Abstract
Purpose
To examine the effect of HIV infection on regional left ventricular dysfunction in cardiovascularly asymptomatic individuals.
Methods
Nineteen HIV-negative and 27 HIV-positive cardiovascularly asymptomatic study participants in Baltimore, Maryland were selected and underwent tagged cardiac magnetic resonance imaging (MRI). Regional left ventricular myocardial mid-wall peak systolic circumferential strain (Ecc) and early diastolic strain rate (SRE) of the left ventricle were assessed with the use of the harmonic phase (HARP) analysis. The average Ecc and SRE measurements were compared between HIV-negative and HIV-positive individuals.
Results
Compared with the HIV-negatives, the HIV-positives had lower average Ecc and SRE measurements in 90% of the 16 standard left ventricular segments. Of the 14 segments with decreased Ecc strain, 3 were statistically significant and of 14 with decreased strain rate (SRE), 6 were statistically significant.
Conclusions
HIV infection may be associated with subclinical regional left ventricular systolic and diastolic dysfunction in individuals free of overt cardiovascular disease.
Keywords: HIV infection, regional left ventricular dysfunction, tagging MRI, Myocardium strain, random-effects model
Individuals with HIV infection represent one of the most rapidly growing groups with cardiovascular disease globally [1–4]. AIDS has been increasingly recognized as an important cause of dilated cardiomyopathy and cardiovascular complications tend to occur late in the disease course of HIV infection [5–6]. However, HIV infection has not been implicated in the occurrence of regional left ventricular (LV) dysfunction in HIV-infected, cardiovascularly asymptomatic individuals.
Most of the cardiac ventricular pathogenesis is usually regional in nature before becoming globally apparent. Regional ventricular functions in humans can be noninvasively measured to great precision by cardiac tagged magnetic resonance imaging (MRI) [7–9]. Harmonic phase MRI (HARP) provides a fast and accurate assessment of myocardial strains from tagged MRI images in both normal human subjects and patients with heart disease [10].
The objective of this study was to examine whether HIV infection is associated with regional LV dysfunction in cardiovascularly asymptomatic individuals. To better understand the pathogenesis of HIV-related myocardial disease at its early stages, HARP-MRI was used to investigate the regional function of the left ventricular myocardium in both HIV-positive and HIV-negative study participants. The regional systolic and diastolic functions were evaluated by measuring the average circumferential strain (Ecc) in the myocardial mid-wall during the systolic and diastolic phases of the left ventricular contraction cycle.
SUBJECTS AND METHODS
Study subjects
Between August 2004 and April 2005, 46 study participants, 27 HIV-positive and 19 HIV-negative, were enrolled in a study investigating the effects of HIV infection on regional LV function. All the study participants are African American (self-designated) and permanent residents of Baltimore, Maryland. Inclusion criteria for this study were (1) HIV positivity: for HIV-infected individuals, HIV-1 infection was determined by ELISA and confirmed by Western blot test; for HIV-negative individuals, ELISA was performed to confirm seronegativity; (2) age between 25 and 54 years; and (3) race: African American (self-designated). Exclusion criteria were (1) any evidence of hypertension or ischemic heart disease as indicated by clinical history, previous hospitalization for myocardial infarction, angina pectoris, or EKG and/or echocardiographic evidence of previous myocardial damage by ischemic heart disease; (2) any symptoms believed to be related to cardiovascular disease; (3) any evidence of renal insufficiency; (4) pregnancy; (5) having a pacemaker, an implanted defibrillator, or certain other implanted electronic or metallic devices; (6) having had brain surgery for a cerebral aneurysm, implanted medical or metallic devices, shrapnel, or other metal, such as metal in the eye; (7) having claustrophobia; and (8) having a history of allergic reaction to contrast media administered intravascularly.
Information about sociodemographics, medical history, and medication use was obtained by interviewer-administered questionnaires. The Committee on Human Research at the Johns Hopkins School of Medicine approved the study protocol, and all study participants provided written informed consent. All procedures used in this study were in accordance with institutional guidelines.
