Abstract
Objective
To describe and compare the causal beliefs and attributions about breast and colorectal cancer among unaffected women in the general population.
Methods
A total of 439 unaffected women in the general population were recruited to complete a web-based survey assessing causal beliefs for either breast (N = 211) or colorectal cancer (N = 228).
Results
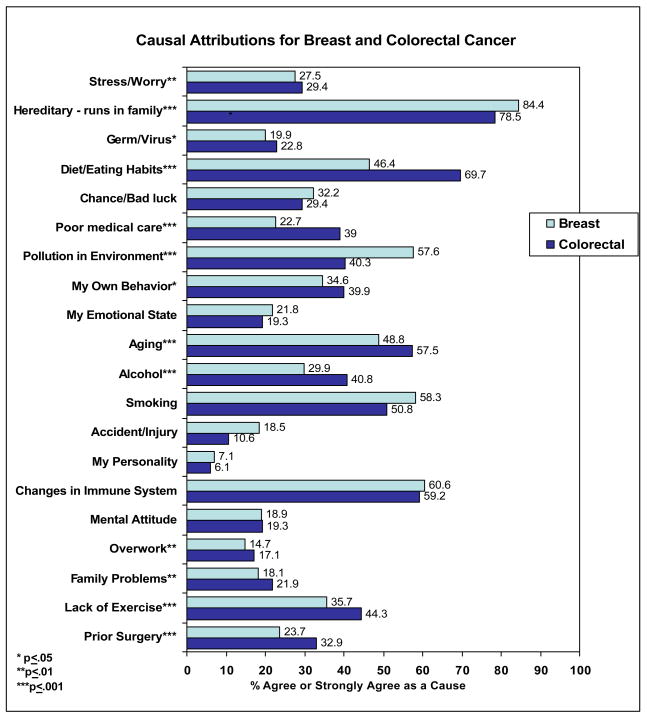
Heredity was ranked as the most important causal factor, followed by diet or eating habits for both cancer sites. Women endorsed the following causes of breast or colorectal cancer respectively: heredity (84.4%, 78.5%), diet or eating habits (46.4%, 69.7%), pollution in the environment (57.6%, 40.3%), aging (48.8%, 57.5%), alcohol (29.9%, 40.8%), smoking (58.3%, 50.8%), stress (27.5%, 29.4%), and lack of exercise (35.7%, 44.3%). Other factors such as prior surgery on the breast (23.7%) and colon (32.9%), or changes in one’s immune system (60.6% - breast; 59.2% - colon) were also endorsed by some women. Significant differences in the degree of endorsement for various causes of breast and colorectal cancer were identified.
Conclusions
Both genetic and environmental causes for breast and colorectal cancer are endorsed by unaffected women. Misconceptions about the causes of these cancers are important targets for public education and risk communication efforts.
Keywords: breast and colorectal cancer, causal beliefs, attributions, risk factors, knowledge
Introduction
In 2009, approximately 713,220 women will be diagnosed and 269,800 will die from cancer in the United States (1). Breast and colorectal cancer account for over one third of the total number of cancers diagnosed in women each year. Yet, an astonishing 38% of breast cancers and 45% of colon cancers in the United States are estimated to be preventable through appropriate diet, physical activity, and weight management (2). Although health communication efforts have often promoted the importance of engaging in healthy lifestyles to reduce one’s risk for these cancers, it is unclear to what extent women in the general population endorse modifiable lifestyle behaviors as risk factors or “causes” of breast and colorectal cancer.
In general, the notion that breast and colorectal cancers are preventable, is not readily endorsed by the general public, especially in comparison to other chronic diseases such as heart disease or diabetes (3). Beliefs about the causes of cancer in general appear to vary widely and are often inaccurate. For example, fewer than half of the respondents in one national survey were able to correctly identify a single risk factor for a variety of cancers (4). Similarly, fewer than half of the female cancer survivors in another study believed that physical inactivity, obesity, and diet were important causes of breast or colorectal cancer (5). Findings from the National Cancer Institute’s Health Information National Trends Survey (HINTS 2005: http://hints.cancer.gov/) further demonstrate that beliefs about the causal role of lifestyle behaviors vary across cancer sites. In particular, respondents were less likely to endorse a person’s behavior or lifestyle as causing colorectal cancer compared to other cancers such as skin or lung (42% versus 70–80%), suggesting that some modifiable risk factors (e.g., smoking, sun exposure) are more readily associated with disease causation than others (e.g., diet, physical activity). In sum, these findings suggest that individuals are often unaware or may not believe in cancer causes or risk factors that are modifiable through behavior change.
The ramifications of causal beliefs on health outcomes are vast. The beliefs women hold about the causes of cancer have been shown to influence the preventive actions they undertake to reduce their risk (6–8). In part, this association may be due to the role of causal beliefs and attributions on shaping subsequent beliefs about disease controllability and/or the efficacy of medical interventions (9). Moreover, the manner in which women respond to communication messages emphasizing cancer risk factors depends, in part, on their prior beliefs and cognitive representations about the disease (10–12). As such, the prior beliefs women have about the causes of cancer may determine the impact of a health communication message on desired behavioral outcomes.
