Abstract
Remnants of triglyceride-rich lipoproteins containing apolipoprotein (apo) B-48 accumulate in apo E-deficient mice, causing pronounced hypercholesterolemia. Mice doubly deficient in apo E and hepatic lipase have more pronounced hypercholesterolemia, even though remnants do not accumulate appreciably in mice deficient in hepatic lipase alone. Here we show that the doubly deficient mice manifest a unique lamellar hyperlipoproteinemia, characterized by vesicular particles 600 Å–1,300 Å in diameter. As seen by negative-staining electron microscopy, these lipoproteins also contain an electron-lucent region adjacent to the vesicle wall, similar to the core of typical lipoproteins. Correlative chemical analysis indicates that the vesicle wall is composed of a 1:1 molar mixture of cholesterol and phospholipids, whereas the electron-lucent region appears to be composed of cholesteryl esters (about 12% of the particle mass). Like the spherical lipoproteins of doubly deficient mice, the vesicular particles contain apo B-48, but they are particularly rich in apo A-IV. We propose that cholesteryl esters are removed from spherical lipoproteins of these mice by scavenger receptor B1, leaving behind polar lipid-rich particles that fuse to form vesicular lipoproteins. Hepatic lipase may prevent such vesicular lipoproteins from accumulating in apo E-deficient mice by hydrolyzing phosphatidyl choline as scavenger receptor B1 removes the cholesteryl esters and by gradual endocytosis of lipoproteins bound to hepatic lipase on the surface of hepatocytes.
Keywords: cholesterol, phospholipids, scavenger receptor B1, endocytosis
Chylomicrons transport dietary fat and cholesterol into the blood and are metabolized in two discrete steps (1). First, chylomicron triglycerides and phospholipids are partially removed in extrahepatic tissues by lipoprotein lipase to yield chylomicron remnants (CR); second, the remnants are adsorbed onto hepatic parenchymal cells and undergo receptor-mediated endocytosis. Binding of CR to hepatocytes is mediated chiefly by the low-density lipoprotein (LDL) receptor and hepatic lipase (HL) (1). The LDL receptor-related protein can also bind CR that have been enriched with apolipoprotein (apo) E. Endocytosis of CR, leading to lysosomal catabolism, is primarily mediated by the LDL receptor. The LDL receptor and LDL receptor-related protein bind CR by means of apo E. Thus, apo E-deficient mice (2, 3) and humans (4) manifest massive accumulation of CR. Although CR clearance is impaired in HL-deficient mice (5), and conversion of very low-density lipoprotein (VLDL) remnants to LDL and LDL concentrations are reduced in HL-deficient humans (6) and mice (5), accumulation of remnant lipoproteins is limited (5–7). Recently, Mezdour et al. (8) have reported that mice doubly deficient in apo E and HL have considerably higher levels of cholesterol in lipoproteins with remnant characteristics than mice deficient only in apo E. Here we show that the doubly deficient mice uniquely manifest a pronounced lamellar hyperlipoproteinemia, in which lipoprotein particles resembling liposomes accumulate. The formation of these lamellar lipoproteins evidently requires deficiency of HL in the setting of the gross remnant accumulation associated with apo E deficiency. HL thus provides an alternative system for hepatic metabolism of remnant particles that cannot be recognized by the LDL receptor or the LDL receptor-related protein, in the absence of which a distinct third pathway of remnant processing is uncovered.
METHODS
Animals.
Mice deficient in apo E (developed on a strain 129 background and backcrossed with C57BL/6J mice for five generations) were bred with mice deficient in HL (5, 7). Deficient heterozygotes and homozygotes were identified by blood plasma immunoassays specific for rat HL (9) and apo E (10). Unless otherwise noted, mice were studied at 12–20 weeks of age and were fed standard chow.
Separation and Characterization of Lipoproteins.
Blood samples were obtained as described (5). To inhibit hepatic lipase, tetrahydrolipostatin (final concentration 0.1 μg/ml) (5) was added to plasma from mice deficient only in apo E. Plasma pools were subjected to sequential ultracentrifugation and lipids and total protein were estimated in plasma and lipoprotein fractions (5). Individual apolipoproteins were quantified by an SDS gel electrophoretic procedure (11). The electrophoretic mobility of lipoprotein fractions was determined after separation in agarose gel (12). Nondenaturing 2%–14% gradient gel electrophoresis of plasma, with lipid staining, and calculation of lipoprotein particle sizes were performed as described (13). For preparative purposes, lipoprotein fractions were size-fractionated on a 0.9 × 60 cm column of 4% agarose gel (14); individual fractions of 0.7 ml were pooled based on absorbance at 280 nM. Phospholipid species were quantified as lipid P after separation of plasma extracts (15) by thin–layer chromatography (16). Fatty acids of cholesteryl esters in extracts (15) of lipoprotein fractions separated by thin-layer chromatography (17) were converted to methyl esters (18) and quantified in a Packard model 5890A gas chromatograph. Plasma and lipoprotein fractions were examined by electron microscopy after negative staining (19).
