Abstract
Purpose
Here we assessed whether silibinin, a nontoxic chemopreventive agent, inhibits spontaneous intestinal tumorigenesis in APCmin/+ mouse model, a genetically predisposed animal model of human familial adenomatous polyposis (FAP).
Materials and Methods
Six-week old APCmin/+ mice were divided into four groups and orally gavaged with 0.2 ml vehicle, or 250, 500 and 750 mg silibinin/kg body weight in 0.2 ml vehicle for five days/week. After 6 weeks, polyp burden was analyzed and tissues examined for molecular alterations.
Results
Silibinin treatments decreased total number of intestinal polyps by 34% (P<0.01), 42% (P<0.01) and 55% (P<0.001), respectively. Immunohistochemical analysis showed that silibinin dose-dependently decreases (P<0.001) proliferation and induces (P<0.001) apoptosis only in intestinal polyps without any considerable effects on normal crypt-villi in APCmin/+ or wild-type mice. Further analysis of polyps showed that silibinin decreases β-catenin, cyclin D1, c-Myc and phospho-glycogen synthase kinase-3β expression. Silibinin treatment also decreased phospho-Akt, cyclooxygenase-2, inducible nitric oxide synthase, nitrotyrosine and nitrite levels in polyps, the well-known mediators of intestinal/colon carcinogenesis.
Conclusion
Together, these results establish silibinin efficacy in a well-established genetic model of FAP, APCmin/+ mouse, and suggest that this natural agent modulates various molecular pathways including β-catenin in its overall chemopreventive efficacy against intestinal carcinogenesis.
Keywords: colon cancer, chemoprevention, silibinin, COX-2, beta-catenin
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the United States (1). Risk factors for CRC are multi-factorial including a combination of hereditary, dietary and environmental factors (1). Particularly, colon cancer progression is a consequence of accumulation of multiple genetic alterations and mutations. Especially, mutation in tumor suppressor gene adenomatous polyposis coli (APC) is reported in familial and most of the sporadic CRC, which plays a pivotal role in early stages of CRC development by altering β-catenin pathway (2). Recent efforts to control CRC incidence and to minimize cytotoxicity of chemotherapeutic agents have mainly focused on chemoprevention strategies (3). Since colon carcinogenesis is a multistage process involving progression of normal epithelium to malignant phenotype that exhibits various alterations in cellular and molecular signaling cascades (2), chemoprevention by natural products is expected to delay neoplastic promotion/progression to more advanced malignancy (3). However, the efficacy of chemopreventive agents needs to be established in well defined preclinical models of CRC before embarking on to clinical trials.
APCmin/+ mouse is one of the CRC animal models which harbors dominant germline mutation at codon 850 of the APC, the mouse homologue of human APC gene (4). APCmin/+ mice are heterozygous for a mutant allele of APC that has lost its function by loss of heterozygosity (5). APCmin/+ mice develop multiple adenomas primarily in their small intestine and only fewer tumors arise in the colon, mostly due to aberrant β-catenin signaling (4, 5). Intestinal polyps in APCmin/+ mice are similar to those in duodenum of familial adenomatous polyposis (FAP) patients (6), suggesting that APCmin/+ mouse represents a clinically relevant animal model mimicking human disease. This mouse model is indeed extensively used to study molecular mechanisms involved in intestinal tumorigenesis and cancer chemopreventive efficacy of different agents (6, 7).
Silibinin, a flavonolignan constituent in silymarin extracted from milk thistle (Silybum marianum) plant (8), has received significant attention in recent years due to its strong cancer chemopreventive and anti-cancer efficacy against a wide-range of epithelial cancers (9-20). Recent studies by others and us have shown silibinin efficacy in various in vitro and in vivo experimental models of skin (9), lung (11,12), prostate (10,13,14) and bladder (15,16) cancers. Regarding CRC, in vitro studies showed that silibinin causes cell cycle arrest and induces apoptosis in human CRC cells (17), and that its oral feeding to nude mice inhibits HT-29 xenograft growth by inhibiting tumor cell proliferation and angiogenesis (18). Regarding its CRC chemopreventive efficacy, we showed that silibinin feeding inhibits azoxymethane (AOM)-induced colonic aberrant crypt foci formation in Fisher 344 rats (19). Kohno et al (20) showed that crude extract of milk thistle namely silymarin prevents AOM-induced colon carcinogenesis in rat. More recently, 0.2% silibinin feeding in diet to APCmin/+ mice starting at 11-week of age for 7 weeks is shown to produce marginal efficacy against gastrointestinal carcinogenesis which may be due to the fact that silibinin administration was started at later age (21). The present study, therefore, was designed using three different doses of silibinin (250, 500 and 750 mg/kg body weight) gavage feeding to APCmin/+ mice starting at 6-week of age, to address whether this agent inhibits intestinal polyposis in APCmin/+ mice, and if it does, what are the potential mechanisms of its efficacy towards β-catenin pathway and other molecular regulators known to be involved in tumorigenesis in APCmin/+ mouse model.
Materials and Methods
Animals and treatment protocol
Heterozygous male Min (C57BL/6J-APCmin/+) and wild-type C57BL/6J male mice (5-week old) were purchased from Jackson Laboratory. Silibinin was from Sigma, and its purity was checked as >98% as described earlier (22). After one week of acclimatization, APCmin/+ mice (6-week old) were divided into four groups of 12 animals each. Group 1 was given 0.2 ml vehicle consisting of 0.5% carboxymethyl cellulose and 0.025% Tween 20 in distilled water. Group 2-4 animals received 250, 500 and 750 mg silibinin/kg body weight, respectively, by oral gavage in 0.2 ml vehicle for five days/week for 6 weeks. Two negative control groups (n=12/group) of wild-type C57BL/6J mice were given vehicle (Group 5) or 500 mg silibinin/kg body weight (Group 6) as for APCmin/+ mice. Mice in all groups received AIN-76A pellet diet and water ad libitum throughout experimental period. Food consumption and body weights of mice in all groups were recorded weekly, and animals monitored regularly for general health. Animal care and treatments were in accordance with approved protocol and adhered to the “principles of laboratory animal care” and was approved by IACUC.
The rationale for selecting these silibinin doses (250, 500 and 750 mg/kg bw) was based on recent publications by us and others (12-14, 19, 23). These silibinin doses (250-750 mg/kg bw/day) have been extrapolated from the mice consuming up to 1% w/w silibinin in diet that did not show any apparent toxicity, which we have used in many animal studies (12-14). In the present study, the highest dose of silibinin (750 mg/kg bw/day) corresponds to 0.5% w/w silibinin in diet (average mouse body weight 20 g; average diet consumption 3 g/mouse/day or 15 mg silibinin/mouse/day) (13, 14).