To date, the beliefs that women have about the causes of breast and colorectal cancer have not been systematically documented, particularly among those without a prior medical history of either cancer. The purpose of this study was twofold: 1) to describe causal beliefs and attributions for breast and colorectal cancer among unaffected women in the general population and 2) to examine how these causal attributions might differ between the two cancer sites. Breast and colorectal cancers were chosen as the prototypic cancer sites to study because they are characterized by a well-documented hereditary component (13, 14) and thus have a underlying “genetic” cause. Moreover, unlike other hereditary cancers such as melanoma where environmental risk factors (i.e., sun exposure) are readily known to the general public, the role of environmental risk factors in breast and colorectal cancer (e.g., diet, physical activity) are less apparent and well-known (15). A greater understanding of the causal attributions women have towards these cancers will help to identify those beliefs that might facilitate, or undermine, adaptive health behaviors and lifestyle change.
Materials and methods
Study eligibility was as follows: 1) females in the general population with no prior personal history of breast and/or colorectal cancer, 2) over the age of 18, 3) able to read and write in English. All study participants were recruited from the Knowledge Networks (KN) Panel. Knowledge Networks is a full-service research provider with capabilities for customer Internet surveys, public policy and attitudinal research, concept and segmentation research, moderated online focus groups, market sciences and analytics, and statistical weighting and estimation. The KN Panel, recruited randomly through Random Digit Dialing, represents the broad diversity and key demographic dimensions of the U.S. population and provides the only probability sample of U.S. households that participate in a research panel using the internet. The web-enabled panel tracks closely the U.S. population on age, race, Hispanic ethnicity, geographical region, employment status, and other demographic elements. The current web-enabled research panel consists of approximately 40,000 adults actively participating in public opinion research.
A total of 439 unaffected women were recruited to complete a web-based survey assessing causal beliefs for either breast (N = 211) or colorectal cancer (N = 228). Recruitment of participants occurred based on a straight random sample, stratified by age (<50, ≥ 50) of panel households from the web-enabled panel. (This stratification allowed for the over-sampling of individuals over the age of 50 in order to provide a sufficient number of participants in the appropriate age range to examine separate research questions pertaining to breast and colorectal cancer screening behaviors.) One randomly sampled adult per household was included in the study provided they met the study eligibility criteria. Participants were contacted via an online web-based solicitation to determine if they were interested in taking part in a survey that set out to examine people’s beliefs about their health. As part of the web-based solicitation, those who initially agree to be contacted for the study (N=655) were then provided a secure link to access the survey. Participants then read a detailed study description and consented online (N=439, 67%). Following the consent process, participants were randomized to receive either the breast cancer or colorectal cancer version of the survey for completion.
Surveys assessed participant demographics including age, education, ethnicity, and family history of breast or colorectal cancer. In addition, causal attributions for breast and colorectal cancer were assessed using the 18-item causal attribution subscale on the Revised Illness Perception Questionnaire (IPQ-R) (16). Causal items include heredity, diet or eating habits, stress or worry, chance or bad luck, a germ or virus, and aging. Additional causes relevant to breast and colorectal cancer (e.g., lack of exercise) and as well as those that are medically inaccurate (e.g., prior surgery on colon) were also included based on prior findings in the literature (17–20). Responses to causal attribution items were provided on a 5-point scale (strongly disagree to strongly agree). As part of the subscale, participants were also asked to rank order the three most important causes of breast or colorectal cancer.
Following the completion of the survey, participants were provided with complete information on the well-known risk factors and general screening and lifestyle recommendations for breast or colorectal cancer prevention. Study methods and procedures were approved by both research review and institutional review board committees at Fox Chase Cancer Center.
Descriptive statistics were used to describe participant demographics and causal attributions. Mann-Whitney tests were used to investigate whether there were differences in the causal attributions between the two cancer sites. Statistical significance was assessed using two-sided tests of significance with 5% Type I error rates. Significance tests were based on ordinal responses, but responses were dichotomized in tables and figures (agree versus neutral/disagree) for ease of presentation.
Multiple linear regressions with robust standard errors were conducted to assess the relationship of demographics with causal attribution responses. Because the number of demographic comparisons resulted in five hypothesis tests – age, education, race/ethnicity (white vs. black, white vs. Hispanic) and family history – a bonferroni correction was implemented, setting the cut-point for statistical significance at p≤.01 (.05/5 = .01).
Results
The demographics for the women who completed the breast cancer version of the survey (N = 211) or the colorectal cancer version (N=228) are presented in Table 1. Overall, across both survey versions, 52.6% of women were over age 45, 31.1% had at least a Bachelor’s degree, and 73.3% were White/Non-Hispanic. Among women completing the breast cancer survey, 11% had at least one first degree relative with breast cancer. Similarly, 10% of women completing the colorectal cancer survey had a first degree relative with colorectal cancer. No demographic differences were noted between the cancer groups with the exception of race and ethnicity. Specifically, there were more African American women who completed the colorectal cancer version of the survey compared to the breast cancer version (χ2 = 5.83, p<.05).