RESULTS
Plasma cholesterol concentrations in doubly deficient male mice were approximately 2-fold higher than in mice deficient only in apo E. This difference was much less pronounced in females (Table 1). Differences in the concentration of lipoprotein lipids were most pronounced for intermediate-density lipoproteins (IDL) and LDL, the overall concentration of which was increased about 3-fold in the doubly deficient mice. Moreover, the fraction of cholesterol not esterified was increased, particularly in LDL. The concentration of lipids in high-density lipoprotein (HDL) fractions was also substantially higher in the doubly deficient animals, as expected (5, 7). Plasma phospholipids of doubly deficient mice were poorer in sphingomyelin than were those from apo E-deficient animals. The phosphatidyl choline:sphingomyelin ratio was 7.9 and 10.1 in two doubly deficient mice and 3.5, 3.6, and 3.1 in three apo E-deficient mice. Agarose gel electrophoretograms from doubly deficient mice showed three species in IDL and two in LDL; only a single species was found in apo E-deficient mice (Fig. 1). Negative-staining electron microscopy (Fig. 2) showed little difference among VLDL species, but the IDL and LDL of doubly deficient mice contained, in addition to the spherical particles found in apo E deficiency, much larger particles with a mainly electron-opaque center surrounded by a more electron-lucent wall. This structure is characteristic of lamellar lipoprotein vesicles, in which a bilayer of polar lipids encloses an aqueous compartment, as in liposomes (19). Many of the vesicular particles contained an eccentrically placed nubbin of electron-lucent material, suggestive of a residual nonpolar lipid core, but the particles varied greatly in size and appearance and, in some, more than one surface lamella was evident. The light HDL class of doubly deficient mice contained some vesicular particles but otherwise resembled those of apo E deficiency. The heavy HDL classes of the two groups were similar and lacked lamellar structures (vesicles or disks, not shown). The heterogeneity of IDL and LDL classes of the doubly deficient mice was also evident on gradient gel electrophoresis (Fig. 3). In both, considerable material was present at or near the top of the gradient, in addition to the smaller particles characteristic of the IDL and LDL of apo E deficiency.
Table 1.
Lipoprotein-lipid concentrations, mg/dl, in Apo E (−/−) and Apo E (−/−) + hepatic lipase (−/−) mice
| Fraction | Apo E (−/−)
|
Apo E (−/−) + hepatic lipase (−/−)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Total cholesterol | Free cholesterol | Phospholipids | Triglycerides | Total cholesterol | Free cholesterol | Phospholipids | Triglycerides | |
| Females | ||||||||
| Plasma | 498 (206) | 203 | 336 | 121 (37) | 735 (354) | 321 | 791 | 136 (56) |
| VLDL | 274 | 102 | 176 | 69 | 299 | 113 | 202 | 96 |
| IDL | 62 | 21 | 33 | 11 | 137 | 57 | 98 | 11 |
| LDL | 102 | 43 | 60 | 14 | 182 | 124 | 204 | 16 |
| HDLL | 7 | 3 | 13 | 6 | 34 | 8 | 37 | 5 |
| HDLH | 18 | 5 | 65 | 4 | 72 | 3 | 210 | 8 |
| Males | ||||||||
| Plasma | 613 (123) | 300 | 573 | 242 (155) | 1302 (436)*† | 573 | 1143 | 288 (150)† |
| VLDL | 405 | 156 | 260 | 191 | 684 | 251 | 473 | 249 |
| IDL | 63 | 27 | 41 | 12 | 225 | 94 | 174 | 16 |
| LDL | 125 | 58 | 103 | 18 | 253 | 165 | 317 | 12 |
| HDLL | 12 | 7 | 27 | 4 | 34 | 25 | 38 | 7 |
| HDLH | 23 | 7 | 83 | 3 | 89 | 19 | 124 | 4 |
Data for Apo E (−/−) mice are from single pools of seven males and four females (age 24–28 weeks); data from apo E (−/−)+ hepatic lipase (−/−) mice are means from two pools of plasma from seven to ten mice each. Standard deviations (in parentheses) for total cholesterol and triglycerides in plasma are from individual analyses from the mice used to make these pools.