Necropsy
After 6 weeks of treatment (at 12-week age), all animals were sacrificed and blood collected in heparinized tubes by cardiac puncture, and plasma separated by centrifugation and stored at −80°C. Small and large intestinal tracts were removed, opened longitudinally and rinsed with saline. The small intestinal samples were divided by length into three equal segments (proximal, middle and distal) and spread onto microscope slides. The total number and size of polyps in all intestinal and colonic segments were examined under a dissecting microscope and measured with digital caliper. A portion of small intestinal and colonic tissues were fixed in 10% (v/v) phosphate-buffered formalin for histopathology and immunohistochemical (IHC) analyses. Tissue sections (5 μm) were cut, processed, and stained with H&E for histopathological evaluation. Remainder of the small intestine and colon samples were snap frozen in liquid nitrogen and stored at −80°C.
Immunohistochemistry and quantification of immunostaining
Paraffin-embedded sections (5-μm thick) were deparaffinized and stained using specific primary antibodies followed by 3,3-diaminobenzidine (DAB) staining, as previously described (14,18). Primary antibodies used were anti-PCNA (1:250 dilution; Dako), anti-β-catenin (1:100 dilution; SantaCruz), anti-nitrotyrosine (1:100 dilution; SantaCruz), anti-c-Myc (1:50 dilution; SantaCruz), rabbit polyclonal anti-cyclin D1 (1:100 dilution; Neomarkers), anti-inducible nitric oxide synthase (iNOS) (1:100 dilution, Abcam), anti-phospho-glycogen synthase kinase (GSK)-3β (Ser 9) (1:50 dilution, Cell Signaling), anti-phospho-Akt (1:50 dilution, Cell Signaling), and goat polyclonal anti-COX-2 (1.100 dilution; SantaCruz). Biotinylated secondary antibodies used were rabbit anti-mouse IgG (Dako) and goat anti-rabbit IgG (Santa Cruz Biotechnology). Apoptotic cells were identified by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining using Dead End Colorometric TUNEL System (Promega Corp). In addition to polyps, all IHC studies also included the crypt and villous regions from all three portions of small intestine. Positive cells were quantified by counting brown-stained cells among total number of cells at 5 randomly selected fields at 400× magnification. iNOS, nitrotyrosine and COX-2 were quantified by immunoreactivity (represented by intensity of brown staining) and scored as 0 (no staining), +1 (very weak), +2 (weak), +3 (moderate), and +4 (strong).
Assay of nitrite/nitrate levels in mouse plasma
iNOS activity in plasma was measured using nitric oxide production by estimating nitrate and nitrite after complete conversion of nitrate in to nitrite by nitrate reductase employing nitrate/nitrite colorimetric assay kit (R&D systems). The final nitrite concentration was the sum of nitrite plus reduced nitrate in each sample and was calculated as an index of iNOS activity as described previously (24).
Statistical and microscopic analyses
Statistical analyses were done using SigmaStat software version 3.5 (Jandel Scientific). Quantitative data are presented as mean ± SEM. Statistical significance of differences between control and silibinin-fed groups was determined by unpaired Student's t-test and P<0.05 was considered statistically significant. Microscopic IHC analyses were done by Zeiss Axioskop 2 microscope (Carl Zeiss, Inc.) and photomicrographs were captured by Carl Zeiss AxioCam MRC5 camera.
Results
Silibinin feeding prevents spontaneous intestinal polyposis in APCmin/+ mice
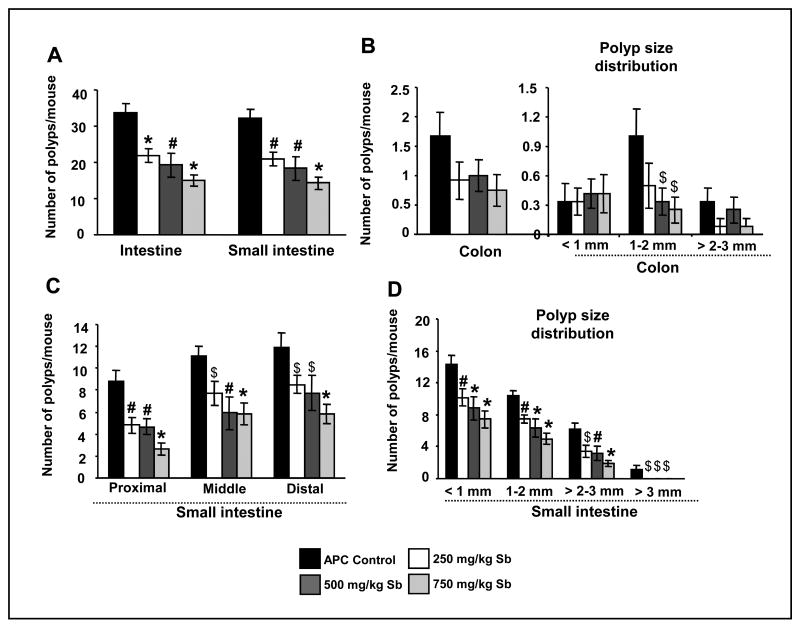
At the end of the study (12-week age), control APCmin/+ mice developed an average of 20-48 polyps in small intestine and ∼0-4 polyps in colon. Six weeks silibinin treatment, however, decreased total number of intestinal polyps by 34% (P<0.01), 42% (P<0.01) and 55% (P<0.001) at 250, 500 and 750 mg/kg body weight doses, respectively (Fig. 1A). Though, silibinin at three dose levels significantly reduced the total number of polyps in small intestinal, however, this effect (∼50% decrease) was statistically non-significant (due to a fewer number of polyps) in colon (Fig. 1B). Importantly, all silibinin treatments significantly reduced number of polyps in proximal, middle and distal portions of small intestine, which was specifically prominent at 750 mg/kg dose where number of polyps was reduced by 70% (P<0.001), 48% (P<0.001), and 51% (P<0.001), respectively (Fig. 1C). In wild-type C57BL/6J mice, both control and silibinin-fed groups did not show any polyps throughout the intestine (data not shown).
Fig. 1.
Silibinin feeding prevents spontaneous intestinal polyposis in APCmin/+ mice. At the end of the study detailed in Materials and Methods, intestinal segments were examined for total number of polyps as well as their sizes. Number of polyps/mouse in APCmin/+ mice is shown for (A) whole intestinal tract and small intestine and (B) large intestine with polyp size distribution (<1 mm, 1-2 mm and >2-3 mm) in colon having no polyps larger than 3 mm. C, number of polyps/mouse in proximal, middle and distal portions of small intestine. D, size distribution (<1 mm, 1-2 mm, >2-3 mm and >3 mm) of polyps in small intestine. The bars shown in each case are mean ± SEM of 12 animals in each group. Significance was analyzed by unpaired Student's t-test. $, P<0.05; #, P<0.01; *, P<0.001 versus control; Sb, silibinin.