Table 1.
| Demographics - N (%) | Breast Cancer (N = 211) | Colorectal Cancer (N = 228) |
|---|---|---|
| Age | ||
| 18–29 | 35 (17.1) | 56 (24.6) |
| 30–44 | 56 (26.5) | 60 (26.3) |
| 45–59 | 57 (27.0) | 55 (24.1) |
| 60–95 | 62 (29.4) | 57 (25.0) |
| Education | ||
| Less than high school | 17 (8.1) | 22 (9.6) |
| High school | 62 (29.4) | 68 (29.8) |
| Some college | 59 (28.0) | 70 (30.7) |
| Bachelor’s degree or higher | 73 (34.6) | 68 (29.8) |
| Ethnicity | ||
| White, Non-Hispanic | 158 (74.9) | 164 (71.9) |
| Black, Non-Hispanic | 16 (7.6) | 34 (14.9) |
| Other, Non-Hispanic | 5 (2.4) | 3 (1.3) |
| Hispanic | 28 (13.3) | 20 (8.8) |
| Multiracial, Non-Hispanic | 4 (1.9) | 7 (3.1) |
| Family History (# 1st degree relatives) | ||
| 0 | 188 (89.1) | 205 (89.9) |
| 1 | 21 (10.0) | 20 (8.6) |
| 2 | 2 (.9) | 2 (.9) |
| 3 | 0 (0) | 1 (.4) |
The causes endorsed by women for breast and colorectal cancer, respectively, are presented in Figure 1. The majority of women endorsed heredity as a cause of both breast (84.4%) and colorectal (78.5%) cancer. Among breast cancer survey respondents, the majority also agreed or strongly agreed with the following as causes of the disease: changes in immune system (60.6%) and pollution in the environment (57.6%). Almost half of the women endorsed aging as a risk factor for breast cancer (48.8%). Causes of breast cancer pertaining to personal behaviors were also endorsed by a subset of women including smoking (58.3%), diet (46.4%), lack of exercise (35.7%) and alcohol (29.9%).
Figure 1. Causal Attributions for Breast and Colorectal Cancer.
(% agreed or strongly agreed with cause)
Note: Reported significance values obtained from Mann-Whitney tests have been adjusted for age, education, race and ethnicity, and family history.
Among colorectal cancer survey respondents, over half of the women (57.5%) indicated that aging was a risk factor for colorectal cancer. Similar to breast cancer, a sizable percentage of women endorsed immune system changes (59.2%) and pollution in the environment (40.3%) as causes of colorectal cancer. Many women also agreed or strongly agreed that personal behaviors such as diet (69.7%), smoking (50.8%), lack of exercise (44.3%) and alcohol (40.8) play a role in causing colorectal cancer. Almost a third of women (32.9%) endorsed prior surgery on the colon as a cause of colorectal cancer.
When asked to rank order the most important causes for breast cancer, heredity was ranked as the most important causal factor, with 59.7% of women ranking this first for breast cancer. Diet or eating habits and pollution in the environment were ranked as the second most important cause of breast cancer with 14.8% and 13.4% endorsing each cause respectively. Immune system changes were ranked as the third most important cause with 14.7%. For colorectal cancer, the ranking pattern was similar but not identical. Approximately 44.7% of women ranked heredity as the most important cause of colorectal cancer, followed by diet which was ranked as the second most important cause with 12.4%. In contrast to breast cancer, aging was ranked highest as the third most important risk factor for colorectal cancer (14.8%).
Significant differences in causal attributions were noted between cancer sites. Analyses comparing breast and colorectal cancer attributions adjusted for participant demographics including age, education, race and ethnicity, and family history. As shown in Figure 1, women were significantly more likely to endorse heredity (p=.001) and pollution (p<.001) as a cause of breast cancer. In contrast, they were significantly more likely to endorse diet (p<.001), aging (p=.001), alcohol (p<.001), and lack of exercise (p=.001) as a cause of colorectal cancer. Other causes such as a prior surgery (p<.001), germ or virus (p<.05), stress (p<.01), poor medical care (p<.001), overwork (p=.01), and family problems or worries (p<.01) were endorsed more as causes of colorectal cancer compared to breast cancer.
Demographic differences in causal attributions were also examined (see Tables 2 and 3). Overall, causal attributions were found to vary as a function of age. More specifically, women age 50 and over were less likely to endorse hereditary has a cause of both colorectal and breast cancer, compared to women under 50 (p=.01). In contrast, they were more likely to endorse other behavioral (diet, p<.01) and psychological (one’s emotional state, personality, & mental attitude, all p<.01) causes of colorectal cancer. Older women were also more likely to believe that germs/virus, accidents/injury, and one’s personality were causes of breast cancer (all p<.005).
Table 2. Causal attributions for breast cancer – by demographics.
