hypothesized that BMCs also regulate capillary arterialization. In order to test this hypothesis, we used a non-ischemic saphenous artery occlusion model of capillary arterialization on chimeric C57Bl/6J mice that were engrafted with BMCs from EGFP+ and CCR2−/− mice. The CCR2−/− BMC chimeras were used to test whether the BMC-specific expression of CCR2 is required for capillary arterialization, while the EGFP+ BMC chimeras were used to test whether BMCs transdifferentiate into smooth muscle.
Materials and methods
Bone marrow transplants
All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Virginia and conformed to the American Heart Association Guidelines for the Use of Animals in Research. EGFP+ [C57BL/6-Tg(ACTB–EGFP)1Osb/J] and CCR2−/− (B6.129S4-Ccr2tm1lfc/J) donor mice were from Jackson Laboratory (Bar Harbor, ME, USA). C57Bl/6J wild-type (WT) mice were used as donors for control groups. The bone marrow of host C57Bl/6J WT mice was abrogated via two doses of radiation (550 rad). Host mice were then reconstituted with donor marrow and allowed 6–8 weeks for donor marrow engraftment to create the following groups (donor–host): EGFP+–WT, CCR2−/−–WT, and WT–WT. Mice were kept on sulfa drugs to boost immunity. Group “n” values are given in the figure legends.
Non-ischemic and ischemic arterial occlusion models
Wild-type (WT), WT–WT, EGFP+–WT, and CCR2−/−–WT mice were anesthetized with inhaled 2.5% isoflurane. The saphenous artery was ligated at two points downstream of where the femoral artery bifurcates into the saphenous and popliteal arteries. This “non-ischemic” occlusion scheme was designed to alter hemodynamics in the gracilis adductor muscle without creating ischemia in the distal hindlimb. For comparison, in another set of WT mice (n = 4), an occlusion was placed just distal to the superficial epigastric artery, to create an “ischemic” hindlimb model. Sham surgeries, consisting of all interventions but the arterial occlusion, were performed on the contralateral side.
Laser Doppler perfusion imaging
Anesthetized (i.p 120 mg/kg ketamine, 12 mg/kg xylazine, and 0.08 mg/kg atropine) WT mice were placed on a surgical heating pad. The feet were scanned with a Lisca PIM laser Doppler imager at 512 × 512 pixels resolution to produce a perfusion image. Mean voltage per region was used to calculate relative perfusion ratio (ligated/unligated).
MCP-1 ELISA
Gracilis muscles were harvested from WT mice at days 0 (before surgery), 1, 4, and 7 after “non-ischemic” saphenous artery occlusion. A protein assay was first conducted to determine the total amount of protein per sample. A mouse-specific mCCL2/JE ELISA kit (R&D Systems, Minneapolis, MN, USA) was used according to the manufacturer’s instructions. MCP-1 levels were standardized to pg/ml and normalized to the total amount of sample protein.
Gracilis muscle fixation for immunochemistry
Mice were anesthetized (2.5% isoflurane) and gracilis muscles were topically superfused with 10−4 M adenosine in sterile 37°C Ringer’s solution for 30 min. Mice were euthanized by overdose of sodium pentobarbital, and ice cold 4% paraformaldehyde in PBS was infused through the left ventricle at 100 mmHg until blood was cleared from the muscle. After 30 additional minutes, gracilis muscles were dissected free and washed in ice cold PBS.
Immunochemistry methods
Whole WT–WT and CCR2−/−–WT gracilis muscles were treated with 3 mg/ml type 1 collagenase in PBS for 30 min, followed by PBS washes, and a 3 day incubation at 4°C in 1:200 Cy3-conjugated monoclonal anti-smooth muscle (SM) α-actin (clone 1A4; Sigma) and 0.1% saponin in PBS. Muscles were imaged by confocal microscopy. All visible arterioles were traced using ImageJ software to determine arteriole length density, defined as total arteriole length per unit area of tissue. Muscles that had already been immunolabeled for SM α-actin as whole-mounts were then cryosectioned and incubated with 1:200 AlexaFluor647® (Molecular Probes) conjugated Bandeiraea simplicifolia-1 (BS-I) lectin. BS-I lectin+ vessels and SM α-actin+ vessels were counted and normalized to the number of muscle fibers. Gracilis muscles from EGFP+–WT chimeric mice were harvested at day 7, perfusion fixed as previously described, cross-sectioned, and stained overnight with 1:200 IA4 monoclonal anti-SM α-actin Cy3 conjugate. Approximately 30 cross-sections were examined per mouse to determine whether EGFP+ was co-localized with SM α-actin.