In size distribution analysis of small intestinal polyps, silibinin at 500 and 750 mg/kg doses reduced the occurrence/development of <1 mm diameter size polyps by 38% (P<0.001) and 48% (P<0.001) and of 1-2 mm diameter size polyps by 39% (P<0.001) and 52% (P<0.001), respectively (Fig. 1D). Lowest silibinin dose (250 mg/kg) also showed statistically significant inhibition (Fig. 1D)., Silibinin at 250, 500 and 750 mg/kg doses reduced the occurrence of 2-3 mm diameter size polyps by 44% (P<0.05), 49% (P<0.01) and 69% (P<0.001), respectively, and more importantly caused a complete suppression (P<0.05) of the occurrence of >3 mm diameter size polyps (1.08 ± 0.6 polyps in control versus no polyps in all three silibinin-treated groups) (Fig. 1D). In colon, these silibinin doses decreased the number of larger polyps by 50% (P>0.05), 67% (P<0.05) and 75% (P<0.05) for 1-2 mm size, respectively, and 24-76 % (P>0.05) for >2-3 mm size; there were no polyps beyond 3 mm size (Fig. 1B). Collectively, these results clearly showed that silibinin treatment strongly and dose-dependently reduces the number as well as growth of polyps in intestine of APCmin/+ mice.
Effects of silibinin on histopathology of intestine and gross toxicity
In APCmin/+ mice, all polyps were histologically identified as adenomas (Fig. 2A); however, the number and diameter of adenomas were reduced in silibinin administered animals as described above. We did not observe any other histological changes in the crypt-villus axis between control and silibinin-treated APCmin/+ mice (Fig. 2B), which was also histologically similar to that of wild-type mice (Fig. 2C). Further, control and silibinin-treated wild-type C57BL/6J mice showed normal intestinal histology (Fig. 2C). During the experiment, all mice were monitored for body weight and diet consumption which could be either due to intestinal obstruction caused by polyps or silibinin treatment. Food consumption and body weight gain did not show any considerable change between control and silibinin groups throughout the study (Fig. 2D), and no mortality was observed in any group. Silibinin treatment also did not produce any signs of toxicity or any gross changes indicative of toxicity in the organs (liver, lung and kidney) examined. Together, these results suggest that silibinin treatment exerts significant chemopreventive effect selectively against intestinal polyps.
Fig. 2.
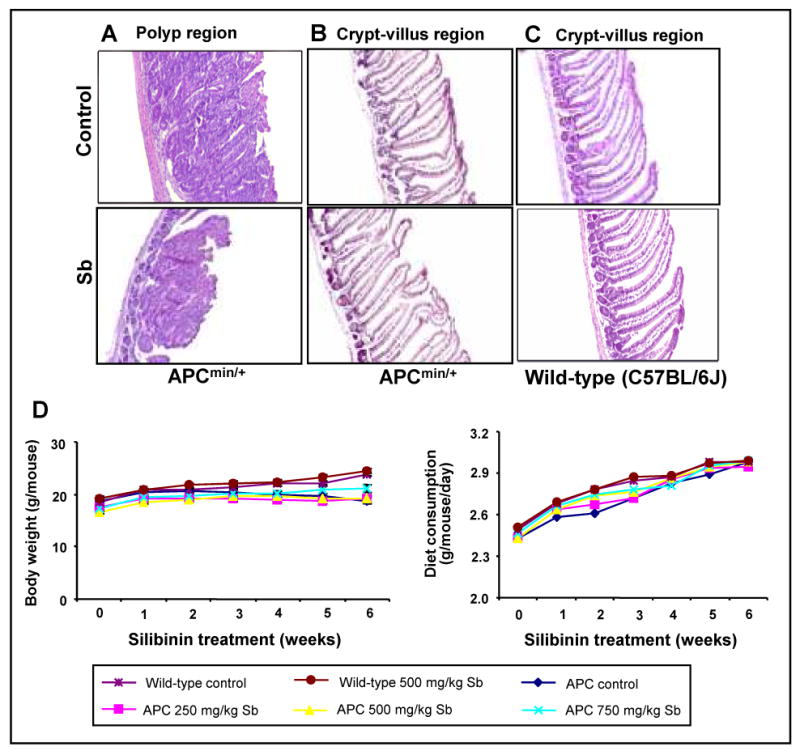
Silibinin feeding selectively prevents polyp burden in small intestine of APCmin/+ mice without any apparent toxicity. At the end of the study detailed in Materials and Methods, small intestine from wild-type C57BL/6J and APCmin/+ mice was processed for H&E staining followed by histological evaluation. A, control and silibinin-treated polyp area from small intestine of APCmin/+ mice (×100). Normal crypt-villus axis from (B) control and silibinin (750mg/kg bw)-treated APCmin/+ mice, and (C) control and silibinin (500 mg/kg bw)-treated wild-type C57BL/6J mice (×100). During experiment, (D) body weight (gram/mouse) and diet consumption (gram/mouse/day) were monitored. Sb, silibinin.
Silibinin treatment reduces proliferation but induces apoptosis selectively in small intestinal polyps of APCmin/+ mice
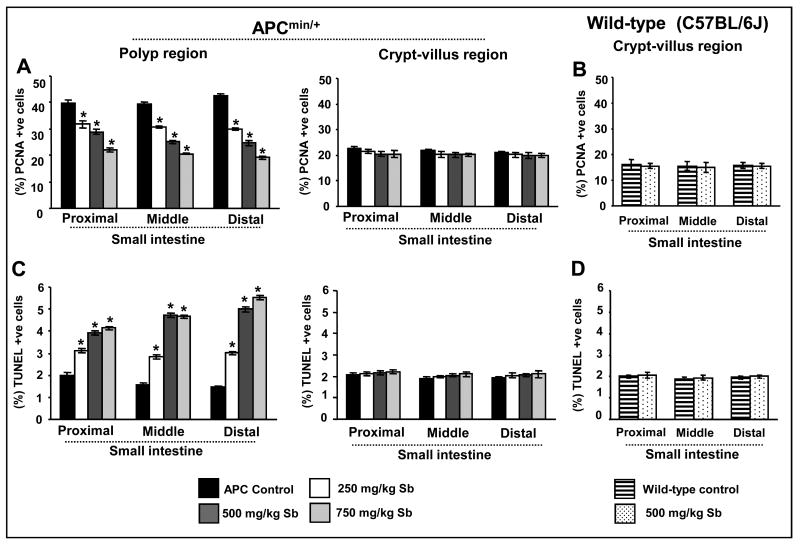
To assess in vivo antiproliferative effect of silibinin, proximal, middle and distal segments of small intestine were analyzed immunohistochemically for PCNA; a marker for cell proliferation (25). Microscopic examination of tissue sections showed a decreased number of PCNA-positive cells only in the polyps from silibinin-treated animals compared to APCmin/+ controls (data not shown). Quantification of PCNA staining showed that silibinin treatment decreases PCNA-positive cells by 20-27% in proximal, 22-36% in middle and 30-42% in distal small intestinal polyps at 250 and 500 mg/kg dose levels compared to APCmin/+ controls (Fig. 3A). A much stronger inhibitory effect of silibinin was observed at its highest dose (750 mg/kg), which reduced proliferation index by 45% (P<0.001), 48% (P<0.001) and 54% (P<0.001) in the polyps in proximal, middle and distal small intestinal segments, respectively (Fig. 3A). Together, these results clearly suggest an in vivo anti-proliferative effect of silibinin selectively in the polyps during spontaneous intestinal tumorigenesis in APCmin/+ mice, as we did not observe any difference in proliferation indices in crypt and villus regions of the all three small intestinal segments from control and silibinin-treated APCmin/+ and wild-type C57BL/6J mice (Fig. 3A and B).