(% agreed or strongly agreed with cause)
| Cause | Age | Education | Ethnicity | Family History | |||||
|---|---|---|---|---|---|---|---|---|---|
| <50 | 50+ | <Bachelors | Bachelors+ | Black | White | Hispanic | No | Yes | |
| Stress or worry | 25.7 | 29.6 | 24.6 | 32.9 | 12.6 | 24.0 | 42.8 | 26.1 | 39.1 |
| Hereditary – it runs in my family | 89.4a | 78.6a | 82.6 | 87.7 | 62.5 | 88.0 | 75.0 | 84.6 | 82.6 |
| A germ or virus | 15.1b | 25.5b | 23.2 | 13.7 | 50.0c | 15.8c | 21.5 | 19.2 | 26.1 |
| Diet or eating habits | 46.9 | 45.9 | 45.7 | 48.0 | 37.6 | 45.5 | 46.4 | 46.3 | 47.8 |
| Chance or bad luck | 36.3 | 27.5 | 31.2 | 34.3 | 18.8 | 35.5 | 21.4 | 31.9 | 34.8 |
| Poor medical care in my past | 25.7 | 19.4 | 26.0 | 16.4 | 37.6 | 20.3 | 21.4 | 24.5 | 8.7 |
| Pollution in the environment | 61.9 | 52.6 | 55.8 | 61.1 | 62.6 | 55.4 | 64.3 | 58.8 | 47.8 |
| My own behavior | 40.7 | 27.5 | 36.2 | 31.5 | 25.0 | 32.2 | 42.8 | 34.5 | 34.8 |
| My emotional state (e.g., feeling down, lonely, anxious, empty) | 23.1 | 20.4 | 19.5 | 26.0 | 31.3 | 17.7 | 32.1 | 19.7 | 39.1 |
| Aging | 56.7 | 39.8 | 44.2 | 57.5 | 31.3 | 47.5 | 57.1 | 47.9 | 56.5 |
| Alcohol | 30.1 | 29.6 | 30.5 | 28.8 | 37.6 | 27.2 | 35.7 | 30.3 | 26.0 |
| Smoking | 57.6 | 59.2 | 56.6 | 61.6 | 62.6 | 57.6 | 57.1 | 56.4 | 73.9 |
| Accident or injury | 12.4b | 25.5b | 21.7 | 12.3 | 12.6 | 19.0 | 14.3 | 18.1 | 21.7 |
| My personality | 5.3b | 9.2b | 6.8 | 8.2 | 12.5 | 6.3 | 7.1 | 6.9 | 8.7 |
| Changes in my immune system | 61.1 | 60.2 | 58.7 | 64.4 | 56.3 | 58.9 | 71.4 | 63.3 | 39.1 |
| My mental attitude (e.g., thinking about life negatively) | 18.6 | 19.4 | 20.2 | 16.4 | 25.1 | 14.6 | 39.3 | 18.1 | 26.0 |
| Overwork | 16.0 | 13.2 | 15.2 | 13.7 | 25.0 | 10.2 | 28.6 | 14.9 | 13.0 |
| Family problems or worries | 17.9 | 18.3 | 17.5 | 19.2 | 25.0 | 13.9 | 29.6 | 17.1 | 26.1 |
| Lack of exercise | 38.4 | 32.6 | 30.6 | 45.2 | 18.8 | 35.7 | 35.7 | 35.8 | 34.7 |
| Prior surgery (on breast/on colon) | 26.6 | 20.4 | 26.1 | 19.2 | 37.5 | 19.6 | 32.2 | 25.6 | 8.6 |
= p≤.01
= p≤.005
= p≤.001
Table 3. Causal attributions for colorectal cancer – by demographics.
(% agreed or strongly agreed with cause)
| Cause | Age | Education | Ethnicity | Family History | |||||
|---|---|---|---|---|---|---|---|---|---|
| <50 | 50+ | <Bachelors | Bachelors+ | Black | White | Hispanic | No | Yes | |
| Stress or worry | 26.1 | 34.0 | 27.5 | 33.8 | 32.4 | 26.8a | 40.0a | 30.2 | 21.7 |
| Hereditary – it runs in my family | 85.0a | 69.1a | 73.8 | 89.7 | 70.6 | 80.5 | 85.0 | 80.0 | 65.2 |
| A germ or virus | 24.6 | 20.2 | 24.4 | 19.1 | 32.3 | 19.5 | 30.0 | 22.9 | 21.7 |
| Diet or eating habits | 64.2a | 77.6a | 69.4 | 70.6 | 64.7 | 71.9 | 60.0 | 70.2 | 65.2 |
| Chance or bad luck | 37.3 | 18.2 | 22.6 | 45.6 | 11.8a | 34.2a | 25.0 | 30.7 | 17.4 |
| Poor medical care in my past | 39.5 | 38.3 | 36.9 | 44.1 | 47.1 | 36.0 | 45.0 | 42.0a | 13.0a |
| Pollution in the environment | 41.1 | 39.4 | 35.0 | 53.0 | 44.1 | 38.5 | 35.0 | 42.5 | 21.7 |
| My own behavior | 41.8 | 37.3 | 33.2 | 55.9 | 44.1 | 37.8 | 50.0 | 41.5 | 26.0 |
| My emotional state (e.g., feeling down, lonely, anxious, empty) | 17.1b | 22.3b | 16.3 | 26.5 | 14.7 | 17.7a | 35.0a | 20.0 | 13.0 |
| Aging | 60.5 | 53.2 | 52.5 | 69.1 | 35.3 | 61.6 | 65.0 | 60.5 | 30.4 |
| Alcohol | 39.6 | 42.6 | 35.6 | 53.0 | 35.3 | 39.6 | 50.0 | 41.0 | 39.1 |
| Smoking | 50.0 | 52.2 | 46.9 | 60.3 | 50.0 | 50.0 | 55.0 | 51.7 | 43.5 |
| Accident or injury | 11.2 | 9.7 | 10.7 | 10.3 | 11.8 | 10.4 | 5.0 | 11.2 | 4.5 |
| My personality | 4.5a | 8.6a | 4.4 | 10.3 | 2.9 | 6.7 | 0.0 | 5.9 | 8.7 |
| Changes in my immune system | 54.5 | 66.0 | 58.1 | 61.8 | 58.8 | 56.7 | 75.0 | 59.5 | 56.5 |
| My mental attitude (e.g., thinking about life negatively) | 14.1c | 26.6c | 15.7 | 27.9 | 11.7 | 19.5 | 25.0 | 19.0 | 21.7 |
| Overwork | 17.9 | 16.1 | 14.4 | 23.5 | 14.7 | 14.1 | 35.0 | 18.1 | 8.7 |
| Family problems or worries | 17.2 | 28.8 | 21.2 | 23.5 | 14.7 | 20.7 | 40.0 | 22.0 | 21.7 |
| Lack of exercise | 44.7 | 43.6 | 38.1a | 58.9a | 38.2 | 45.7 | 50.0 | 45.8 | 30.4 |
| Prior surgery (on breast/on colon) | 34.3 | 30.9 | 32.5 | 33.8 | 38.2 | 30.5 | 45.0 | 32.6 | 34.7 |
= p≤.01
= p≤.005
= p≤.001
Women who were more educated (i.e., at least a Bachelor’s degree or higher) were more likely to endorse a lack of exercise as a cause of colorectal cancer (p<.01).