Blood Pressure Measurement
Systolic and diastolic sitting blood pressure (BP) was measured twice with a standard mercury sphygmomanometer. A nurse at the clinic measured the study participant's arm circumference and applied a correctly sized cuff. The participant sat quietly for 5 minutes, and then the nurse obtained the systolic and diastolic blood pressure measurements; a second measurement was made 3 minutes later. The average of the two readings was reported.
Echocardiographic Measurements
Each study participant was asked not to use tobacco or caffeine on the day of echocardiographic examinations. Two-dimensional transthoracic echocardiographic images, including M mode, cross-sectional and pulsed wave Doppler images, were obtained with the person in the left lateral decubitus position. The echocardiographic examinations were performed by an experienced operator using a Hewlett-Packard Sonos 5500 ultrasound instrument with 3.0 and 3.5 MHz transducers. For each study participant, the following views were obtained: parasternal long axis, parasternal short axis, apical 2-chamber, and apical 4-chamber. Transmitral flow was assessed in the apical 4-chamber view with the pulsed Doppler sample volume at the leaflet tips. Echocardiographic images were recorded on VHS videotapes and measurements were made in accordance with the recommendations of the American Society of Echocardiography [11]. Three individual sets of measurements were obtained from each cross-sectional echocardiogram, and the results were averaged. The discrepancy between measurements was less than 10% in every case. The following variables were measured: left ventricular dimensions in end diastole and end systole, interventricular septum and left ventricular posterior wall thickness in diastole, left ventricular ejection fraction, early peak velocity (E wave), late peak velocity (A wave), E to A ratio (E/A), isovolumic relaxation time, and deceleration time of the early filling period. Left ventricular mass and left ventricular mass index were calculated according to the American Society of Echocardiography's corrected formula [11].
Measurement of Lipids
After overnight fasting, venous blood samples were obtained in participants from a large antecubital vein. Routine blood cell analyses were assessed with a blood cell analyzer. Serum was separated by centrifugation at 2000 g for 15 minutes at 4 °C and stored at −75 °C until assayed. Serum lipid parameters, including total cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, and very-low-density lipoprotein, were determined by enzymatic colorimetric methods on a Hitachi 747 analyzer with standard reagents (Roche Diagnostics, Indianapolis, Indiana).
Measurement of Regional Left Ventricular Strain by HARP-MRI
Cardiac MRI images were acquired by 1.5T MR scanners (SIGNA LX and CVi, GE Electric Medical Systems, Waukesha, Wisconsin, and Somatom Vision and Sonata, Siemens Medical Solutions, Erlangen, Germany). Dedicated phase array coils were used for signal reception. After completion of the standard protocol, 3 tagged ventricular short axis slices (basal, mid-ventricular and apical) were obtained. All images were obtained by ECG-triggered segmented k-space fast gradient-echo (SPGR or FLASH) pulse sequence during breath holds (12–18 seconds). Parallel stripe tags were prescribed in 2 orthogonal orientations (0° and 90°) using identical pulse sequences with additional spatial modulation of magnetization (SPAMM) encoding gradients.
The parameters for tagged images were: field of view 40 cm; slice thickness 8–10 mm; repetition time 6 ms (range 3.5–7.2 ms); echo time 3.0 ms (range 2.0–4.2 ms); flip angle 12°; matrix size 256*96–140; 4–9 phase encoding views per segment, and tag spacing of 7 mm. With use of these parameters, 19–27 phases per cycle were acquired yielding a temporal resolution of 40 ms (range 20–41 ms).
Tagged ventricular MRI images of the standard 3 short-axis slices [12] were analyzed by HARP (Diagnosoft, Inc, Palo Alto, California), which enables a fast determination of regional myocardial strain [13]. The average peak mid-wall circumferential strain (Ecc) in the systolic phase and peak early diastolic strain rate (SRE), respectively, were determined in all standardized myocardial segments in the 3 short axis slices (11), except the apical segment 17. The values of Ecc are normally negative during the contraction of the ventricle as they express circumferential shortening [14–17, Figure 1]. Less negative Ecc indicates diminished regional LV function. HARP-MRI has an excellent reproducibility, with the correlation coefficients (R) for interobserver and intraobserver agreement for peak systolic midwall Ecc being 0.81 and 0.84, respectively [18].