HDLL, light HDL; HDLH, heavy HDL.
Significantly different from Apo E (−/−) mice (P < 0.001).
Significantly different from females of the same genotype (P < 0.001).
Figure 1.
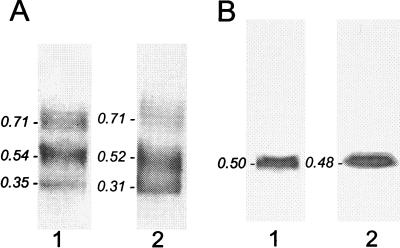
Agarose gel electropherograms of IDL (1) and LDL (2) from an apo E- and HL-doubly deficient mouse (A) and an apo E-deficient mouse (B). A single component with β-mobility is evident in both fractions from the apo E-deficient mouse, whereas three components with slow β, β, and pre-β mobilities are evident in fractions from the doubly deficient mouse. (The latter pattern was observed with IDL and LDL from two other mice.) Rf values relative to albumin marker (not shown) are indicated for each lipoprotein component.
Figure 2.
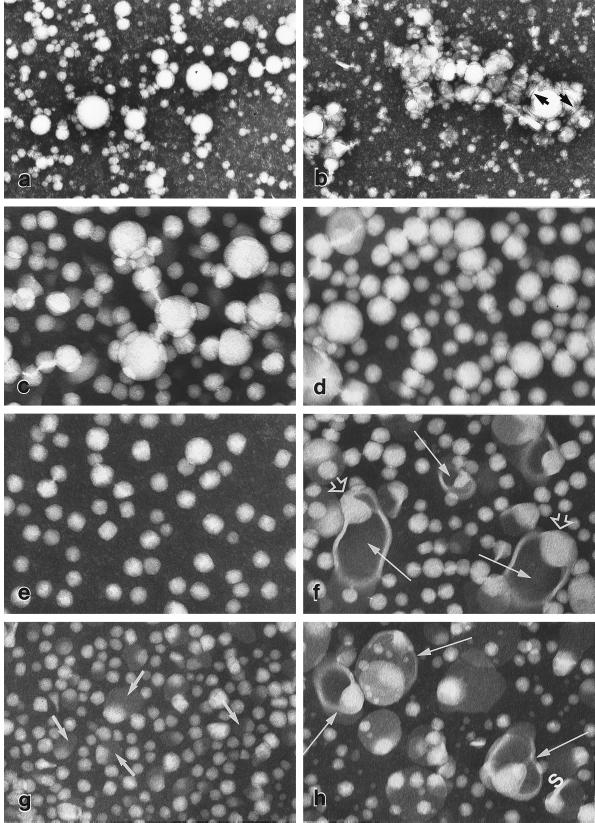
Plasma lipoproteins, visualized by negative-staining electron microscopy from apo E-deficient (Left: a, c, e, g) and doubly deficient mice (Right: b, d, f, h). Whole plasma from apo E-deficient mice (a) contains mainly large spherical particles of 200 Å–1,600 Å in diameter; that from doubly deficient mice (b) contains, in addition, clusters of polymorphic structures, some of which are flattened (arrows). VLDL fractions from apo E-deficient mice (c) and doubly deficient mice (d) contain spherical particles of 250 Å–1,600 Å in diameter with electron-lucent cores as seen in whole plasma. IDL from apo E-deficient mice (e) contain round to rectangular particles of 225 Å–450 Å diameter with electron-lucent cores whereas IDL from doubly deficient mice (f) contain, in addition, larger polymorphic particles with electron-opaque cores (arrows); in many of these an electron-lucent region (open arrowhead) is seen abutting the wall. The LDL fraction from apo E-deficient mice (g) contains somewhat smaller particles (170 Å–300 Å diameter) than those in e; some larger particles are partially electron-opaque (arrows). LDL from doubly deficient mice (h) contain a similar mixture of particles to those seen in (f) but with a greater proportion of the larger species (arrows). (×87,300, except for a and b (×29,100.)
Figure 3.