Statistics
One-way (Fig. 1c) or two-way (Figs. 1b, 2c, 3b, c) ANOVAs were performed followed by pairwise comparisons with the Student–Newman–Keuls method. Significance was assessed at P < 0.05.
Fig. 1.
Animal model characterization. a Line graph of laser doppler perfusion imaging (LDPI) ratios for “non-ischemic” saphenous (n = 3) and “ischemic” (n = 3) femoral artery occlusion models in C57Bl/6J mice. Values are mean ± standard deviation (SD). * Significantly different than non-ischemic at same time point (P <0.05). b Bar graph of MCP-1 expression levels in WT gracilis muscle after non-ischemic saphenous artery occlusion at days 0, 1, 4, and 7 (n = 4 per group). Values are mean ± SD. * Significantly different than all other time points (P < 0.05)
Fig. 2.
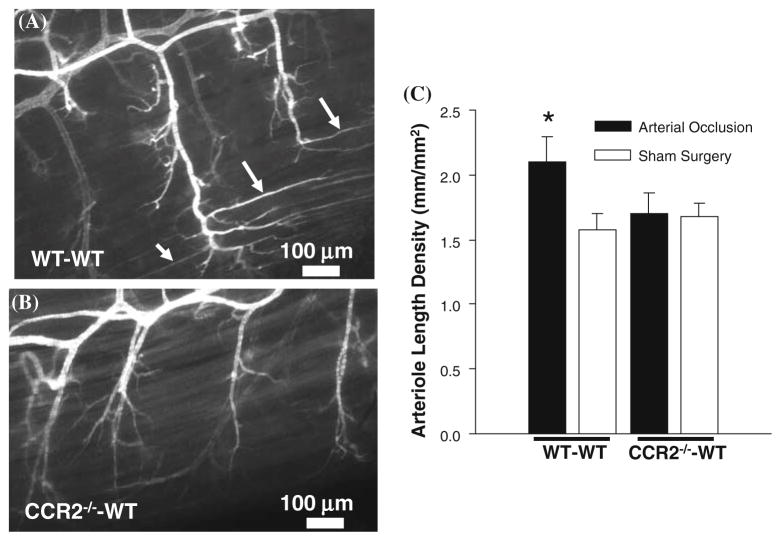
The deletion of CCR2 from BMCs inhibits capillary arterialization as assessed in whole-mounts. a, b Confocal images of SM α-actin labeled gracilis muscles exposed to arterial occlusion from WT–WT (a) and CCR2−/−–WT (b) mice at day 7. Arrows denote long extensions of SM α-actin expression along capillaries running parallel to the muscle fiber direction. c Bar graph of arteriole length density for WT–WT (n = 5) and CCR2−/−–WT (n = 5) mice. * Significantly different than all other groups (P <0.05)
Fig. 3.