Fig. 3.
Silibinin treatment reduces proliferation but induces apoptosis selectively in small intestinal polyps of APCmin/+ mice. At the end of the study detailed in Materials and Methods, small intestinal segments were processed for PCNA and TUNEL staining. The quantitative data for (A,B) PCNA and (C,D) TUNEL are shown for intestinal polyps and crypt-villus region of APCmin/+ mice (A and C) and crypt-villus region of C57BL/6J wild type mice (B and D), respectively. Data represent mean ± SEM of 8 animals in each group in each case. *, P<0.001 versus control; Sb, silibinin.
To assess the effects of silibinin on in vivo apoptosis induction, we next assessed apoptotic index by TUNEL staining. Qualitative microscopic examination of TUNEL-stained intestinal sections showed an increase in TUNEL-positive cells only in the polyps from silibinin-treated APCmin/+ mice compared to APCmin/+ controls (data not shown). Quantification of TUNEL staining showed that silibinin treatment increases apoptotic cell population by 1.5, 1.9 and 2.0 folds in proximal, by 1.8, 3.0 and 3.0 folds in middle, and by 2.0, 3.0 and 4.0 folds in distal small intestinal polyps at 250, 500 and 750 mg/kg dose levels, respectively, over that of APCmin/+ controls (Fig. 3C). Similar to PCNA, we did not observe any difference in apoptotic indices in the crypt and villus regions of the all three small intestinal segments from control and silibinin-treated APCmin/+ and wild-type C57BL/6J mice (Fig. 3C and D). Overall, these results suggest an important role of pro-apoptotic effect of silibinin in suppressing polyps development in APCmin/+ mice, which could be another potential mechanism underlying its chemopreventive effect on intestinal tumorigenesis in APCmin/+ mice. Since our further IHC analysis showed no significant changes in the expression of various molecular markers in the crypt and villus regions throughout the small intestine of control and silibinin-treated APCmin/+ mice, the results of further analyses in these normal tissues or wild-type mice are not discussed in subsequent sections.
Silibinin treatment reduces phospho-Akt levels selectively in small intestinal polyps of APCmin/+ mice
Akt is known to activate various molecules involved in cell cycle progression, cellular growth and survival, and its phosphorylation represents activation state during carcinogenesis (26). To identify silibinin effect on Akt activation, we examined phospho-Akt expression by IHC. Microscopic observation of all three small intestinal segments indicated strong phospho-Akt-positive cells in the polyps of APCmin/+ controls, which decreased strongly with silibinin treatments (Fig. 4A). The quantification of phospho-Akt-positive cells showed a reduction by 23-28% in proximal, 28-44% in middle and 35-53% in distal portions of small intestinal polyps in silibinin-treated samples compared to APCmin/+ controls (Fig. 4A).
Fig. 4.
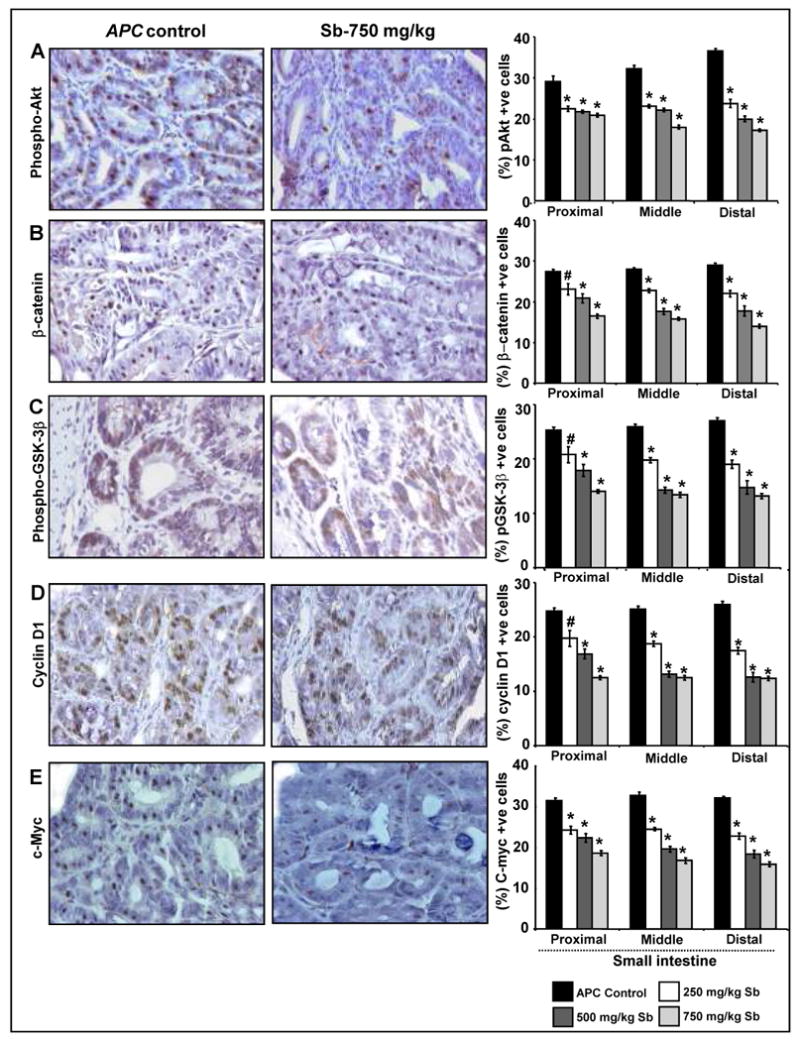
Silibinin treatment modulates aberrant β-catenin pathway and associated molecules selectively in small intestinal polyps of APCmin/+ mice. At the end of the study detailed in Materials and Methods, small intestinal segments were processed for IHC staining for (A) phospho-Akt, (B) β-catenin, (C) phospho-GSK-3β, (D) cyclin D1 and (E) c-Myc. The qualitative IHC (×400) and quantitative analyses of staining are shown only for small intestinal polyps, as no effect was observed for silibinin in normal crypt-villus axis. Quantitative data shown as percent positive cells represent mean ± SEM of 8 animals in each group in each case. #, P<0.01; *, P<0.001 versus control; Sb, silibinin.