Compared to white women, Hispanic women were more likely to believe that stress and one’s emotional state were causes of colorectal cancer (p<.01). Black women were less likely to believe that chance was a cause of colorectal cancer compared to white women (p=.01). In contrast, Black women were more likely to believe that a germ or virus causes breast cancer, compared to white women (p<.001).
Women with family history of colorectal cancer were significantly less likely to believe that poor medical care was a cause of colorectal cancer compared to women without a family history (p<.01). Beliefs about heredity as a cause of colorectal or breast cancer did not vary as a function of family history.
Discussion
Women in the general population endorse both genetic and environmental causes of breast and colorectal cancer. In this study, an overwhelming majority of women (approximately 80%) endorsed heredity as a cause for both breast and colorectal cancer. Although the percentages reported in this study are higher than those found by other researchers, the findings endorsing heredity are consistent with several prior studies and highlight the relative importance of this risk factor compared to others (4, 9, 20–22). The higher estimates found in the present study may result from two main trends. First, awareness and acknowledgement of the role of heredity may have changed over time consistent with scientific advances. This may account for some of the differences noted, in particular, between this study and those conducted prior to the mid-late 1990s. Second, previous work tended to assess beliefs using open ended listing approaches (22–26), which relies more heavily on recall abilities of respondents and results in a tendency for fewer causes to be reported.
The implications of endorsing heredity as a cause are not yet well understood, especially in relation to health behaviors that are undertaken to reduce cancer risk (27, 28). For example, are beliefs about the role of heredity associated with fatalistic beliefs and subsequent lack of effort by some women to take lifestyle precautions to reduce disease risk (11, 29, 30)? Or, will heredity attributions result in women taking some precautions such as increased surveillance and screening rather than lifestyle behavior change? Moreover, because the majority of unaffected women do not have a family history or documented genetic mutation conferring increased risk for breast or colorectal cancer, this also raises the possibility that the salience of this risk factor may result in a discounting of personal risk among women in the general population.
Results from our study suggest that endorsing heredity does not preclude women from also endorsing modifiable risk factors for breast and colorectal cancer. As such, it is conceivable that attribution profiles exist between women wherein a subset of women endorse only heredity as a cause that cannot be undone by behaviors, while another subset ascribe to the possibility that behaviors can ‘override genetics’. (The latter profile would be akin to the notion of gene-environment interactions.) The types of attribution profiles that exist and their association with cancer prevention behaviors warrant further research.
The present study demonstrates the variability in the causal beliefs women have about breast and colorectal cancer. Although heredity and family history were considered to be primary risk factors for both cancers, women were significantly more likely to agree that they are important in the development of breast cancer compared to colorectal cancer. This finding is consistent with others (4, 21), and suggests that the predominant coverage of breast cancer in the media compared to other cancer sites (31, 32) raises public awareness about the role of genetic contributions in breast cancer through greater exposure to cognitively salient news stories.
Women differed in their endorsement of environmental causes for each cancer. For example, pollution was endorsed by significantly more women as a cause of breast cancer than as a cause of colorectal cancer. Current medical evidence suggests a possible role of some environmental pesticides such as dichlorodiphenyltrichloroethane (DDT) in breast cancer risk, which may be accurately reflected in the beliefs of participants (33). Media coverage and framing of cancer clusters (e.g., Long Island Breast Cancer Cluster; see 34) may also contribute to the heightened endorsement of pollution as a cause of breast cancer.