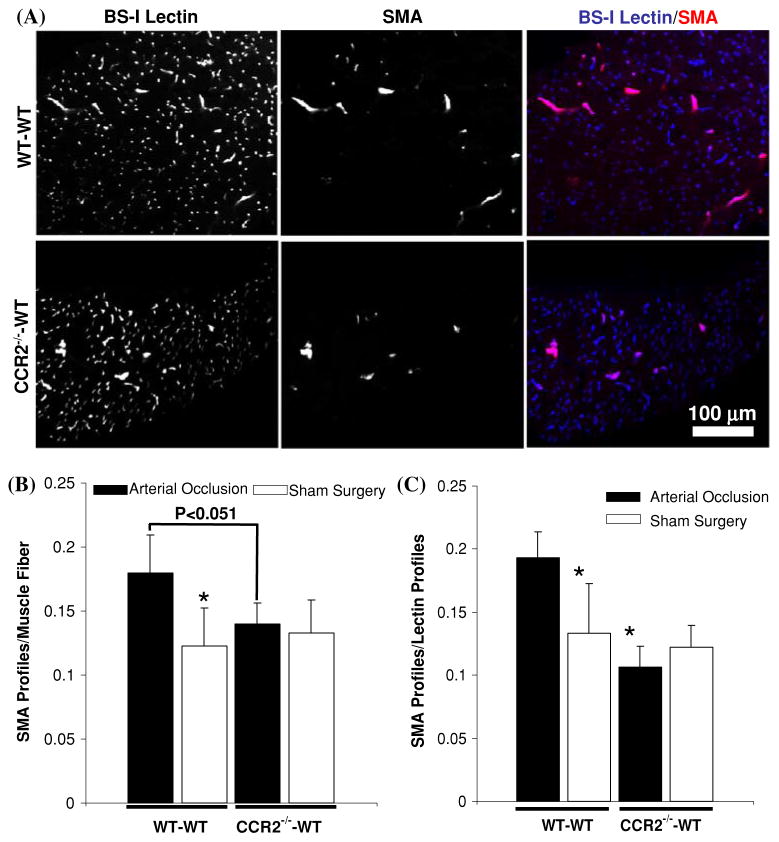
The deletion of CCR2 expression from BMCs inhibits capillary arterialization as assessed in cross-sections. a Confocal images of cross-sectioned gracilis muscles from WT–WT and CCR2−/−–WT mice at 7 days after arterial occlusion. Arterioles and venules are labeled with SM α-actin, while all small microvessels are labeled with BS-I lectin. Co-localization of BS-I lectin and SM α-actin is shown in the merge panel. b Bar graph of SM α-actin+ profiles per muscle fiber for WT–WT (n = 5) and CCR2−/−–WT (n = 5) mice. * Significantly different than WT–WT arterial occlusion group (P < 0.05). c Bar graph of the ratio of SM α-actin+ profiles to BS-I lectin profiles. * Significantly different than WT–WT saphenous occlusion group (P <0.05)
Results
Characterization of the non-ischemic occlusion model
In order to characterize the saphenous artery occlusion model, Laser Doppler perfusion imaging (LDPI) scans were performed and compared to a standard ischemic hindlimb (Fig. 1a and b). Immediately post-op, perfusion ratio did not change for the “non-ischemic” group, but fell markedly in the ischemic group. Over 7 days, perfusion ratio increased in the ischemic group but remained significantly below the non-ischemic group. MCP-1 protein levels were significantly elevated after 1 day and then returned to baseline by day 4 (Fig. 1c).
BMC-specific CCR2 expression is required for capillary arterialization
Figure 2a and b shows representative confocal images from WT–WT and CCR2−/−–WT specimens exposed to arterial occlusion for 7 days, respectively. Long thin extensions of SM α-actin expression originating at the terminal arterioles and running parallel to the muscle fiber direction were observed in the WT–WT specimens (Fig. 2a), but largely absent from the CCR2−/−–WT specimens (Fig. 2b). We observed a 36% increase in arteriole length density with arterial occlusion in WT–WT mice. In contrast, within the CCR2−/−–WT group, no changes in arteriole length density were observed (Fig. 2c).