Silibinin treatment modulates aberrant β-catenin pathway selectively in small intestinal polyps of APCmin/+ mice
Alteration in β-catenin pathway due to loss of APC function is implicated in CRC initiation and progression (27-29). To assess silibinin effect on β-catenin pathway, we analyzed by IHC the expression of β-catenin together with other molecular players associated with this pathway including phospho-GSK-3β, cyclin D1 and c-Myc in all three small intestinal segments. Microscopic examination of tissue sections showed increased number of β-catenin, phospho-GSK-3β, cyclin D1 and c-Myc-positive cells in the polyps of control APCmin/+ mice, which substantially decreased by silibinin treatment (Fig. 4B-E). Quantification of immunostained sections showed that silibinin dose-dependently decreases β-catenin-positive cells by 15-40% (P<0.01-0.001) in proximal, 18-43% (P<0.001) in middle, and 24-52% (P<0.001) in distal segments of small intestinal polyps (Fig. 4B). Phosphorylation of GSK-3β at serine 9 residue down-regulates its activity and subsequently leads to both cytoplasmic stabilization and increased nuclear translocation of β-catenin (30). In our study, quantification of phospho-GSK-3β (ser 9) staining showed that silibinin treatment (250-750 mg/kg) reduces phospho-GSK-3β-positive cells by 22-44% (P<0.01-0.001), 24-48% (P<0.001) and 30-52% (P<0.001) in the polyps in proximal, middle and distal portions of small intestine, respectively (Fig. 4C).
Both cyclin D1 and c-Myc are downstream transcriptional targets of β-catenin pathway (31,32), and are implicated in initiation of intestinal tumorigenesis (29). Over expression of cyclin D1 and c-Myc in tumor cells is frequently common and positively correlates with proliferative index (29). In the present study, compared to control APCmin/+ mice, we observed the reduction in cyclin D1-positive cells by 20-49% (P<0.01-0.001) in proximal, 25-50% (P<0.001) in middle, and 33-52% (P<0.001) in distal portions of small intestinal polyps by silibinin treatments (Fig. 4D). Silibinin treatments also decreased c-Myc-positive cells by 23-41% (P<0.001) in proximal, 25-49% (P<0.001) in middle, and 29-50% (P<0.001) in distal portions of small intestinal polyps (Fig. 4E).
Silibinin treatment decreases COX-2 and iNOS expression selectively in small intestinal polyps of APCmin/+ mice
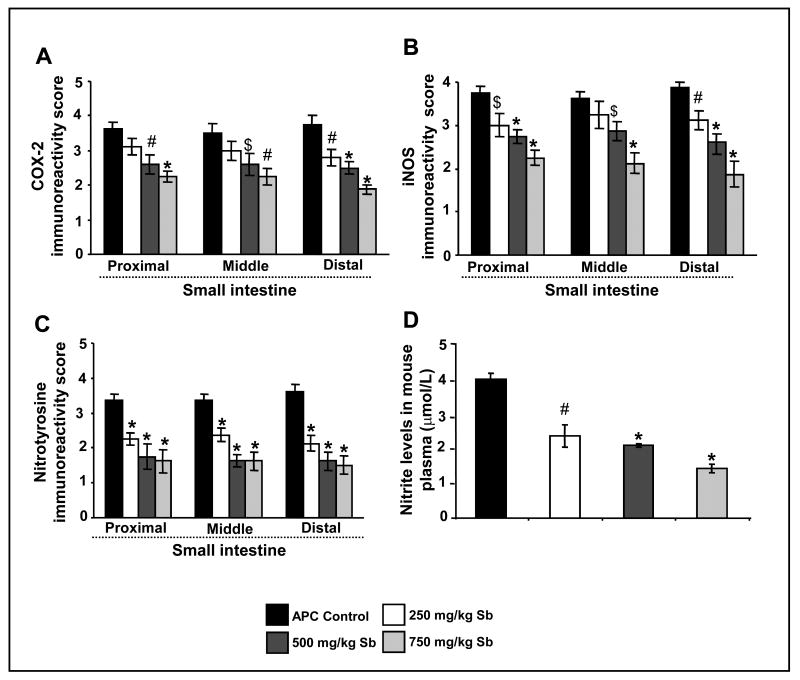
COX-2 and iNOS have been strongly implicated in both colon and small intestinal carcinogenesis (33). Accordingly, we next studied silibinin effect on COX-2 and iNOS expression by IHC. Our results showed strong immunoreactivity for both COX-2 and iNOS in small intestinal polyps of APCmin/+ mice, which decreased strongly by silibinin treatments (IHC staining data are not shown). Quantification of immunostained-cells showed that silibinin, particularly at 750 mg/kg dose, decreases COX-2 and iNOS levels (P<0.01-0.001) by 38% and 40% in proximal, 36% and 41% in middle, and 50% and 51% in distal portions of small intestinal polyps, respectively (Fig. 5A and B). Together, these results indicate that COX-2 and iNOS could be potential molecular targets for the chemopreventive effects of silibinin.
Fig. 5.
Silibinin treatment decreases COX-2, iNOS and nitrotyrosine protein levels selectively in small intestinal polyps, and nitrite levels in plasma of APCmin/+ mice. At the end of the study detailed in Materials and Methods, small intestinal segments were immunohistochemically analyzed for (A) COX-2, (B) iNOS and (C) nitrotyrosine. D, iNOS activity in plasma was estimated as described in Methods, and is expressed as μmol/L nitrite. The quantitative analyses of staining are shown only for small intestinal polyps, as no effect was observed for silibinin in normal crypt-villus axis. Data represent mean ± SEM of 8 animals in each group in each case. Significance was analyzed by unpaired Student's t-test. $, P<0.05; #, P<0.01; *, P<0.001 versus control; Sb, silibinin.
Silibinin treatment decreases iNOS activity in small intestine of APCmin/+ mice
Based on our results showing a strong decrease in iNOS levels by silibinin in small intestinal polyps of APCmin/+ mice, next we analyzed its effect on iNOS activity in terms of nitrotyrosine levels in small intestinal segments (Fig. 5C) and nitrite levels in the plasma (Fig. 5D) of APCmin/+ mice. iNOS is known to produce high and sustained concentration of NO that reacts with superoxide radical to produce peroxynitrite, which in turn mediates the nitration of protein tyrosine residues to form nitrotyrosine (34). Thus, nitrotyrosine expression and nitrite levels have been used as markers for iNOS activity (34, 35). Our IHC analysis showed that silibinin treatment decreases nitrotyrosine expression evidenced by low intensity of cytoplasmic staining (data not shown). Quantification of stained-cells showed that silibinin at all three dose levels decreases nitrotyrosine expression in the polyps by 33-52% (P<0.001) in proximal, 30-52% (P<0.001) in middle, and 41-55% (P<0.001) in distal regions of small intestine (Fig. 5C). We also assessed silibinin effect on NO formation by measuring total nitrite levels in plasma by ELISA (Fig. 5D). Silibinin decreased iNOS activity as evidenced by plasma total nitrite levels of 1.67 ± 0.07 μmol/L in control APCmin+ mice to 0.99 ± 0.13, 0.87 ± 0.02 and 0.6 ± 0.05 μmol/L in 250, 500 and 750 mg/kg silibinin-treated mice, respectively. These silibinin treatments thus decreased the total/combined levels of nitrate and nitrite by 41% (P<0.01), 48% (P<0.001) and 64% (P<0.01), respectively (Fig. 5D). These results indicate that silibinin reduces iNOS activity mostly via the decreased expression of iNOS; further suggesting its usefulness as iNOS inhibitor in chemoprevention studies.