Several erroneous beliefs about the causes of breast and colorectal cancer were also endorsed by the women in this study. Approximately one third of women endorsed prior surgery on the colon as a risk factor for colorectal cancer. Prior studies have reported a tendency for women to believe that some form of physical “injury” is responsible for causing breast and colorectal cancer (5, 7, 35). It is possible that women might also have interpreted “prior surgery” to mean prior disease in the breast or colon that required surgery, which may explain the findings noted. Other causal beliefs, such as immune system changes, were endorsed by approximately 60% of women for both breast and colorectal cancer. It is unclear why this cause was so readily endorsed by women. Perhaps the notion that a healthy immune system can fight off diseases, including cancer, is a cause that seems scientifically and medically plausible from a lay perspective. In contrast, only half of the women believed that aging was a risk factor for either cancer. Other national surveys have similarly noted the poor recognition of aging as an actual risk factor for these cancers (4).
Beliefs about the role of a sedentary lifestyle (i.e., lack of exercise) in colorectal cancer development were more likely endorsed among those who were more educated. Others have similarly reported that those who are more educated are more likely to identify behavioral risk factors for cancer in general (36). These findings suggest the need to focus on developing cancer prevention messages that better target those in the population with lower levels of education and literacy to ensure an adequate understanding of modifiable risk factors for cancer and to address cancer disparities that may result from knowledge gaps in awareness.
Colorectal cancer causal beliefs pertaining to stress, one’s emotional state, and chance varied as a function of race and ethnicity. Unlike other studies which found that only White women reported stress as a cause of colorectal cancer (19), our study suggests the opposite; that is, Whites are least likely to believe in this risk factor, especially in comparison to Hispanic women. Few significant differences were noted across racial categories for causal beliefs about breast cancer. Although others have reported varying breast cancer beliefs between African Americans and Caucasians, the findings have been inconsistent (9, 20). For example, Kwate et al. (20) found that African Americans were less likely to believe in the role of heredity in causing breast cancer, whereas Parrott et al. (9) noted the opposite. The findings in the present study, though not statistically significant based on our stringent criteria, tend to support the results reported by Parrott et al. Future studies are very much needed to better clarify potential racial/ethnic differences in the causal beliefs women have about breast and colorectal cancer.
There were three main limitations of the present study. First, different groups of women completed surveys about causal attributions for breast or colorectal cancer. We, therefore, cannot determine whether the results would have been similar had the all women responded to both breast and colorectal cancer causal belief items. Second, we did not collect information on behaviors and related risk factors (e.g., smoking, BMI, overweight, etc.) that might be associated with causal attributions. We are therefore unable to report how attributions would vary as a function of women’s health behaviors. Third, causal attributions were assessed for causes of cancer in general, and not worded to focus on “risk to oneself” as is often the case when assessing causal beliefs, particularly among cancer survivors. However, preliminary data indicate that the focus of the attribution “target” has implications for how people respond to the survey questions (i.e., lower agreement/endorsement for all causes when focused on risk to oneself - Shoshana Shiloh, personal communication) and suggests that assessment of causal attributions with a focus on risk to oneself may be prone to bias (37).
In addition, research has shown that individuals have a tendency to defensively discount or downplay cancer causal attributions when they are at high risk (e.g., genetically susceptible) (23, 37). For example, Blalock and colleagues (23) found that only 20% of siblings of colon cancer patients acknowledged that heredity was a risk-increasing factor for themselves. Others have noted that individuals tend to believe that their risk for cancer is reduced (e.g., “I eat a healthy diet”) rather than increased (e.g., “I smoke”) by various causes (22, 25, 26) and are often less willing to report their risky lifestyle behaviors (38, 39). Consequently, future work is necessary to better understand the possible discounting that takes place when individuals think about causes of cancer, and identify methods to counter these defensive biases such that people can recognize and modify any maladaptive behaviors that put them at increased risk. Research is also needed to determine the implications of each respective causal attribution target (i.e., oneself versus general) for understanding the health protective behaviors people engage in.
Conclusion
Breast and colorectal cancers among women are preventable to a great extent through modifiable lifestyle behaviors associated with weight management (2). Yet, women must first endorse that lifestyle behaviors such as diet and physical activity play a role in the onset of these cancers before they actively take preventive action to reduce their risk. Overall, women in the general population endorse both genetic and environmental risk factors as causes of breast and colorectal cancer. The implications of these causal beliefs (and the interplay between them) on health behaviors will be critical to examine in upcoming years, particularly as advances are made in our understanding of both genetic and environmental influences on cancer. Efforts to reduce the burden of breast and colorectal cancer should focus not only on clarifying misconceptions about the causes of cancers in the general public, but also on devising approaches to counteract defensive biases that may impede message processing and the adoption of cancer preventive behaviors.
Acknowledgments
This research was supported in part by grants from the American Cancer Society (IRG-92-027-14) and the National Cancer Institute (P30 CA06927). Catharine Wang is also supported by a career development award from the National Cancer Institute (K07 CA131103). We would like to acknowledge Knowledge Networks (KN) and the participants from the KN panel, as well as the Fox Chase Cancer Center Behavioral Research Core facility, for their contribution to this research.