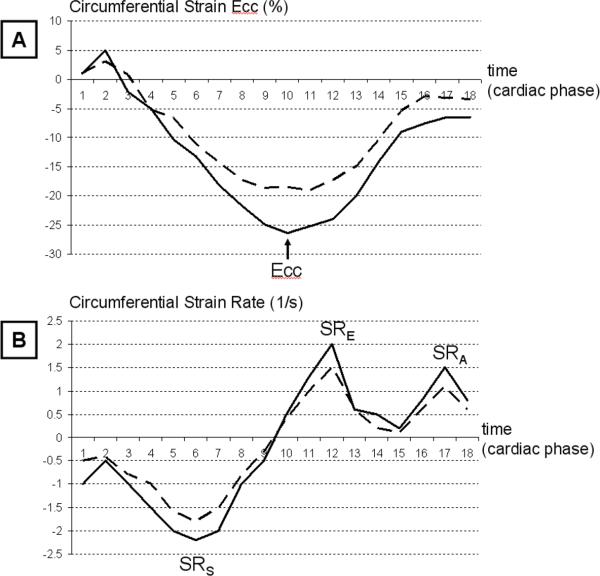
Figure 1.
Segmental Mid-Wall Circumferential Myocardial Strain (Ecc), and Strain Rate in HIV-negative and HIV-positive Participants.
The Ecc is calculated as a Lagrangian strain and defined as the ratio of circumferential myocardial shortening from the initial length. The systolic phase is the time period from zero to the peak Ecc who appears negative. Because it is a measure of shortening better contraction is seen as more negative Ecc values; The diastolic phase is the time period from peak Ecc to the end of the cardiac cycle. While differences can be visually observed, in general the observations made are based on group averages rather than individual comparisons thus limiting the need for presentation of individual patient data.
Figure 1A shows Ecc temporal curves for an HIV-negative (plain) and HIV-positive participant (dotted). Peak systolic Ecc strain is defined as as the maximum negative strain during systole (arrow). In this example peak Ecc is lower for this segment in the HIV-positive compared to the HIV-positive participant. Note: No systematic difference between R-R duration between HIV patients and controls was detected.
Figure 1B shows strain rate temporal curves for an HIV-negative (plain) and HIV-positive participant (dotted). Diastolic strain rate is defined as the maximum positive strain rate during early diastole (SRE) and late diastole (SRA). Only the early diastolic strain rate (SRE) was considered in this analysis. In this case, peak SRE is lower for this segment in the HIV-positive compared to the HIV-positive participant.
Statistical Analysis
Statistical analysis was performed with SAS statistical software [19]. Continuous parameters were described as mean ± standard deviation, and categorical parameters were described as percentages. Both the Student's t test (for continuous variables) and the chi-square test (for categorical variables) were used to compare potential risk factors for cardiovascular heart disease and echocardiographic indices in the HIV-positive and HIV-negative groups. A random-effects model was used to control for potential confounding factors and compare the average midwall Ecc and SRE in each left ventricular segment in HIV-positive and HIV-negative groups since when repeated measurements of strain are taken on the same study subject these repeated measurements are highly correlated [20]. The significance level was set at p <0.05.
RESULTS
General Characteristics
Of the 46 participants in this study, 18 (39.1%) were women and 27 (58.7%) were infected with HIV. The mean age (±SD) was 40±5.6 years. Among the 27 HIV positives, 14 (52%) were on HAART. The median CD4 cell count (interquartile range) in the HIV-positive group was 329 cells/mm3 (266–457), and the median HIV viral load (interquartile range) was 787 copies/mL (174 – 6868).