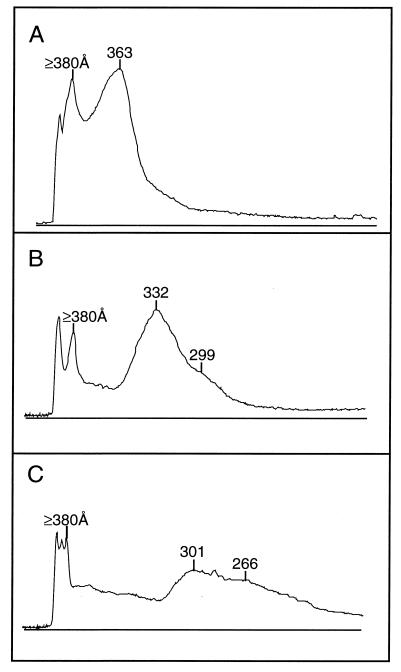
Densitometric tracings of lipid-stained 2%–l4% polyacrylamide gels after electrophoresis of VLDL (A), IDL (B), and LDL (C) from an apo E- and HL-doubly deficient mouse. Components near the top of the gel are evident in all cases, together with included components of progressively decreasing size. Comparable patterns were observed with lipoproteins from two other doubly deficient mice. The early components for VLDL represent large spherical particles that were excluded from, or barely entered, the gel, whereas those for IDL and LDL represent lamellar particles. The more rapidly migrating components represent spherical microemulsion particles in all cases (see Fig. 4) and may include lipoprotein species containing or lacking apo B.
The protein components of VLDL, IDL, and LDL classes from doubly deficient mice, like those from apo E-deficient mice, were characterized by an overwhelming predominance of apo B-48 over apo B-100 (Table 2). Apo A-IV, however, was a much more prominent constituent of all lipoprotein classes of the doubly deficient mice and its concentration in plasma (determined only in females) was increased 5-fold over that of apo E-deficient animals The concentration of apo A-I was also increased in the doubly deficient mice (chiefly in heavy HDL), as was the concentration of combined A-II + C apoproteins (chiefly in VLDL).
Table 2.
Apolipoprotein concentrations, mg/dl, in Apo E (−/−) and Apo E (−/−)+ hepatic lipase (−/−) mice
| Fraction | Apo E (−/−)
|
Apo E (−/−)+ hepatic lipase (−/−)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B-100 | B-48 | A-IV | A-I | A-II+Cs | B-100 | B-48 | A-IV | A-I | A-II+Cs | |
| Females | ||||||||||
| Total | 2.9 | 28.5 | 7.3 | 21.3 | 29.8 | 5.0 | 39.6 | 35.4 | 103.8 | 92.5 |
| VLDL | 0.6 | 13.5 | 5.2 | 5.5 | 17.8 | 0.9 | 9.9 | 10.3 | 3.1 | 14.7 |
| IDL | 0.7 | 6.7 | 1.1 | 4.5 | 4.6 | 0.9 | 9.0 | 7.3 | 4.1 | 7.7 |
| LDL | 1.6 | 6.6 | 0.3 | 3.6 | 1.6 | 2.7 | 14.9 | 9.9 | 8.3 | 9.4 |
| HDLL | — | 1.7 | 0.1 | 0.3 | 0.9 | 0.5 | 3.9 | 5.3 | 5.8 | 3.8 |
| HDLH | — | — | 0.6 | 7.4 | 4.9 | — | 1.9 | 2.6 | 82.5 | 56.9 |
| Males | ||||||||||
| Total | 6.1 | 54.2 | 45.6 | 95.7 | 158.5 | |||||
| VLDL | 4.0 | 26.7 | 8.0 | 11.3 | 34.9 | 2.1 | 24.7 | 21.0 | 8.1 | 49.4 |
| IDL | 0.7 | 6.8 | 1.0 | 4.2 | 3.6 | 1.1 | 10.2 | 8.0 | 5.0 | 11.4 |
| LDL | 2.7 | 13.9 | 9.8 | 9.0 | 11.9 | |||||
| HDLL | 0.2 | 3.9 | 4.3 | 7.3 | 7.6 | |||||
| HDLH | — | 1.5 | 2.5 | 66.3 | 78.2 | |||||
Data from Apo E (−/−) mice are from single pools of plasma from eight males and four females; data from Apo E (−/−)+ hepatic lipase (−/−) mice are means from two pools of seven to ten mice each.
To define further the properties of the lamellar lipoproteins, IDL and LDL were size-fractionated by gel chromatography. Two fractions were obtained. For both IDL and LDL, the early peak of material contained highly purified vesicular particles (Fig. 4). These lipoproteins, in contrast to the mainly spherical particles eluting in the second peak, were primarily composed of free cholesterol and phospholipids in an approximately 1:1 molar ratio (Table 3), as in the vesicular lipoprotein of cholestasis (LP-X) (20), but also contained about 12% by mass of cholesteryl esters and a small amount of triglycerides (Table 3). Apo B-48 and apo A-IV together accounted for about 75% of the protein mass of the lamellar particles. The appearance and composition of the spherical particles in IDL closely resembled those of apo E deficiency. The smaller LDL species of doubly deficient mice were more polymorphic, with some particles characterized by apparent excess of surface material and a few discoidal particles. The composition of this fraction suggested a mixture of mainly spherical particles with some admixture of phospholipid-rich particles, as compared with the LDL from apo E-deficient mice.