Discussion
APCmin/+ mice develop numerous tumors in whole intestinal tract, primarily small intestine, and this model has genetic similarity to human CRC. The quick development of intestinal polyps also signifies the use of APCmin/+ mouse model in chemoprevention studies (6, 7). Using this model, our findings demonstrate the chemopreventive efficacy of silibinin against spontaneous intestinal tumorigenesis in APCmin/+ mice. Notably, we observed that all three doses of silibinin significantly and dose-dependently decreased number and size of intestinal polyps (including both small and large intestine), and this efficacy of silibinin was accompanied by anti-proliferative and pro-apoptotic activities. In molecular studies employing small intestinal tissues from control and silibinin-treated APCmin/+ mice, silibinin showed a significant down regulation in Wnt/β-catenin pathway proteins and phospho-Akt level, together with a decrease in COX-2, iNOS and nitrotyrosine levels, and iNOS activity, selectively in the polyps. We did not observe any considerable effect of silibinin in normal crypt-villus regions of APCmin/+ or wild type mice.
The role of hyper proliferation and evasion of apoptosis in cancer development has been extensively studied, and increased cell proliferation and resistance to apoptosis in premalignant intestinal epithelial cells have been shown to cause the development and progression of colon tumors (36, 37). Thus, identification of chemopreventive agents that down-regulate cell proliferation and/or up-regulate apoptotic processes is recognized as useful strategy to control colon tumor growth. Our results clearly showed an in vivo anti-proliferative and pro-apoptotic efficacy of silibinin selectively in intestinal polyps of APCmin/+ mice together with a decrease in the number as well as size of polyps, which further underscore the overall chemopreventive effects of silibinin against CRC.
Activation of Wnt/β-catenin pathway in colonic epithelium is considered as one of the key events in polyp formation (27-29). Under normal conditions, APC protein binds with β-catenin together with axin and GSK-3β thereby promoting proteolytic degradation of β-catenin (27). However, mutation in APC causes a constitutive activation of β-catenin pathway (28). In addition, phosphorylation of GSK-3β at serine 9 residue decreases its activity and also causes cytoplasmic stabilization and nuclear translocation of β-catenin resulting in T-cell factor-(TCF)/lymphoid enhancer factor (LEF)-dependent transcriptional activation of target genes such as cyclin D1 and c-Myc (28, 30). Besides this, activation of Akt has been reported to promote the phosphorylation of GSK-3β that inhibits its activity, which then leads to accumulation of β-catenin and consequently β-catenin-TCF-dependent activation of more than 30 different genes including cyclin D1 and c-Myc (27, 29). Both cyclin D1 and c-Myc are critical oncogenes, and their concurrent activation produces synergistic effects resulting in more aggressive cancer phenotype (38, 39). It has been shown that upregulation of β-catenin/TCF transcription is involved in the development of macroscopic tumors in APCmin/+ mice (40). In the present study, high levels of β-catenin, phospho (ser9)-GSK-3β, cyclin D1 and c-Myc were observed in the small intestinal polyps of control APCmin/+ mice, clearly showing the presence of altered and activated β-catenin pathway due to the functional loss of APC in these mice. Consistent with this and the inhibitory effect of silibinin on number and size of polyps in APCmin/+ mice, we observed a selective in vivo down-regulation of these established molecular targets, cyclin D1 and c-Myc, in polyps of silibinin-treated mice, supporting the reduced activity of β-catenin pathway by silibinin treatment. Moreover, silibinin also decreased the phosphorylation of both Akt and, one of its downstream targets, GSK-3β together with decreased β-catenin levels selectively in polyps. Overall, in APCmin/+ mouse model, it is well established that due to lack of functional APC protein, aberrant/uncontrolled β-catenin signaling is the main causative event in the development of polyps (40). Therefore, inhibition of β-catenin pathway would inhibit tumor initiation as well as growth in this mouse model. Thus, our results indicate that silibinin may down-regulate β-catenin levels either directly or indirectly through Akt-GSK-3β for its chemopreventive efficacy. However, further studies are needed to elucidate the mechanisms.
Several preclinical and clinical studies have shown that both iNOS and COX-2 pay a central role in cancer development (33, 41). Higher levels of both iNOS and NO have been reported in various cancers including colon (34, 42). The knocking out of iNOS gene in APCmin/+ mice is shown to markedly reduce number of intestinal polyps further supporting a major role of iNOS and NO in colon carcinogenesis (43). Consistent with this report, we also observed higher levels of iNOS protein as well as it activity in polyps and elevated levels of nitrite in plasma of APCmin+ mice. Elevated levels of COX-2 expression have been reported in intestinal adenomas in APCmin/+ mice (45, 46). Covey et al (47) have reported that pancreatic cells over expressing COX-2 cause oxidation-mediated inactivation of tumor suppressor phosphatase and tensin homolog because of increased arachidonic acid metabolism resulting in the activation of Akt pathway. Activation of Akt as a result of increased COX-2 expression may in turn cause stabilization and activation of β-catenin-mediated TCF-signaling (48). In the present study, we observed a positive correlation for immunoreactivity of iNOS, nitrotyrosine and COX-2 in polyps of APCmin/+ mice which was decreased by silibinin treatment. However, the mechanisms of silibinin-caused decrease in iNOS and COX-2 levels in polyps, the two important molecules implicated in promoting intestinal tumorigenesis (33, 45, 46), remain to be established. Nevertheless, this effect of silibinin could have contributed in inhibiting the growth of polyp.