The general characteristics of the study participants by HIV status are presented in Table 1. There were no significant differences in demographic and risk factors between the HIV-positive and HIV-negative groups except that the serum levels of triglyceride and very-low-density lipoprotein were significantly higher in the HIV-positive group.
Table 1.
General Characteristics of 46 Study Participants by HIV infection Status in Baltimore, Maryland
| Variable | HIV− (N = 19) | HIV+ (N = 27) | p-value* |
|---|---|---|---|
| Age (y) | 38.8 ± 6.9 | 41.0 ± 4.4 | 0.23 |
| Women (%) | 42 | 37 | 0.73 |
| Smoker (%) | 74 | 85 | 0.56 |
| Body mass index (kg/m2) | 25.3±4.7 | 25.6±5.3 | 0.85 |
| Body surface area (m2) | 1.85 ± 0.19 | 1.89 ± 0.24 | 0.57 |
| Heart rate (beat/min) | 76 ± 25 | 77 ± 24 | 0.66 |
| Diastolic blood pressure (mmHg) | 76 ± 8 | 79 ± 10 | 0.30 |
| Total cholesterol (mg/dL) | 172 ± 33 | 174 ± 41 | 0.87 |
| Triglycerides (mg/dL) | 66 ± 24 | 135 ± 107 | 0.003 |
| Very-low-density lipoprotein (mg/dL) | 13 ± 5 | 21 ± 14 | 0.01 |
| Low-density lipoprotein (mg/dL) | 102 ± 32 | 94 ± 37 | 0.49 |
| High-density lipoprotein (mg/dL) | 57 ± 17 | 54 ± 22 | 0.61 |
| Abnormal C-reactive protein** (%) | 44 | 46 | 0.91 |
HIV, human immunodeficiency virus; HIV+, HIV-positive group; HIV-, HIV-negative group.
Between-group differences were compared by Student's t tests for continuous variables and chi-square tests for categorical variables.
The abnormal C-reactive protein level was defined as > 1.9 mg/L.
Global and Regional Left Ventricular Function
All the global left ventricular function measurements by echocardiography were within the normal ranges. There were no significant differences in the major structural and functional measurements of global left ventricular systolic and diastolic function by echocardiography between the HIV-negative and the HIV-positive groups (Table 2).
Table 2.
Comparison of echocardiographic indices between HIV− and HIV+ groups
| Variable | HIV− (N = 19) | HIV+ (N = 27) | p-value* |
|---|---|---|---|
| Posterior wall (cm) | 1.02 ± 0.17 | 1.06 ± 0.22 | 0.50 |
| Interventricular septum (cm) | 1.07 ± 0.15 | 1.08 ± 0.29 | 0.87 |
| LV mass (g) | 174.4 ± 40.1 | 185.8 ± 63.3 | 0.53 |
| LV mass index (g/m2) | 59.9 ± 15.0 | 61.7 ± 21.2 | 0.76 |
| LV Ejection fraction (%) | 59 ± 7 | 60 ± 7 | 0.61 |
| Mitral Flow Deceleration time (sec) | 0.26 ± 0.08 | 0.25 ± 0.06 | 0.68 |
| E wave (cm/sec) | 80.9 ± 13.2 | 75.0 ± 21.1 | 0.34 |
| A wave (cm/sec) | 57.3 ± 8.8 | 57.3 ± 17.3 | 1.00 |
| E/A ratio | 1.42 ± 0.19 | 1.35 ± 0.37 | 0.51 |
HIV+, HIV−positive group; HIV−, HIV−negative group; LV, left ventricular.
Between-group differences were compared by Student's t tests for continuous variables and chi-square tests for categorical variables.