Representative confocal images from cross-sectioned WT–WT and CCR2−/−–WT gracilis muscles exposed to arterial occlusion are shown in Fig. 3a. As expected, SM α-actin was always colocalized with BS-I lectin, indicating that smooth muscle cells, pericytes, and/or their precursors only express SM α-actin when they come in contact with endothelium. In addition, the total density of SM α-actin+ vessels appears significantly enhanced in the WT–WT specimen. Within the WT–WT group, arterial occlusion caused a 46% increase in SM α-actin+ vessels over sham surgery control (Fig. 3b). In contrast, within the CCR2−/−–WT group, no changes occurred with arterial occlusion. The number of SM α-actin+ vessels/muscle fiber between arterially ligated gracilis muscles of WT–WT and CCR2−/−–WT mice was close to achieving statistical significance (P < 0.051). Finally, we also determined the fraction of total microvessels (i.e., BS-I lectin+) that were also SM α-actin+ (Fig. 3c). These results generally mirrored those in Fig. 3b. Here, arterial occlusion induced a 45% increase in SM α-actin+ vessel fraction over sham surgery within the WT–WT group, but no differences were observed for this metric within the CCR2−/−–WT group.
BMCs do not contribute to capillary arterialization by transdifferentiating into smooth muscle
We tested whether BMCs contribute to this response by directly transdifferentiating into smooth muscle, pericytes, and/or their precursors through the use of EGFP+–WT chimeras. Several cross-sections from EGFP+–WT gracilis muscles 7 days after arterial occlusion were exhaustively examined for evidence of colocalization between EGFP and SM α-actin. While EGFP+ cells were clearly present in the muscle (Fig. 4), despite examining hundreds of SM α-actin+ vessels, we never observed the colocalization of EGFP with SM α-actin.
Fig. 4.
Representative confocal images of EGFP+ BMCs in SM α-actin immunolabeled gracilis muscle at 7 days after “non-ischemic” saphenous artery occlusion. The colocalization of EGFP with SM α-actin was never observed in EGFP+–WT bone marrow chimeric mice (n = 3)
Discussion
There are three major new findings in this study. First, we showed that new arterioles form via capillary arterialization in the gracilis adductor muscle in response to occlusion of the saphenous artery. In this model, saphenous artery occlusion did not create ischemia in the distal hindlimb. MCP-1 levels were, however, markedly increased in the gracilis muscle 1 day after arterial occlusion. Second, by using this saphenous occlusion model in conjunction with three different metrics of capillary arterialization, we determined that capillary arterialization is completely blocked in CCR2−/−–WT bone marrow chimeric mice. Third, through examination of SM α-actin labeled specimens from EGFP+–WT mice, we observed that the BMCs which are recruited to gracilis muscle tissue do not express SM α-actin. Overall, based on these findings, we conclude that the BMC-specific expression of CCR2 is required for capillary arterialization in response to saphenous artery occlusion in skeletal muscle; however, the contribution of BMCs to capillary arterialization does not include their transdifferentiation into smooth muscle. While other investigators have shown that the MCP-1/CCR2 axis is a critical regulator of both arteriogenesis and angiogenesis, the current study is the first to show that CCR2 expression on BMCs is also essential for new terminal arteriole formation.
CCR2+ BMCs and the regulation of capillary arterialization
Although not nearly as well studied as arteriogenesis and angiogenesis, capillary arterialization has been shown to occur during maturation [27, 28], muscle stretch [26], altered hemodynamics [29, 30], electrical stimulation [31], exercise training [32, 33], and in response to growth factor gene and protein delivery [25, 34]. A recurring theme in many of these studies is that mechanical forces likely play a significant role, a concept that is further supported by computational modeling studies [36–38]. Although microvascular hemodynamics were not explicitly measured in this study, an occlusion was placed along the saphenous artery to alter blood flow patterns in the gracilis muscle and stimulate capillary arterialization in control WT–WT mice. Since ischemia is another putative stimulus for capillary arterialization, we used LDPI to confirm that no changes in distal hindlimb perfusion occurred in this arterial occlusion model (Fig. 1a). With regard to the upper hindlimb, Deindl et al. [40] have shown that, even in more severe femoral artery occlusion models in which ischemia is created in the distal hindlimb, the upper hindlimb is not affected. Overall, we argue that it is unlikely that ischemia was a contributing factor in the more moderate arterial occlusion model used here.