Figure 4.
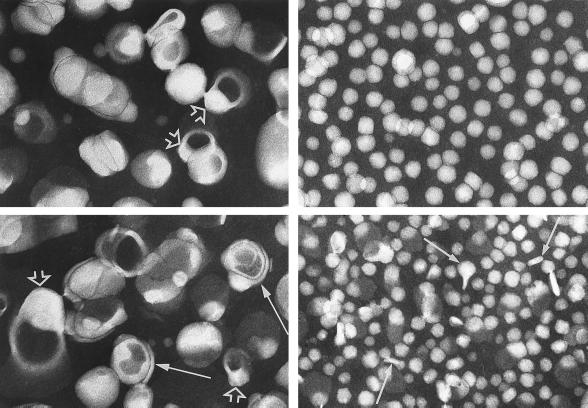
Species of lipoproteins in IDL (Upper) and LDL (Lower) fractions from apo E- and HL-doubly deficient mice, separated by gel filtration. The larger species of 600 Å–1,300 Å diameter (Left) are characterized by an electron-opaque core, with an electron-lucent region abutting the wall (open arrowheads). Some particles are multilamellar (arrows). The smaller species (Right) resemble the spherical/rectangular particles of total IDL and LDL (see Fig. 2), with a few oblong or irregular particles in the latter (arrows). (×87,300.)
Table 3.
Composition of lipoprotein species in Apo E (−/−)+ hepatic lipase (−/−)+ Apo E (−/−) mice
| Component | IDL, % mass
|
LDL, % mass
|
||||
|---|---|---|---|---|---|---|
| Double deficiency
|
Apo E (−/−) | Double deficiency
|
Apo E (−/−) | |||
| Lamellar | Spherical | Lamellar | Spherical | |||
| CE | 11.0 | 46.8 | 40.8 | 12.4 | 28.3 | 35.7 |
| TG | 4.1 | 1.9 | 7.7 | 1.4 | 2.3 | 5.4 |
| FC | 28.5 | 10.3 | 15.7 | 28.3 | 11.3 | 16.8 |
| PL | 50.0 | 30.2 | 23.8 | 52.6 | 43.7 | 26.6 |
| Protein | 6.3 | 10.6 | 12.1 | 5.1 | 14.1 | 15.6 |
| B-100 | 0.2 | 0.1 | 0.5 | 0.1 | 0.6 | 1.8 |
| B-48 | 2.2 | 3.1 | 4.9 | 1.5 | 4.9 | 7.5 |
| A-IV | 2.4 | 1.2 | 0.7 | 2.5 | 1.5 | 0.3 |
| A-I | — | 2.9 | 3.1 | 0.5 | 5.9 | 4.1 |
| A-II + Cs | 1.5 | 3.3 | 2.9 | 0.5 | 1.2 | 1.9 |
CE, cholesteryl esters; TG, triglycerides; FC, free cholesterol; PL, phospholipids.
To evaluate the provenance of the cholesteryl esters of the lamellar lipoproteins, we determined their fatty acid composition (Table 4). Lamellar particles were richer in cholesteryl palmitate, stearate, and oleate and poorer in cholesteryl linoleate and arachidonate than were spherical IDL+LDL particles.
Table 4.
Fatty acid composition, mol %, of cholesteryl esters of IDL + LDL in hepatic lipase (−/−) and apo E (−/−) + hepatic lipase (−/−) mice
| Fatty acid | Lamellar particles | Spherical particles |
|---|---|---|
| 14:0 | 2.6 | — |
| 16:0 | 14.8 | 9.5 |
| 16:1 | 5.9 | 5.1 |
| 18:0 | 5.2 | 1.9 |
| 18:1 | 25.7 | 18.4 |
| 18:2 | 31.9 | 44.7 |
| 18:3 | 1.2 | 1.1 |
| 20:4 | 12.6 | 19.3 |
DISCUSSION
On the background of apo E deficiency, with its massive accumulation of remnant lipoproteins containing apo B-48 (2), superimposed deficiency of HL has a dramatic and unexpected effect: the appearance of large numbers of lamellar lipoproteins with a vesicular structure resembling liposomes. These particles, with a density spanning that of IDL and LDL, are much larger than the spherical microemulsion lipoproteins that persist in the IDL and LDL fractions of doubly deficient animals, and even larger than the bulk of VLDL particles that predominate in apo E-deficient mice. The lamellar surface of the vesicles is presumably composed mainly of an equimolar mixture of phospholipids and cholesterol, associated primarily with apo B-48 and apo A-IV. They are more polymorphic and most are larger than LP-X (20), however, and contain about 15% by weight of nonpolar lipids, which presumably compose the electron-lucent nubbin seen in electron photomicrographs.