The regional left ventricular functional measurements by HARP-MRI differed significantly between the HIV-negative and the HIV-positive groups. The differences in the average segmental Ecc between the two groups are listed in Table 3. A negative value of the Ecc difference between the groups indicates a lower segmental Ecc in the HIV-positive group. The overwhelmingly negative values of the Ecc differences revealed that the majority of left ventricular segments in the HIV-positive group had lower average mid-wall Ecc and SRE measurements than that of the HIV-negative group. Lower Ecc and SRE measurements were found in 90% (14/16) of the 16 left ventricular segments in the HIV-positive group. Among the 14 lower Ecc measurements of the HIV-positive group, 3 were significantly lower than their respective counterparts in the HIV-negative group and 5 SRE measurements were significantly lower than their respective counterparts of the HIV-negative group. The segments with significantly lower Ecc and SRE were scattered in the basal and midcavity levels of the left ventricle, and they did not appear to fit into any pattern that may relate to coronary artery distribution. The remaining 10% (2/16) ventricular segments whose strain measurements appeared not to be lower (i.e., the ones with the positive values in the Ecc differences) in the HIV-positive group, in either systole or diastole, were also scattered in the ventricular wall, but every one of them was either adjacent to, or sandwiched between, the segments with significantly lower Ecc in the left ventricle.
Table 3.
Differences in the average mid-wall Systolic Strain (Ecc) and diastolic strain rate (SRE) in each left ventricular segment between the HIV− and HIV+ group
| LV segment* | Systolic Ecc | Diastolic SRE | ||
|---|---|---|---|---|
| Difference** ± SE | p-value*** | Difference ± SE | p-value*** | |
| Basal slice | ||||
| anterior | −0.84 ± 0.68 | 0.23 | 1.65 ± 0.79 | 0.04 |
| anterolateral | −0.12 ± 0.75 | 0.87 | −0.52 ± 0.64 | 0.42 |
| inferolateral | −1.11 ± 0.66 | 0.10 | −2.09 ± 0.65 | 0.002 |
| inferior | −2.44 ± 0.66 | 0.001 | −3.18 ± 0.67 | < 0.001 |
| inferoseptal | −0.86 ± 0.60 | 0.16 | −1.50 ± 0.69 | 0.04 |
| anteroseptal | −0.60 ± 0.70 | 0.40 | −1.32 ± 0.78 | 0.10 |
| Mid-cavity slice | ||||
| anterior | −2.38 ± 0.74 | 0.003 | −3.74 ± 0.85 | < 0.001 |
| anterolateral | 0.80 ± 0.71 | 0.27 | −0.03 ± 0.69 | 0.96 |
| inferolateral | −1.80 ± 0.80 | 0.03 | −2.69 ± 0.62 | < 0.001 |
| inferior | −0.52 ± 0.62 | 0.40 | −0.26 ± 0.53 | 0.62 |
| inferoseptal | −1.20 ± 0.80 | 0.14 | −0.86 ± 0.76 | 0.27 |
| anteroseptal | 0.10 ± 0.75 | 0.90 | −1.03 ± 0.76 | 0.18 |
| Apical slice | ||||
| Anterior | −0.54 ± 0.73 | 0.46 | 0.54 ±0.79 | 0.49 |
| Lateral | −1.38 ± 0.92 | 0.14 | −0.26 ± 0.89 | 0.78 |
| inferior | −0.02 ± 0.73 | 0.98 | −0.62 ± 0.66 | 0.36 |
| septal | −0.71 ± 0.70 | 0.32 | −0.29 ± 0.76 | 0.70 |
Ecc: peak systolic circumferential strain; SRE: peak early diastolic strain rate. HIV+, HIV positive group; HIV−, HIV negative group.
The nomenclature of the left ventricle myocardial segmentations was adopted from reference 11.
The difference is the average strain of the HIV− group minus that of the HIV+ group.
Between-group differences were compared by a random-effects model.
DISCUSSION
This study demonstrates a positive association of HIV infection with regional LV dysfunction. Since the study was performed in cardiovascularly asymptomatic individuals, the findings suggest that HIV infection may be involved in the development of early subclinical regional LV dysfunction. Although HIV infection has been linked to early subclinical atherosclerotic cardiovascular disease [21,22], regional LV dysfunction in relation to HIV infection in cardiovascularly asymptomatic individuals has not been reported. MRI offers a unique opportunity to assess regional left ventricular function in a detailed and quantitative manner through myocardial tagging [7,23]. MRI tagging is an accurate and objective method for assessing regional myocardial dysfunction [24,25].