If hemodynamic changes were indeed the primary stimulus, a linkage between hemodynamics and CCR2+ BMCs most likely exists. Importantly, studies have shown that both shear stress gradients [41] and circumferential wall stress/stretch [42, 43] can activate the expression of MCP-1 by endothelial cells and/or smooth muscle, and we determined that MCP-1 levels were elevated in gracilis muscle after arterial occlusion (Fig. 1b). Therefore, we postulate that alterations in microvascular hemodynamics caused an increase in MCP-1 that, in turn, elicited the recruitment of circulating BMCs via the CCR2 receptor. Our studies neither pinpoint where within the microvascular network BMC capture and emigration occurred, nor do they identify the subsequent molecular mechanism(s) through which these BMCs caused capillary arterialization; however, results from the EGFP+–WT mice suggest that they do not transdifferentiate into smooth muscle. Through the process of elimination, it is likely that BMCs serve as paracrine growth factor sources that facilitate capillary arterialization; however, further studies are clearly needed to adequately address this hypothesis.
Comparisons to arterial occlusion studies using CCR2−/− mice
One day after non-ischemic ligation, MCP-1 protein levels in gracilis muscles from C57Bl/6J WT mice increased from “undetectable” to 17 pg/mg of protein (Fig. 1b). In general, this result agrees with Tang et al. [24], who reported a 6.1-fold increase MCP-1 mRNA levels in the thigh of C57Bl/6J mice, and Capoccia et al. [44] who reported that MCP-1 protein levels increased by 50 pg/ml 24 h after arterial occlusion. In contrast to our results, as well as those of Tang et al. [24] and Capoccia et al. [44], Contreras-Shannon et al. [45] found no change in MCP-1 levels in the thighs of WT mice after arterial occlusion. Although difficult to reconcile, this could be due to differences in the muscle(s) tested, the occlusion model, and/or the timing of measurements. Contreras-Shannon et al. [45] did, however, observe significantly increased MCP-1 levels in CCR2−/− mice, which at least further confirms that MCP-1 levels can be enhanced in the thigh by arterial occlusion.
Arteriogenesis and distal hindlimb reperfusion have been studied in CCR2−/− mice [23, 24, 45]; however, to the best of our knowledge, only Tang et al. [24] have examined SMA+ arteriole density in the thigh region, finding trends toward increased SMA+ density with arterial occlusion in both WT and CCR2−/− mice. In general agreement with this trend, our results from whole-mounted (Fig. 2) and cross-sectioned (Fig. 3) muscles show enhanced capillary arterialization in WT–WT mice; however, we did not observe capillary arterialization in CCR2−/−–WT mice. However, we note that Tang et al. [24] did not observe entire cross-sections, and they used low power microscope fields, so changes in very small diameter SM α-actin+ vessels may have been difficult to detect. Furthermore, in contrast to our study, CCR2 expression was deleted from all cells in the study by Tang et al. [24]. With regard to capillary arterialization, it is possible that the lack of endothelial [46, 47] and smooth muscle cell [48] CCR2 expression could have countered the effects of CCR2 deletion from BMCs.
Comparisons to arterial occlusion studies of BMC transdifferentiation into smooth muscle
Smooth muscle (SM) α-actin is expressed by both mature and immature smooth muscle [27], as well as by myofibroblasts [49] and pericytes [50, 51]; therefore, the choice of SM α-actin as a marker for the potential transdifferentiation of BMCs into a smooth muscle lineage provided a high probability of positive detection. Nonetheless, despite observing hundreds of SM α-actin+ vessels with confocal microscopy at high magnification, we never observed the colocalization of EGFP with SM α-actin (Fig. 4). These results confirm a recent study from our group in which ultrasonic microbubble destruction stimulated the formation of new arterioles in EGFP+–WT mice without the transdifferentiation of BMCs into smooth muscle [8]. In addition, Ziegelhoeffer et al. [9] have shown that BMCs do not transdifferentiate into smooth muscle during hind-limb arteriogenesis. While smooth muscle progenitor cells are present in bone marrow [52, 53] and bone marrow-derived cells can transdifferentiate into smooth muscle [1, 2], there are substantial differences between these studies and ours. Of primary importance, studies in which BMCs transdifferentiate into microvascular smooth muscle have had clear therapeutic goals, and were not necessarily designed to determine the role of endogenous BMCs in physiological capillary arterialization. The protocols in these studies involve ex-vivo enrichment and processing of BMCs, as well as the re-injection or re-seeding of BMCs into either the venous circulation or directly to the tissue of interest. In our studies, EGFP+–WT mice were lethally irradiated and engrafted with BMCs approximately 8 weeks before arterial occlusion; however, BMCs were not enriched ex-vivo, nor were they supplemented with additional cell injections. For this reason, we argue that our experimental model better represents the role of BMCs in physiological capillary arterialization.