The structure and composition of VLDL from the doubly deficient mice resembles that of apo E deficiency alone. Their concentration is increased only in males. The concentration and size of HDL particles is increased in both sexes, as in mice with deficiency of HL alone (5, 7). Thus, the increased hypercholesterolemia found in female doubly deficient mice results primarily from accumulation of the lamellar lipoproteins and, to a lesser extent, from accumulation of HDL; in the males, an increased concentration of β-VLDL also contributes to the augmented hypercholesterolemia. The basis for this sex-specific effect is unclear.
The vesicular particles likely are products of the metabolism of the accumulated β-VLDL and evidently result from the HL deficiency. In apo E-deficient mice, dietary cholesterol, as cholesteryl esters, does reach the liver, as it does in wild-type mice (21), indicating that this component of chylomicron remnants is eventually taken up by the liver without the intervention of the receptors that recognize apo E (LDL receptor and LDL receptor-related protein). In the doubly deficient mice, plasma lipoproteins also reach plateau values, indicating that some removal processes persist, but this is now accompanied by the accumulation of lamellar particles in the blood, suggesting that the remnants cannot be taken up into hepatocytes by endocytosis. HL participates in the initial binding of chylomicron remnants to liver cells in rats (22) and mice (5), binds apo B-48 by means of its amino-terminal region (23), and is concentrated in endosomes in rat liver (24). Thus, we suggest that HL mediates the endocytosis of remnants containing apo B-48 in apo E-deficient mice. The potential for this process is indicated by the substantial reduction of plasma cholesterol concentrations of apo E-deficient mice overexpressing human HL at very high levels (25). The cholesteryl esters of the lamellar lipoproteins contain fewer polyunsaturated fatty acids than do the spherical IDL and LDL, resembling those of VLDL of wild-type mice (26). These cholesteryl esters are thought to be synthesized by acyl CoA cholesterol acyltransferase in intestinal absorptive cells or hepatocytes (27). As compared with the β-VLDL containing apo B-48, however, the lamellar particles are grossly depleted of cholesteryl esters. These observations suggest that the lamellar particles are byproducts of selective uptake of cholesteryl esters by the liver (28) from the β-VLDL. This process is mediated by scavenger receptor B1 (SR-B1) and accounts for a major portion of the plasma clearance of HDL cholesteryl esters in rodents (29). SR-B1 can bind apolipoproteins containing amphipathic helical repeats (30). Apo A-IV, apo A-I, and C apoproteins of the β-VLDL could therefore confer affinity to SR-B1, leading to gradual removal of most of the cholesteryl esters from the core of these lipoproteins. Selective removal is thought to contribute to the generation of core lipid-poor and protein-rich pre-β-HDL from α-HDL. The pre-β-HDL can then acquire more unesterified cholesterol from peripheral cells that is esterified by lecithin-cholesterol acyltransferase, providing a cycle for trafficking of cholesterol from extrahepatic cells to the liver for excretion into the bile (31). In the case of β-VLDL, a product resembling pre-β-HDL is unlikely, given the high molecular weight and low aqueous solubility of apo B-48 (32). We propose that the cholesteryl ester-depleted β-VLDL become reorganized by fusion of several of the core lipid-depleted precursor particles to yield stable vesicles. Cholesteryl esters may also be removed selectively from β-VLDL in mice deficient only in apo E. If such removal occurs, HL could hydrolyze phosphatidylcholine on the lipoprotein surface as cholesteryl esters are removed, thereby reducing the potential for vesicle formation. Others have suggested that HL may normally act on HDL in concert with SR-B1 (33, 34).