The relationship between HIV infection and cardiovascular disease is still under debate. There are two studies that suggest that HIV infection per se might explain the exacerbated atherosclerosis and vasculopathy in HIV-infected individuals. The first of these, a pathological study, showed that HIV directly infected human arterial smooth muscle cells (SMCs). The results of this study indicate that SMCs can be infected by HIV, in vivo and in vitro, by a CD4, chemokine receptor-, and endocytosis-dependent mechanism. This infection also results in increased chemokine CCL2 production by SMCs. On the basis of these data, it is believed that HIV infection of SMCs may contribute to the pathogenesis of atherosclerosis observed in HIV-infected individuals [22]. The second study, the SMART (Strategies for Management of Antiretroviral Therapy) trial, observed that the rate of cardiovascular events was higher in patients in the drug conservation arm (with higher viral load levels) than in patients in the viral suppression arm. This trial could not rule out the possibility that an increase in viral load will increase the cardiovascular risk [26].
The advent of highly active antiretroviral therapy (HAART) has made the population of individuals with HIV infection one of the most rapidly growing groups with cardiovascular disease globally [22]. In addition to its association with accelerated atherosclerosis and vasculopathy, HIV infection has also been linked to myocardial disease, such as dilated cardiomyopathy [5]. AIDS-associated cardiomyopathy was first reported in 1986 [27], but HIV-associated regional left ventricular dysfunction has not been reported. Since regional left ventricular (LV) dysfunction may be the earliest stage of subsequent LV dysfunction, the identification of this early stage is of critical importance in the management of HIV-associated myocardial disease.
This study was conducted in relatively young participants (aged between 25 and 54 years) who were cardiovascularly asymptomatic. The study showed that the left ventricular function in HIV-positive participants in several regions, including inferior, anterior, and inferolateral, was significantly lower than that of the HIV-negative group. Also, more regions were found to have abnormal relaxation in diastole (reduced SRE) than to have abnormal systolic deformation (impaired Ecc) (5/16 vs. 3/16, see Table 3) among HIV-positive study participants. The hypofunctional myocardial regions were scattered on the left ventricular walls, with a few hyperfunctional regions sandwiched between, or adjacent to the hypofunctioning regions, indicating a possible compensatory mechanism for the hypofunctional regions from adjacent normal myocardium.
The mechanism for HIV-associated myocardial pathogenesis remains unclear. A variety of potential etiologies have been postulated in HIV-related heart disease, including myocardial infection with HIV itself, opportunistic infections, drug-related cardiotoxicity, nutritional deficiencies, autoimmune responses, and prolonged immunosuppression [5,6]. It has been reported that HIV virions appear to infect the myocardial tissue in patchy distributions [5,6]. Interestingly a study in non HIV viral myocarditis has showed heterogeneous late gadolinium enhancement patterns with lateral regions being predominantly concerned by PVB19 infection whereas anteroseptal regions were linked to HHV6 infection (Mahrholdt et al Circulation 2006). Hence, the functional abnormalities caused by HIV-associated myocarditis could first appear as regional anomalies before a possible evolution to global dysfunction. The results of this study agree with that assumption. In addition, more abnormal segments in the diastolic than the systolic phase may indicate that, at the same stage of HIV infection, the diastolic function is more prone to disruption than is the systolic function, perhaps because of inflammation and microvascular damage in the affected myocardium.
This study revealed that the average circumferential strain (Ecc) in regional myocardium measured by HARP-MRI is more sensitive for identifying LV dysfunction than the global LV parameters measured by routine echocardiography, since global LV function measured by echocardiography in all the study participants could not reveal any systolic or diastolic function abnormalities (Table 2). In addition, although both ejection fraction and average Ecc are considered systolic functional indices of the ventricle, the ejection fraction, being the measure of relative volumic change during the cardiac cycle, remains a global but rough indicator of the LV pump function whereas average regional Ecc and SRE, measuring the average myocardial deformation throughout the cardiac cycle are therefore a better indicators of local LV contraction and relaxation respectively.