References
- 1.American Cancer Society. Cancer Facts & Figures 2009. Atlanta, GA: American Cancer Society; 2009. [Google Scholar]
- 2.World Cancer Research Fund/American Institute for Cancer Research (2009) Policy and Action for Cancer Prevention. Food, Nutrition, and Physical Activity: a Global Perspective. Washington, DC: AICR; [Google Scholar]
- 3.Wang C, O’Neill SM, Rothrock N, et al. Comparison of risk perceptions and beliefs across common chronic diseases. Prev Med. 2009;48:197–202. doi: 10.1016/j.ypmed.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breslow RA, Sorkin JD, Frey CM, Kessler LG. Americans’ knowledge of cancer risk and survival. Preventive Medicine. 1997;26:170–7. doi: 10.1006/pmed.1996.0136. [DOI] [PubMed] [Google Scholar]
- 5.Wold KS, Byers T, Crane LA, Ahnen D. What do cancer survivors believe causes cancer? (United States) Cancer Causes Control. 2005;16:115–23. doi: 10.1007/s10552-004-2414-0. [DOI] [PubMed] [Google Scholar]
- 6.Costanzo ES, Lutgendorf SK, Bradley SL, Rose SL, Anderson B. Cancer attributions, distress, and health practices among gynecologic cancer survivors. Psychosom Med. 2005;67:972–80. doi: 10.1097/01.psy.0000188402.95398.c0. [DOI] [PubMed] [Google Scholar]
- 7.Rabin C, Pinto B. Cancer-related beliefs and health behavior change among breast cancer survivors and their first-degree relatives. Psycho-Oncology. 2006;15:701–12. doi: 10.1002/pon.1000. [DOI] [PubMed] [Google Scholar]
- 8.Lemon SC, Zapka JG, Clemow L. Health behavior change among women with recent familial diagnosis of breast cancer. Prev Med. 2004;39:253–62. doi: 10.1016/j.ypmed.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 9.Parrott RL, Silk KJ, Condit C. Diversity in lay perceptions of the sources of human traits: genes, environments, and personal behaviors. Social Science and Medicine. 2003;56:1099–109. doi: 10.1016/s0277-9536(02)00106-5. [DOI] [PubMed] [Google Scholar]
- 10.Marteau TM, Senior V. Illness representations after the Human Genome Project: The perceived role of genes in causing illness. In: Petrie KJ, Weinman JA, editors. Perceptions of Health & Illness. Amsterdam: Harwood Academic Publishers; 1997. pp. 241–66. [Google Scholar]
- 11.Marteau TM, Weinman J. Self-regulation and the behavioural response to DNA risk information: A theoretical analysis and framework for future research. Social Science and Medicine. 2006;62:1360–8. doi: 10.1016/j.socscimed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Walter FM, Emery J, Braithwaite D, Marteau TM. Lay understanding of familial risk of common chronic diseases: A systematic review and synthesis of qualitative research. Annals of Family Medicine. 2004;2:583–94. doi: 10.1370/afm.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garber JE, Offit K. Hereditary cancer predisposition syndromes. Journal of Clinical Oncology. 2005;23:276–92. doi: 10.1200/JCO.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 14.Eng C, Hampel H, de la Chapelle A. Genetic testing for cancer predisposition. Annu Rev Med. 2001;52:371–400. doi: 10.1146/annurev.med.52.1.371. [DOI] [PubMed] [Google Scholar]
- 15.Paul C, Tzelepis F, Walsh RA, Girgis A, King L, McKenzie J. Has the investment in public cancer education delivered observable changes in knowledge over the past 10 years? Cancer. 2003;97:2931–9. doi: 10.1002/cncr.11393. [DOI] [PubMed] [Google Scholar]
- 16.Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised illness perception questionnaire (IPQ-R) Psychology and Health. 2002;17:1–16. [Google Scholar]
- 17.Greiner KA, Born W, Nollen N, Ahluwalia JS. Knowledge and perceptions of colorectal cancer screening among urban African Americans. Journal of General Internal Medicine. 2005;20:977–83. doi: 10.1111/j.1525-1497.2005.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCaffery K, Wardle J, Waller J. Knowledge, attitudes, and behavioral intentions in relation to the early detection of colorectal cancer in the United Kingdom. Preventive Medicine. 2003;36:525–35. doi: 10.1016/s0091-7435(03)00016-1. [DOI] [PubMed] [Google Scholar]
- 19.Shokar NK, Vernon SW, Weller SC. Cancer and colorectal cancer: knowledge, beliefs, and screening preferences of a diverse patient population. Fam Med. 2005;37:341–7. [PubMed] [Google Scholar]
- 20.Kwate NO, Thompson HS, Valdimarsdottir HB, Bovbjerg DH. Brief report: etiological attributions for breast cancer among healthy African American and European American women. Psychooncology. 2005;14:421–5. doi: 10.1002/pon.905. [DOI] [PubMed] [Google Scholar]