Acknowledgments
This study was supported by NIH R01 HL74082 to R. J. P. and American Heart Association Pre-Doctoral Fellowships to C. W. B. and J. K. M.
References
- 1.Belmadani S, Matrougui K, Kolz C, Pung YF, Palen D, Prockop DJ, Chilian WJ. Amplification of coronary arteriogenic capacity of multipotent stromal stem cells by epidermal growth factor. Arterioscler Thromb Vasc Biol. 2009;29:802–808. doi: 10.1161/ATVBAHA.109.186189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafe M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leria A, Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 3.Kamota T, Li T-S, Morikage N, Murakami M, Ohshima M, Kubo M, Kobayashi T, Mikamo A, Ikeda Y, Matsuzaki M, Hamano K. Ischemic pre-conditioning enhances the mobilization and recruitment of bone marrow stem cells to protect against ischemia/reperfusion injury in the late phase. J Am Coll Cardiol. 2009;53:1814–1822. doi: 10.1016/j.jacc.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann NY Acad Sci. 2001;938:221–230. doi: 10.1111/j.1749-6632.2001.tb03592.x. [DOI] [PubMed] [Google Scholar]
- 5.Yeh ETH, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;208:2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 6.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 7.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 8.Chappell JC, Song J, Klibanov AL, Price RJ. Ultrasonic microbubble destruction stimulates therapeutic arteriogenesis via the CD18-dependent recruitment of bone marrow derived cells. Arterioscl Throm Vasc Biol. 2008;28:1117–1122. doi: 10.1161/ATVBAHA.108.165589. [DOI] [PubMed] [Google Scholar]
- 9.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 10.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002;283:H2411–H2419. doi: 10.1152/ajpheart.01098.2001. [DOI] [PubMed] [Google Scholar]
- 12.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 13.Rehman J, La J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;207:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 14.Shireman PK, Contreras-Shannon V, Ochoa O, Karia BP, Michalek JM, McManus LM. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leuk Biol. 2007;81:775–785. doi: 10.1189/jlb.0506356. [DOI] [PubMed] [Google Scholar]
- 15.Voskuil M, Hoefer IE, van Royen N, Hua J, de Graaf S, Bode C. Abnormal monocyte recruitment and collateral artery formation in monocyte chemoattractant-1 deficient mice. Vasc Med. 2004;9:287–292. doi: 10.1191/1358863x04vm571oa. [DOI] [PubMed] [Google Scholar]
- 16.Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, Kovacs EJ, DiPietro LA. Wound healing in MIP-1−/− and MCP-1−/− mice. Am J Pathol. 2001;159:457–463. doi: 10.1016/s0002-9440(10)61717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoefer IE, van Royen N, Rectenwald JE, Deindl E, Hua J, Jost M, Grundmann S, Voskuil M, Ozaki CK, Piek JJ, Buschmann IR. Arteriogenesis proceeds via ICAM-1/Mac-1-mediated mechanisms. Circ Res. 2004;94:1179–1185. doi: 10.1161/01.RES.0000126922.18222.F0. [DOI] [PubMed] [Google Scholar]
- 18.Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res. 1997;80:829–837. doi: 10.1161/01.res.80.6.829. [DOI] [PubMed] [Google Scholar]
- 19.Muhs A, Lenter MC, Seidler RW, Zweigert R, Kirchengast M, Weser R, Ruedinger M, Guth B. Nonviral monocyte chemoattractant protein-1 gene transfer improves arteriogenesis after femoral artery occlusion. Gene Ther. 2004;11:1685–1693. doi: 10.1038/sj.gt.3302360. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz ER, Meven DA, Sulemanjee NK, Kersting PH. Monocyte chemoattractant protein-1-induced monocyte infiltration produces angiogenesis but not arteriogenesis in chronically infracted myocardium. J Cardiovasc Pharmacol Ther. 2004;9:279–289. doi: 10.1177/107424840400900408. [DOI] [PubMed] [Google Scholar]