This study has limitations. First, the sample size was relatively small so that the power to detect the differences between the HIV-positive group and the HIV-negative group would be quite limited. Due to its small size, this study could not answer the question of whether HIV-associated regional LV dysfunction has a distinct pattern of anatomic distribution in the left ventricle. An adequate sample size is needed to address this issue. A second limitation is that causal relationships cannot be assessed in this cross-sectional study, and the conclusions derived from it should be drawn with caution. Larger studies with a longitudinal design and including the study of late gadolinium enhancement by MRI are needed to complement our findings. Lastly, LV mass and volume data from MRI were not available so that we could not use MRI for global LV function assessment.
CONCLUSIONS
This study suggests that HIV infection may contribute to impaired regional left ventricular systolic and diastolic function and that cardiac tagged MRI could be used as a sensitive tool for the detection of early HIV-associated cardiomyopathies. Further studies with larger sample sizes and a longitudinal design are warranted.
ACKNOWLEDGEMENTS
We thank the study participants for their contributions. The study was supported by grants from the National Institute on Drug Abuse, National Institutes of Health (NIH R01-DA 12777 and DA15020).
REFERENCES
- 1.Highleyman L. Mortality trends: toward a new definition of AIDS? BETA. 2005;17:18–28. [PubMed] [Google Scholar]
- 2.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S, Wilkinson D, Becker A, Askew D, Ntyintyane L, McMurray JJ, Sliwa K. Mapping the emergence of heart disease in a black, urban population in Africa: the Heart of Soweto Study. Int J Cardiol. 2006;108:101–108. doi: 10.1016/j.ijcard.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Sudano I, Spieker LE, Noll G, Corti R, Weber R, Luscher TF. Cardiovascular disease in HIV infection. Am Heart J. 2006;151:1147–1155. doi: 10.1016/j.ahj.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Barbaro G. Cardiovascular manifestations of HIV infection. Circulation. 2002;106:1420–1425. doi: 10.1161/01.cir.0000031704.78200.59. [DOI] [PubMed] [Google Scholar]
- 6.Barbaro G, Barbaro G. Incidence of the involvement of the cardiovascular system in HIV infection. AIDS. 2003;17(suppl 1):s46–50. [PubMed] [Google Scholar]
- 7.Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging: a method for noninvasive assessment of myocardial motion. Radiology. 1998;169:59–63. doi: 10.1148/radiology.169.1.3420283. [DOI] [PubMed] [Google Scholar]
- 8.Young AA, Axel L. Three-dimensional motion and deformation of the heart wall: estimation with spatial modulation of magnetization: a model-based approach. Radiology. 1992;185:241–7. doi: 10.1148/radiology.185.1.1523316. [DOI] [PubMed] [Google Scholar]
- 9.O'Dell WG, Moore CC, Hunter WC, Zerhouni EA, McVeigh ER. Three-dimensional myocardial deformations calculation with displacement field fitting to tagged MR images. Radiology. 1995;195:829–835. doi: 10.1148/radiology.195.3.7754016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garot J, Bluemke DA, Osman NF, Rochitte CE, McVeigh ER, Zerhouni EA, Prince JL, Lima JA. Fast determination of regional myocardial strain fields from tagged cardiac images using harmonic phase MRI. Circulation. 2000;101(9):981–988. doi: 10.1161/01.cir.101.9.981. [DOI] [PubMed] [Google Scholar]
- 11.Henri WL, De Maria A, Gramiak R. Report of the American Society of Cardiography committee on nomenclature and standards in two-dimensional echocardiography. Circulation. 1980;62:212–217. doi: 10.1161/01.cir.62.2.212. [DOI] [PubMed] [Google Scholar]
- 12.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 13.Osman NF, Prince JL. Visualizing myocardial function using HARP MRI. Phys Med Biol. 2000;45:1665–1682. doi: 10.1088/0031-9155/45/6/318. [DOI] [PubMed] [Google Scholar]