In terms of the cause and results relationship between the protective effect of silibinin and the decrease in the expression of beta catenin and associated molecules, we observed that silibinin treatment results in the reduction in the size and number of intestinal polyps in APCmin/+ mice together with reduction in β-catenin and a resultant decrease in the expression of cyclin D1 and c-Myc; the downstream targets of β-catenin pathway. Since cyclin D1 and c-Myc are oncogenes associated with increased proliferation of cancerous cells, it seems reasonable to assume that a decrease in β-catenin levels by silibinin might be causally responsible for the observed reduction in the number and size of the polyps in the intestinal tract of APCmin/+ mice. With regard to upstream of β-catenin, we found that silibinin also inhibits the phosphorylation of Akt which might be responsible for the activation of GSK-3β in terms of reduced phosphorylation at Ser 9 residue by silibinin (activation of Akt promotes the phosphorylation of GSK-3β at Ser 9 inhibiting its activity), which then promotes β-catenin degradation. Thus, silibinin targeting β-catenin pathway through Akt and GSK-3β by decreasing nuclear β-catenin levels together with down regulation of cyclin D1 and c-Myc could be one of the possible underlying cause and effect mechanisms of its chemopreventive potential against intestinal polyps in APCmin/+ mice as observed in the present study. In summary, we observed that silibinin treatment inhibits multiplicity as well as growth of intestinal polyps in APCmin/+ mice without any apparent adverse health effects by decreasing cell proliferation, inducing apoptosis, and modulating β-catenin pathway, COX-2 and iNOS expression. Since almost 90% of FAP patients develop small intestinal polyps together with colonic polyps that remain a significant risk factor following colectomy (2, 6), findings of the present study have a translational potential in offering a relatively non-toxic alternative for the prevention of intestinal polyps in FAP patients as well as the reduction of associated morbidity and mortality.
Acknowledgments
This work was supported by NCI RO1 grant CA112304.
Abbreviations
- APC
Adenomatous polyposis coli
- COX-2
cyclooxygenase-2
- CRC
colorectal cancer
- FAP
familial adenomatous polyposis
- GSK-3β
glycogen synthase kinase-3β
- IHC
immunohistochemistry
- iNOS
inducible nitric oxide synthase
References
- 1.American Cancer Society. Cancer facts and figures 2007-2008. Atlanta, GA; [Google Scholar]
- 2.Hisamuddin IM, Yang VW. Molecular Genetics of Colorectal Cancer: An Overview. Curr Colorectal Cancer Rep. 2006;2:53–9. doi: 10.1007/s11888-006-0002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonovas S, Tsantes A, Drosos T, Sitaras NM. Cancer chemoprevention: a summary of the current evidence. Anticancer Res. 2008;28:1857–66. [PubMed] [Google Scholar]
- 4.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–24. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 5.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–70. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 6.Preston SL, Leedham SJ, Oukrif D, Deheregoda M, Goodlad RA, Poulsom R, Alison MR, Wright NA, Novelli M. The development of duodenal microadenomas in FAP patients: the human correlate of the Min mouse. J Pathol. 2008;214:294–301. doi: 10.1002/path.2294. [DOI] [PubMed] [Google Scholar]
- 7.Corpet DE, Pierre F. Point: from animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 8.Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther. 2007;6:110–9. doi: 10.1177/1534735407301825. [DOI] [PubMed] [Google Scholar]
- 9.Singh RP, Agarwal R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer. 2005;41:1969–79. doi: 10.1016/j.ejca.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Mol Carcinog. 2006;45:436–42. doi: 10.1002/mc.20223. [DOI] [PubMed] [Google Scholar]
- 11.Chittezhath M, Deep G, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits cytokine-induced signaling cascades and down-regulates inducible nitric oxide synthase in human lung carcinoma A549 cells. Mol Cancer Ther. 2008;7:1817–26. doi: 10.1158/1535-7163.MCT-08-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyagi A, Singh RP, Ramasamy K, Raina K, Redente EF, Dwyer-Nield LD, Radcliffe RA, Malkinson AM, Agarwal R. Growth inhibition and regression of lung tumors by silibinin: modulation of angiogenesis by macrophage-associated cytokines and nuclear factor-kappaB and signal transducers and activators of transcription 3. Cancer Prev Res (Phila Pa) 2009;2:74–83. doi: 10.1158/1940-6207.CAPR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh RP, Raina K, Sharma G, Agarwal R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin Cancer Res. 2008;4:7773–80. doi: 10.1158/1078-0432.CCR-08-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raina K, Rajamanickam S, Singh RP, Deep G, Chittezhath M, Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008;68:6822–30. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyagi A, Agarwal C, Harrison G, Glode LM, Agarwal R. Silibinin causes cell cycle arrest and apoptosis in human bladder transitional cell carcinoma cells by regulating CDKI-CDK-cyclin cascade, and caspase 3 and PARP cleavages. Carcinogenesis. 2004;25:1711–20. doi: 10.1093/carcin/bgh180. [DOI] [PubMed] [Google Scholar]
- 16.Singh RP, Tyagi A, Sharma G, Mohan S, Agarwal R. Oral silibinin inhibits in vivo human bladder tumor xenograft growth involving down-regulation of survivin. Clin Cancer Res. 2008;14:300–8. doi: 10.1158/1078-0432.CCR-07-1565. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, Sclafani RA, Agarwal R. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–82. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- 18.Singh RP, Gu M, Agarwal R. Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Res. 2008;68:2043–50. doi: 10.1158/0008-5472.CAN-07-6247. [DOI] [PubMed] [Google Scholar]
- 19.Velmurugan B, Singh RP, Tyagi A, Agarwal R. Inhibition of azoxymethane-induced colonic aberrant crypt foci formation by silibinin in male Fisher 344 rats. Cancer Prev Res. 2008;1:376–84. doi: 10.1158/1940-6207.CAPR-08-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohno H, Tanaka T, Kawabata K, Hirose Y, Sugie S, Tsuda H, Mori H. Silymarin, a naturally occurring polyphenolic antioxidant flavonoid, inhibits azoxymethane-induced colon carcinogenesis in male F344 rats. Int J Cancer. 2002;101:461–8. doi: 10.1002/ijc.10625. [DOI] [PubMed] [Google Scholar]