In their studies of mice deficient in apo E and HL, Mezdour et al. (8) observed reduced atherosclerotic lesions in females but not in males. They found that pre-β-HDL persisted among the more prevalent HDL particles of the doubly deficient mice and that their HDL had increased capacity to promote efflux of cholesterol from cultured cells. They attributed reduced atherogenesis in the female mice to these changes in concentration and properties of HDL. Our observations suggest additional reasons for reduced atherosclerosis in the doubly deficient female mice. First, the augmented hypercholesterolemia primarily reflects accumulation of the large vesicular lipoproteins, which may have little or no atherogenic potential. Second, the atherogenic potential of the β-VLDL of apo E-deficient mice has been attributed in part to enrichment with sphingomyelin, which increases aggregation of the lipoproteins when exposed to sphingomyelinase secreted by macrophages and endothelial cells (35). We found the lipoprotein-phospholipids of the doubly deficient mice to be relatively deficient in sphingomyelin, which presumably reflects lack of hydrolysis of the major phospholipid, phosphatidyl choline, by HL (36). Thus, their β-VLDL may have reduced atherogenic potential. Third, the lamellar lipoproteins may contribute to reverse transport of arterial cholesterol by sequestering cholesterol transferred initially to HDL (37). Like liposomes prepared in vitro (38), these vesicular particles may be taken up by hepatic Kupffer cells. The high cholesterol–phospholipid ratio of the lamellar particles indicates little potential for such sequestration, but may reflect only saturation with free cholesterol associated with a long residence time in plasma. Evidently, several mechanisms other than alterations within HDL could account for reduced atherogenesis in the doubly deficient female mice. The comparable atherosclerosis observed by Mezdour et al. (8) in doubly deficient males may reflect their much higher β-VLDL concentrations.
In conclusion, HL evidently prevents the accumulation of vesicular lipoproteins in apo E-deficient mice, presumably by hydrolyzing phosphatidyl choline of β-VLDL and by providing a pathway for their removal by means of endocytosis into the liver. The production of these distinct lamellar particles may reflect the selective removal pathway for cholesteryl esters, acting on an atypical substrate, β-VLDL.
Acknowledgments
We thank Edward Rubin and Nobuyo Maeda for providing, respectively, breeding pairs of apo E (−/−) and HL (−/−) mice, Jinny Wong for outstanding electron microscopy, Jon Goerke for compositional analysis of fatty acids, and Edward Rubin for comments on an earlier version of the manuscript. This research was supported by grants from the National Institutes of Health (HL 14990, HL 14237, and HL 18574). M.V. was recipient of a fellowship from the Spanish Ministry of Education and Culture.
ABBREVIATIONS
- apo
apolipoprotein
- CR
chylomicron remnants
- HL
hepatic lipase
- VLDL
very low-density lipoprotein(s)
- IDL
intermediate-density lipoprotein(s)
- LDL
low-density lipoprotein(s)
- HDL
high-density lipoprotein(s)
- SR-B1
scavenger receptor B1
References
- 1.Havel R J. Curr Opin Lipidol. 1995;6:312–316. doi: 10.1097/00041433-199510000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S H, Reddick R L, Piedrahita J A, Maeda N. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 3.Plump A, Smith J, Hayek T, Aalto-Setala K, Walsh A, Verstuyft J, Rubin E, Breslow J. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 4.Ghiselli G, Schaefer E J, Gascon P, Brewer H B., Jr Science. 1981;214:1239–1241. doi: 10.1126/science.6795720. [DOI] [PubMed] [Google Scholar]
- 5.Qiu S, Bergeron N, Kotite L, Krauss R M, Bensadoun A, Havel R J. J Lipid Res. 1998;39:1661–1668. [PubMed] [Google Scholar]
- 6.Demant T, Carlson L A, Holmquist L, Karpe F, Nilsson-Ehle P, Packard C J, Shepherd J. J Lipid Res. 1988;29:1603–1611. [PubMed] [Google Scholar]