- 21.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ Res. 2004;94:671–677. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 24.Tang GL, Chang DS, Sarkar R, Wang R, Messina LM. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J Vasc Surg. 2005;41:312–320. doi: 10.1016/j.jvs.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Benest AV, Stone OA, Miller WH, Glover CP, Uney JB, Baker AH, Harper SJ, Bates DO. Arteriolargenesis and angiogenesis induced by endothelial nitric oxide synthase overexpression results in a mature vasculature. Arterioscler Thromb Vasc Biol. 2008;28:1462–1468. doi: 10.1161/ATVBAHA.108.169375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen-Smith F, Egginton S, Zhou A-L, Hudlicka O. Growth of arterioles precedes that of capillaries in stretch-induced angiogenesis in skeletal muscle. Microvasc Res. 2001;62:1–14. doi: 10.1006/mvre.2001.2308. [DOI] [PubMed] [Google Scholar]
- 27.Price RJ, Owens GK, Skalak TC. Immunohistochemical identification of arteriolar development using markers of smooth muscle differentiation: evidence that capillary arterialization proceeds from terminal arterioles. Circ Res. 1994;75:520–527. doi: 10.1161/01.res.75.3.520. [DOI] [PubMed] [Google Scholar]
- 28.Stingl J, Rhodin JAG. Early postnatal growth of skeletal muscle blood vessels in the rat. Cell Tissue Res. 1994;275:419–434. doi: 10.1007/BF00318812. [DOI] [PubMed] [Google Scholar]
- 29.Van Gieson EJ, Murfee WL, Skalak TC, Price RJ. Enhanced spatial smooth muscle cell coverage of microvessels exposed to increased hemodynamic stresses in vivo. Circ Res. 2003;92:929–936. doi: 10.1161/01.RES.0000068377.01063.79. [DOI] [PubMed] [Google Scholar]
- 30.Price RJ, Skalak TC. Chronic α1 adrenergic blockade stimulates terminal and arcade arteriolar development. Am J Physiol. 1996;271:H752–H759. doi: 10.1152/ajpheart.1996.271.2.H752. [DOI] [PubMed] [Google Scholar]
- 31.Hansen-Smith FM, Egginton S, Hudlicka O. Growth of arterioles in chronically stimulated adult rat skeletal muscle. Microcirculation. 1998;5:49–59. [PubMed] [Google Scholar]
- 32.Binder KW, Song J, Murfee WL, Laughlin MH, Price RJ. Computational network model prediction of hemodynamic alterations due to arteriolar remodeling in interval sprint trained skeletal muscle. Microcirculation. 2007;14:181–192. doi: 10.1080/10739680601139294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lash JM, Bohlen HG. Functional adaptation of rat skeletal muscle arterioles to aerobic exercise training. J Appl Physiol. 1992;72:2052–2062. doi: 10.1152/jappl.1992.72.6.2052. [DOI] [PubMed] [Google Scholar]
- 34.Rissanen TT, Korpisalo P, Markkanen JE, Liimatainen T, Ordén M-R, Kholová I, de Goede A, Heikura T, Gröhn OH, Ylä-Herttuala S. Blood flow remodels growing vasculature during vascular endothelial growth factor gene therapy and determines between capillary arterialization and sprouting angiogenesis. Circulation. 2005;112:3937–3946. doi: 10.1161/CIRCULATIONAHA.105.543124. [DOI] [PubMed] [Google Scholar]
- 35.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 36.Price RJ, Skalak TC. Circumferential wall stress as a mechanism for arteriolar rarefaction and proliferation in a network model. Microvasc Res. 1994;47:188–202. doi: 10.1006/mvre.1994.1015. [DOI] [PubMed] [Google Scholar]