- 14.Castillo E, Osman NF, Rosen BD, El-Shehaby I, Pan L, Jerosch-Herold M, et al. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: Interobserver and intraobserver agreement of fast strain analysis with harmonic phase (HARP) MRI. J Cardiovasc Magn Reson. 2005;7(5):783–91. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 15.Edvardsen T, Rosen BD, Pan L, Jerosch-Herold M, Lai S, Hundley WG, et al. Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imaging--the multi-ethnic study of atherosclerosis (MESA) Am Heart J. 2006 Jan;151(1):109–14. doi: 10.1016/j.ahj.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes VR, Edvardsen T, Rosen BD, Carvalho B, Campos O, Cordeiro MA, et al. The influence of left ventricular size and global function on regional myocardial contraction and relaxation in an adult population free of cardiovascular disease: A tagged CMR study of the MESA cohort. J Cardiovasc Magn Reson. 2007;9(6):921–30. doi: 10.1080/10976640701693824. [DOI] [PubMed] [Google Scholar]
- 17.Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch-Herold M, et al. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: The multi-ethnic study of atherosclerosis. Circulation. 2005 Aug 16;112(7):984–91. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 18.Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch-Herold M, Shantanu S, Kronmal R, Arnett D, Crouse JR, III, Heckbert SR, Bluemke DA, Lima JAC. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi Ethnic Study of Atherosclerosis. Circulation. 2005;112(7):984–91. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 19.SAS Institute, Inc . SAS/STAT User Guide: Version 8. SAS Institute, Inc.; Cary, NC: 1999. [Google Scholar]
- 20.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 21.Lai S, Lima JA, Lai H, Vlahov D, Celentano D, Tong W, Bartlett JG, Margolick J, Fishman EK. Human immunodeficiency virus 1 infection, cocaine, and coronary calcification. Arch Intern Med 28. 2005;165(6):690–5. doi: 10.1001/archinte.165.6.690. [DOI] [PubMed] [Google Scholar]
- 22.Eugenin EA, Morgello S, Klotman ME, Mosoian A, Lento PA, Berman JW, Schecter AD. Human immunodeficiency virus (HIV) infects human arterial smooth muscle cells in vivo and in vitro: implications for the pathogenesis of HIV-mediated vascular disease. Am J Pathol. 2008;172(4):1100–11. doi: 10.2353/ajpath.2008.070457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masood S, Yang GZ, Pennell DJ, Firmin DN. Investigating intrinsic myocardial mechanics: the role of MR tagging, velocity phase mapping, and diffusion imaging. J Magn Reson Imaging. 2000;12:873–883. doi: 10.1002/1522-2586(200012)12:6<873::aid-jmri10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 24.Gerber BL, Garot J, Bluemke DA, Wu KC, Lima JA. Accuracy of contrast-enhanced magnetic resonance imaging in predicting improvement of regional myocardial function in patients after acute myocardial infarction. Circulation. 2002;106:1083–1089. doi: 10.1161/01.cir.0000027818.15792.1e. [DOI] [PubMed] [Google Scholar]
- 25.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lance. 2003;361:374–379. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 26.Phillips AN, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, Williams I, Drummond F, Duprez D, Belloso WH, Goebel F-D, Grund B, Hatzakis A, Vera J, Lundgren JD. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther. 2008;13:177–187. doi: 10.1177/135965350801300215. [DOI] [PubMed] [Google Scholar]
- 27.Cohen IS, Anderson DW, Virmani R, Reen BM, Macher AM, Sennesh J, DiLorenzo P, Redfield RR. Congestive cardiomyopathy in association with the acquired immunodeficiency syndrome. N Engl J Med. 1986;315(10):628–30. doi: 10.1056/NEJM198609043151007. [DOI] [PubMed] [Google Scholar]