- 7.Homanics G E, de Silva H V, Osada J, Zhang S H, Wong H, Borensztajn J, Maeda N. J Biol Chem. 1995;270:2974–2980. doi: 10.1074/jbc.270.7.2974. [DOI] [PubMed] [Google Scholar]
- 8.Mezdour H, Jones R, Dengremont C, Castro G, Maeda N. J Biol Chem. 1997;272:13570–13575. doi: 10.1074/jbc.272.21.13570. [DOI] [PubMed] [Google Scholar]
- 9.Cisar L, Bensadoun A. J Lipid Res. 1985;26:380–386. [PubMed] [Google Scholar]
- 10.Brasaemle D, Cornely-Moss K, Bensadoun A. J Lipid Res. 1993;34:455–465. [PubMed] [Google Scholar]
- 11.Bergeron N, Kotite L, Havel R J. Methods Enzymol. 1996;263:82–94. doi: 10.1016/s0076-6879(96)63006-7. [DOI] [PubMed] [Google Scholar]
- 12.Noble R P. J Lipid Res. 1968;9:693–700. [PubMed] [Google Scholar]
- 13.Krauss R M, Burke D J. J Lipid Res. 1982;23:97–104. [PubMed] [Google Scholar]
- 14.van’t Hooft F, Havel R J. J Biol Chem. 1981;256:3963–3968. [PubMed] [Google Scholar]
- 15.Bligh E G, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 16.Touchstone J C, Chen J C, Beaver K M. Lipids. 1980;15:61–62. [Google Scholar]
- 17.Fielding C J. Biochim Biophys Acta. 1979;573:255–265. doi: 10.1016/0005-2760(79)90059-6. [DOI] [PubMed] [Google Scholar]
- 18.Eder K, Reichlmayer-Lais A M, Kirchgessner M. J Chromatogr. 1992;607:55–67. doi: 10.1016/0021-9673(92)87054-c. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton R L, Jr, Goerke J, Guo L S S, Williams M C, Havel R J. J Lipid Res. 1980;21:981–992. [PubMed] [Google Scholar]
- 20.Hamilton R L, Havel R J, Kane J P, Blaurock A E, Sata T. Science. 1971;172:475–478. doi: 10.1126/science.172.3982.475. [DOI] [PubMed] [Google Scholar]
- 21.Chang S, Zhang S H, Maeda N, Borensztajn J. Biochim Biophys Acta. 1994;1215:205–208. doi: 10.1016/0005-2760(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 22.Shafi S, Brady S E, Bensadoun A, Havel R J. J Lipid Res. 1994;35:709–720. [PubMed] [Google Scholar]
- 23.Choi S Y, Goldberg I J, Curtiss L K, Cooper A D. J Biol Chem. 1998;273:20456–20462. doi: 10.1074/jbc.273.32.20456. [DOI] [PubMed] [Google Scholar]
- 24.Belcher J C, Hamilton R L, Brady S E, Havel R J. Circulation. 1988;28:145. [Google Scholar]
- 25.Dichek H L, Brecht W, Fan J, Ji Z S, McCormick S P, Akeefe H, Conzo L, Sanan D A, Weisgraber K H, Young S G, et al. J Biol Chem. 1998;273:1896–1903. doi: 10.1074/jbc.273.4.1896. [DOI] [PubMed] [Google Scholar]
- 26.Mathur S N, Spector A A. Biochim Biophys Acta. 1976;424:45–56. doi: 10.1016/0005-2760(76)90048-5. [DOI] [PubMed] [Google Scholar]
- 27.Goodman D S. Physiol Rev. 1965;45:747–839. doi: 10.1152/physrev.1965.45.4.747. [DOI] [PubMed] [Google Scholar]
- 28.Glass C, Pittman R C, Weinstein D B, Steinberg D. Proc Natl Acad Sci USA. 1983;80:5435–5439. doi: 10.1073/pnas.80.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rigotti A, Trigatti B L, Penman M, Rayburn H, Herz J, Krieger M. Proc Natl Acad Sci USA. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu S, Laccotripe M, Huang X, Rigotti A, Zannis V I, Krieger M. J Lipid Res. 1997;38:1289–1298. [PubMed] [Google Scholar]
- 31.Fielding C J, Fielding P E. J Lipid Res. 1995;36:211–228. [PubMed] [Google Scholar]
- 32.Kane J P, Havel R J. In: Metabolic and Molecular Basis of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1994. pp. 1841–1852. [Google Scholar]
- 33.Wang N, Weng W, Breslow J L, Tall A R. J Biol Chem. 1996;271:21001–21004. doi: 10.1074/jbc.271.35.21001. [DOI] [PubMed] [Google Scholar]
- 34.Rigotti A, Trigatti B, Babitt J, Penman M, Xu S, Krieger M. Curr Opin Lipidol. 1997;8:181–188. doi: 10.1097/00041433-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Jeong T-S, Schissel S L, Tabas I, Pownall H J, Tall A R, Jiang X-C. J Clin Invest. 1998;101:905–912. doi: 10.1172/JCI870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson R L. The Enzymes. XVI. New York: Academic; 1983. pp. 141–181. [Google Scholar]
- 37.Rodrigueza W V, Klimuk S K, Prichard P H, Hope M J. Biochim Biophys Acta. 1998;1368:306–320. doi: 10.1016/s0005-2736(97)00198-3. [DOI] [PubMed] [Google Scholar]
- 38.Roerdink F, Dijkstra J, Hartman G, Bolscher B, Scherphof G. Biochim Biophys Acta. 1981;677:79–89. doi: 10.1016/0304-4165(81)90148-3. [DOI] [PubMed] [Google Scholar]