- 21.Verschoyle RD, Greaves P, Patel K, Marsden DA, Brown K, Steward WP, Gescher AJ. Evaluation of the cancer chemopreventive efficacy of silibinin in genetic mouse models of prostate and intestinal carcinogenesis: relationship with silibinin levels. Eur J Cancer. 2008;44:898–906. doi: 10.1016/j.ejca.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Zhao J, Agarwal R. Tissue distribution of silibinin, the major active constituent of silymarin, in mice and its association with enhancement of phase II enzymes: implications in cancer chemoprevention. Carcinogenesis. 1999;20:2101–8. doi: 10.1093/carcin/20.11.2101. [DOI] [PubMed] [Google Scholar]
- 23.Giacomelli S, Gallo D, Apollonio P, Ferlini C, Distefano M, Morazzoni P, Riva A, Bombardelli E, Mancuso S, Scambia G. Silybin and its bioavailable phospholipid complex (IdB 1016) potentiate in vitro and in vivo the activity of cisplatin. Life Sci. 2002;70:1447–59. doi: 10.1016/s0024-3205(01)01511-9. [DOI] [PubMed] [Google Scholar]
- 24.Chen T, Nines RG, Peschke SM, Kresty LA, Stoner GD. Chemopreventive effects of a selective nitric oxide synthase inhibitor on carcinogen-induced rat esophageal tumorigenesis. Cancer Res. 2004;64:3714–7. doi: 10.1158/0008-5472.CAN-04-0302. [DOI] [PubMed] [Google Scholar]
- 25.Maga G, Hübscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116:3051–60. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 26.Saglam O, Garrett CR, Boulware D, Sayegh Z, Shibata D, Malafa M, Yeatman T, Cheng JQ, Sebti S, Coppola D. Activation of the serine/threonine protein kinase AKT during the progression of colorectal neoplasia. Clin Colorectal Cancer. 2007;6:652–6. doi: 10.3816/CCC.2007.n.034. [DOI] [PubMed] [Google Scholar]
- 27.Gavert N, Ben-Ze'ev A. beta-Catenin signaling in biological control and cancer. J Cell Biochem. 2007;102:820–8. doi: 10.1002/jcb.21505. [DOI] [PubMed] [Google Scholar]
- 28.Morin PJ. beta-catenin signaling and cancer. Bioessays. 1999;21:1021–30. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 29.Liao DJ, Thakur A, Wu J, Biliran H, Sarkar FH. Perspectives on c-Myc, Cyclin D1, and their interaction in cancer formation, progression, and response to chemotherapy. Crit Rev Oncog. 2007;13:93–158. doi: 10.1615/critrevoncog.v13.i2.10. [DOI] [PubMed] [Google Scholar]
- 30.Luo J. Glycogen synthase kinase 3β (GSK3 β) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273:194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–47. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- 32.Garte SJ. The c-myc oncogene in tumor progression. Crit Rev Oncog. 1993;4:435–49. [PubMed] [Google Scholar]
- 33.Watanabe K, Kawamori T, Nakatsugi S, Wakabayashi K. COX-2 and iNOS, good targets for chemoprevention of colon cancer. Biofactors. 2000;12:129–33. doi: 10.1002/biof.5520120120. [DOI] [PubMed] [Google Scholar]
- 34.Kojima M, Morisaki T, Tsukahara Y, Uchiyama A, Matsunari Y, Mibu R, Tanaka M. Nitric oxide synthase expression and nitric oxide production in human colon carcinoma tissue. J Surg Oncol. 1999;70:222–9. doi: 10.1002/(sici)1096-9098(199904)70:4<222::aid-jso5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 35.Halliwell B, Zhao K, Whiteman M. Nitric oxide and peroxynitrite. The ugly, the uglier and the not so good: a personal view of recent controversies. Free Radic Res. 1999;31:651–69. doi: 10.1080/10715769900301221. [DOI] [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 37.Seong J, Chung EJ, Kim H, Kim GE, Kim NK, Sohn SK, Min JS, Suh CO. Assessment of biomarkers in paired primary and recurrent colorectal adenocarcinomas. Int J Radiat Oncol Biol Phys. 1999;45:1167–73. doi: 10.1016/s0360-3016(99)00302-8. [DOI] [PubMed] [Google Scholar]
- 38.Michaux L, Wlodarska I, Theate I, Stul M, Scheiff JM, Deneys V, Ferrant A, Hagemeijer A. Coexistence of BCL1/CCND1 and CMYC aberrations in blastoid mantle cell lymphoma: a rare finding associated with very poor outcome. Ann Hematol. 2004;83:578–83. doi: 10.1007/s00277-004-0879-2. [DOI] [PubMed] [Google Scholar]
- 39.Shiina H, Igawa M, Shigeno K, Terashima M, Deguchi M, Yamanaka M, Ribeiro-Filho L, Kane CJ, Dahiya R. Beta-catenin mutations correlate with over expression of C-myc and cyclin D1 Genes in bladder cancer. J Urol. 2002;168:2220–6. doi: 10.1016/S0022-5347(05)64359-5. [DOI] [PubMed] [Google Scholar]
- 40.Oyama T, Yamada Y, Hata K, Tomita H, Hirata A, Sheng H, Hara A, Aoki H, Kunisada T, Yamashita S, Mori H. Further upregulation of beta-catenin/Tcf transcription is involved in the development of macroscopic tumors in the colon of ApcMin/+ mice. Carcinogenesis. 2008;29:666–72. doi: 10.1093/carcin/bgn001. [DOI] [PubMed] [Google Scholar]
- 41.Kawai N, Tsujii M, Tsuji S. Cyclooxygenases and colon cancer. Prostaglandins Other Lipid Mediat. 2002;68-69:187–96. doi: 10.1016/s0090-6980(02)00030-8. [DOI] [PubMed] [Google Scholar]
- 42.Thomsen LL, Miles DW. Role of nitric oxide in tumor progression: lessons from human tumours. Cancer Metastasis Rev. 1998;17:107–18. doi: 10.1023/a:1005912906436. [DOI] [PubMed] [Google Scholar]
- 43.Ahn B, Ohshima H. Suppression of intestinal polyposis in Apc(Min/+) mice by inhibiting nitric oxide production. Cancer Res. 2001;61:8357–60. [PubMed] [Google Scholar]
- 44.Washo-Stultz D, Hoglen N, Bernstein H, Bernstein C, Payne CM. Role of nitric oxide and peroxynitrite in bile salt-induced apoptosis: relevance to colon carcinogenesis. Nutr Cancer. 1999;35:180–8. doi: 10.1207/S15327914NC352_13. [DOI] [PubMed] [Google Scholar]
- 45.Swamy MV, Patlolla JM, Steele VE, Kopelovich L, Reddy BS, Rao CV. Chemoprevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APC Min mice. Cancer Res. 2006;66:7370–7. doi: 10.1158/0008-5472.CAN-05-4619. [DOI] [PubMed] [Google Scholar]
- 46.Issa AY, Volate SR, Muga SJ, Nitcheva D, Smith T, Wargovich MJ. Green tea selectively targets initial stages of intestinal carcinogenesis in the AOM-ApcMin mouse model. Carcinogenesis. 2007;28:1978–84. doi: 10.1093/carcin/bgm161. [DOI] [PubMed] [Google Scholar]
- 47.Covey TM, Edes K, Fitzpatrick FA. Akt activation by arachidonic acid metabolism occurs via oxidation and inactivation of PTEN tumor suppressor. Oncogene. 2007;26:5784–92. doi: 10.1038/sj.onc.1210391. [DOI] [PubMed] [Google Scholar]
- 48.Little D, Jones SL, Blikslager AT. Cyclooxygenase (COX) inhibitors and the intestine. J Vet Intern Med. 2007;21:367–77. doi: 10.1892/0891-6640(2007)21[367:cciati]2.0.co;2. [DOI] [PubMed] [Google Scholar]