- 21.Wardle J, Waller J, Brunswick N, Jarvis MJ. Awareness of risk factors for cancer among British adults. Public Health. 2001;115:173–4. doi: 10.1038/sj/ph/1900752. [DOI] [PubMed] [Google Scholar]
- 22.Aiken LS, Fenaughty AM, West SG, Johnson JJ, Luckett TL. Perceived determinants of risk for breast cancer and the relations among objective risk, perceived risk, and screening behavior over time. Women’s Health: Research on Gender, Behavior, and Policy. 1995;1:27–50. [PubMed] [Google Scholar]
- 23.Blalock SJ, DeVellis BM, Afifi RA, Sandler RS. Risk perceptions and participation in colorectal cancer screening. Health Psychology. 1990;9:792–806. doi: 10.1037//0278-6133.9.6.792. [DOI] [PubMed] [Google Scholar]
- 24.Lipkus IM, Biradavolu M, Fenn K, Keller P, Rimer BK. Informing women about their breast cancer risks: Truth and consequences. Health Communication. 2001;13:205–26. doi: 10.1207/S15327027HC1302_5. [DOI] [PubMed] [Google Scholar]
- 25.Lipkus IM, Rimer BK, Lyna PR, Pradhan AA, Conaway M, Woods-Powell CT. Colorectal screening patterns and perceptions of risk among African-American users of a community health center. Journal of Community Health. 1996;21:409–27. doi: 10.1007/BF01702602. [DOI] [PubMed] [Google Scholar]
- 26.Robb KA, Miles A, Wardle J. Perceived risk of colorectal cancer: sources of risk judgments. Cancer Epidemiol Biomarkers Prev. 2007;16:694–702. doi: 10.1158/1055-9965.EPI-06-0151. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Miller SM. Psychological issues in genetic testing. In: Miller SM, Bowen DJ, Croyle RT, Rowland JH, editors. Handbook of Cancer Control and Behavioral Science: A Resource for Researchers, Practitioners, and Policy Makers. Washington, DC: American Psychological Association; 2008. [Google Scholar]
- 28.Wang C, Bowen DJ, Kardia SLR. Research and practice opportunities at the intersection of genomics and health education, health behavior, and genomics. Health Education & Behavior. 2005;32:686–701. doi: 10.1177/1090198105278827. [DOI] [PubMed] [Google Scholar]
- 29.Frosch DL, Mello P, Lerman C. Behavioral consequences of testing for obesity risk. Cancer Epidemiology, Biomarkers & Prevention. 2005;14:1485–9. doi: 10.1158/1055-9965.EPI-04-0913. [DOI] [PubMed] [Google Scholar]
- 30.Senior V, Marteau TM, Weinman J. Impact of genetic testing on causal models of heart disease and arthritis: An analogue study. Psychology and Health. 2000;14:1077–88. doi: 10.1080/08870440008407368. [DOI] [PubMed] [Google Scholar]
- 31.Gerlach KK, Marino C, Weed DL, Hoffman-Goetz L. Lack of colon cancer coverage in seven women’s magazines. Women Health. 1997;26:57–68. doi: 10.1300/j013v26n02_04. [DOI] [PubMed] [Google Scholar]
- 32.Gerlach KK, Marino C, Hoffman-Goetz L. Cancer coverage in women’s magazines: what information are women receiving? Journal of Cancer Education. 1997;12:240–4. doi: 10.1080/08858199709528496. [DOI] [PubMed] [Google Scholar]
- 33.Clapp RW, Jacobs MM, Loechler EL. Environmental and occupational causes of cancer: new evidence 2005–2007. Rev Environ Health. 2008;23:1–37. doi: 10.1515/reveh.2008.23.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson D. Cancer clusters: Findings vs feelings. Med Gen Med. 2002;4:16. [PubMed] [Google Scholar]
- 35.Consedine NS, Magai C, Spiller R, Neugut AI, Conway F. Breast cancer knowledge and beliefs in subpopulations of African American and Caribbean women. Am J Health Behav. 2004;28:260–71. doi: 10.5993/ajhb.28.3.7. [DOI] [PubMed] [Google Scholar]
- 36.Sanderson SC, Waller J, Jarvis MJ, Humphries SE, Wardle J. Awareness of lifestyle risk factors for cancer and heart disease among adults in the UK. Patient Educ Couns. 2009;74:221–7. doi: 10.1016/j.pec.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Shiloh S, Drori E, Orr-Urtreger A, Friedman E. Being ‘at-risk’ for developing cancer: cognitive representations and psychological outcomes. J Behav Med. doi: 10.1007/s10865-008-9178-z. (in press) [DOI] [PubMed] [Google Scholar]
- 38.Weinstein ND. Why it won’t happen to me: Perceptions of risk factors and susceptibility. Health Psychology. 1984;3:431–57. doi: 10.1037//0278-6133.3.5.431. [DOI] [PubMed] [Google Scholar]
- 39.Weinstein ND. Unrealistic optimism about susceptibility to health problems: Conclusions from a community-wide sample. Journal of Behavioral Medicine. 1987;10:481–500. doi: 10.1007/BF00846146. [DOI] [PubMed] [Google Scholar]