- 37.Price RJ, Skalak TC. A circumferential wall stress-growth rule predicts arcade arteriole formation in a network model. Microcirculation. 1995;2:41–51. doi: 10.3109/10739689509146758. [DOI] [PubMed] [Google Scholar]
- 38.Price RJ, Less JR, Van Gieson EJ, Skalak TC. Hemodynamic stresses and structural remodeling of anastomosing arteriolar networks: design principles of collateral arterioles. Microcirculation. 2002;9:111–124. doi: 10.1038/sj/mn/7800127. [DOI] [PubMed] [Google Scholar]
- 39.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 40.Deindl E, Buschmann I, Hoefer IE, Podzuweit T, Boengler K, Vogel S, van Royen N, Fernandez B, Schaper W. Role of ischemia and of hypoxia-inducible genes in arteriogenesis after femoral artery occlusion in the rabbit. Circ Res. 2001;89:779–786. doi: 10.1161/hh2101.098613. [DOI] [PubMed] [Google Scholar]
- 41.Shyy Y, Hsieh HJ, Usami S, Chien S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Nat Acad Sci USA. 1994;91:4678–4682. doi: 10.1073/pnas.91.11.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demicheva E, Hecker M, Korff T. Stretch-induced activation of the transcription factor activator protein-1 controls monocyte chemoattractant protein-1 expression during arteriogenesis. Circ Res. 2008;103:477–484. doi: 10.1161/CIRCRESAHA.108.177782. [DOI] [PubMed] [Google Scholar]
- 43.Wung BS, Cheng JJ, Hsieh HJ, Shyy YJ, Wang DL. Cyclic strain–induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ Res. 1997;81:1–7. doi: 10.1161/01.res.81.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Capoccia BJ, Gregory AD, Link DC. Recruitment of the inflammatory subset of monocytes to sites of ischemia induces angiogenesis in a monocyte chemoattractant protein-1-dependent fashion. J Leukoc Biol. 2008;84:760–768. doi: 10.1189/jlb.1107756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Contreras-Shannon V, Ochoa O, Reyes-Reyna SM, Sun D, Michalek JE, Kuziel WA, McManus LM, Shireman PK. Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2−/− mice following ischemic injury. Am J Physiol Cell Physiol. 2007;292:C953–C967. doi: 10.1152/ajpcell.00154.2006. [DOI] [PubMed] [Google Scholar]
- 46.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 47.Weber KS, Nelson PJ, Grone HJ, Weber C. Expression of CCR2 by endothelial cells: implications for MCP-1 mediated wound injury repair and in vivo inflammatory activation of endothelium. Arterioscler Thromb Vasc Biol. 1999;19:2085–2093. doi: 10.1161/01.atv.19.9.2085. [DOI] [PubMed] [Google Scholar]
- 48.Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24:1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- 49.Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C. Smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell. 2003;14:2508–2519. doi: 10.1091/mbc.E02-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol. 1991;113:147–157. doi: 10.1083/jcb.113.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verbeek MM, Otte-Höller I, Ruiter DJ, de Waal RM. Induction of alpha-smooth muscle actin expression in cultured human brain pericytes by transforming growth factor-beta 1. Am J Pathol. 1994;144:372–382. [PMC free article] [PubMed] [Google Scholar]
- 52.Foubert P, Matrone G, Souttou B, Lére-Déan C, Barateau V, Poluët J, Ricousse-Roussane SL, Lévy BI, Silvestre J-S, Tobelem G. Coadministration of endothelial and smooth muscle progenitor cells enhances the efficacy of proangiogenic cell-based therapy. Circ Res. 2008;103:751–760. doi: 10.1161/CIRCRESAHA.108.175083. [DOI] [PubMed] [Google Scholar]
- 53.Melero-Martin JM, De Obaldia ME, Kang S-Y, Khan ZA, Yuan L, Oettgen P, Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]