Abstract
Agonist-selective signaling or ligand-biased signaling of G protein-coupled receptor (GPCR) has become the focus of an increasing number of laboratories. The principle of this concept is that agonist possesses different abilities to activate different signaling pathways. Current review summarizes the observations of agonist-selective signaling of various GPCRs, indicating the significance of agonist-selective signaling in biological processes. In addition, current review also provides an overview on how agonist-selective signaling is initiated. Especially, the relationship between GPCR-G protein interaction and GPCR-β-arrestin interaction is discussed in depth.
Keywords: ERK, G protein, β-arrestin, μ-opioid receptor (OPRM1)
1. Introduction
The abilities [intrinsic activities (1) or intrinsic efficacies (2)] of G protein-coupled receptor (GPCR) ligands to initiate downstream signaling events can vary. Depending on the efficacies, receptor ligands can be classified into: full agonists, partial agonists, neutral antagonists and inverse agonists (3, 4). This classification is normally made under specific experimental conditions, because the classification can be affected by the level of receptor expressed and the strength of stimulus-response coupling. In addition, accumulating evidences have suggested that agonists can induce different conformation changes of one particular GPCR, which then lead to the activation of different signaling pathways (5), even when the expression level of receptor and the strength of stimulus-response coupling are the same. Similar theory has been suggested by Kenakin years ago as the “agonist trafficking theory” (6).
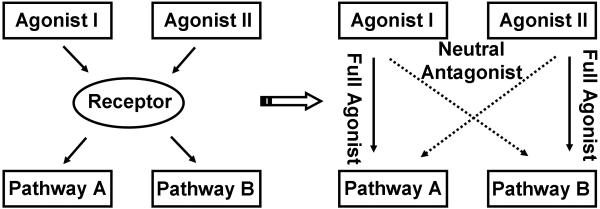
Recently, a new “agonist-selective” (or ligand-biased or functional-selective) theory is proposed to accommodate reported observations that receptor ligands can activate the same signaling pathway differently and these differential activations are not necessarily correlated with those on another signaling pathway. The observations were summarized in a schematic diagram (Fig. 1). Agonist I and agonist II activate the receptor, which transduces the signal to two pathways, pathway A and pathway B. Agonist I is a full agonist on pathway A, while is a neutral antagonist on pathway B. Agonist II is just opposite: full agonist on pathway B, while neutral antagonist on pathway A. This phenomena has been observed with various GPCRs, including 5-HT2 serotonin receptors, adrenergic receptors, dopamine receptors and opioid receptors (5).
Figure 1. Schematic representation of agonist-selective signaling.
Agonist I and II exhibit differential efficacies in pathway A and B. The “Full Agonist” and “Neutral Antagonist” were used to indicate the different “intrinsic efficacies” of these agonists on the two pathways. Also, they can be “Partial Agonist” or “Inverse Agonist”.
2. Agonist-selective signaling
The direct agonist-selective signaling is that different agonists induce signaling pathways differentially. The indirect agonist-selective signaling is that the activation of different signaling pathways leads to the different cellular responses in vitro or physiological responses in vivo.
2.1 μ-opioid receptor (OPRM1)
OPRM1 is the opioid receptor type that mediates the majority of the in vivo effects of morphine, such as analgesia. As a typical Gi/o coupled receptor, its signals are mediated by adenylyl cyclase, extracellular signal-regulated kinase (ERK) pathway, intracellular calcium stores and ion channels on cell membrane (7, 8). Because of the large volume of literature and cognate ligands, OPRM1 was used as an example to provide a general view of agonist-selective signaling.
2.1.1 Agonist-selective signaling of OPRM1
The agonists-selective signaling has been reported with OPRM1 from a variety of studies. Several signaling pathways can be activated by nearly all OPRM1 agonists, but to different extents. For instance, morphine induces minimal receptor phosphorylation while other agonists such as etorphine, fentanyl and [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO) induce robust receptor phosphorylation (9). Similarly, morphine induces minimal β-arrestin recruitment (10) and receptor internalization (11) as compared to the other agonists. These differences have been attributed to morphine being a partial agonist, while the others are full agonists in activating these signaling pathways. However, morphine induced higher level of adenylyl cyclase superactivation than etorphine (12). Morphine also has higher ability to induce analgesia tolerance than etorphine and fentanyl (13, 14). Thus morphine is a full agonist, while etorphine seems to be a partial agonist in these responses. In addition, similar abilities of morphine and other agonists to induce ERK phosphorylation and to inhibit the adenylyl cyclase activity suggest that these agonists are all full agonists (12, 15). Hence, the classification of these opioid agonists indicates the strength of stimulus-response coupling (or the interaction between agonist efficacy and system sensitivity) could not be used to address all the observations. Moreover, the cellular content could not be used in addressing the divergent observations because some of these studies were carried out with the same cell model (12). Hence, agonist-selective signaling theory is more suitable in interpreting the observations on OPRM1 signaling.
2.1.2 Pathway-selective signaling of OPRM1
Agonists can activate various signaling pathways differentially. If the differences among agonists are large enough on one signaling pathway, a special phenomenon will be observed, i.e., only selective agonists can activate this signaling pathway. Furthermore, if the differences are large enough on two signaling pathways, agonist-selective activation on these two pathways will occurred (similar to that summarized in Fig. 1); i.e., a group of agonists only activate one signaling pathway, while another group of agonists only activate another signaling pathway. Hence the agonist-selective signaling can be considered as the “selection between signaling pathways” or “pathway-selective signaling”.
This selection is actually observed with a number of GPCRs, including OPRM1. Although all agonists activate ERK to similar extent, morphine uses the Protein Kinase C (PKC) pathway to induce ERK phosphorylation, while etorphine and DAMGO utilize the β-arrestin pathway (16). The receptor desensitization on the G protein-coupled inwardly rectifying potassium channel currents and the release of intracellular calcium are also agonist-selective, morphine via the PKC pathway and DAMGO via the β-arrestin pathway (17, 18). Hence, morphine selectively utilizes the PKC pathway, while etorphine and DAMGO selectively utilizes the β-arrestin pathway.
2.1.3 Downstream signaling events
The different signaling pathway activated by agonists may result in different physiological effects. Although it is difficult to correlate the signaling cascades (normally within seconds to minutes) to the measurable physiological effects (normally within minutes to hours or days) directly, the regulations on transcriptional factors, gene expression, posttranslational modification, and even the subcellular distribution of signaling molecules can serve as the reporters for pathways selected for signaling.
Take ERK phosphorylation induced by OPRM1 agonists for example, the phosphorylated ERK induced by morphine through the PKC pathway remains in the cytosol, while the phosphorylated ERK induced by etorphine through the β-arrestin pathway translocates into the nucleus (16). The different subcellular locations of phosphorylated ERK result in the activation of different transcriptional factors. phosphorylated ERK in cytosol activates the cytosol-located p90 ribosomal S6 kinase, while phosphorylated ERK in nucleus activates the Elk-1 (16). In addition, etorphine, but not morphine, modulates the expression of β-arrestin2 and G protein-coupled receptor kinase 2 (GRK2), which are highly related to OPRM1 functions (16, 19). These modulations are attenuated when etorphine-induced ERK phosphorylation is blocked (16). OPRM1 agonists also affect the expression of microRNA differentially. Especially the agonist-selective regulation on miR-190 is related with the agonist-selective ERK phosphorylation (20). Therefore, OPRM1 agonists do have different abilities to regulate the transcriptional factors and gene expression.
OPRM1 agonists have different influences on the dendritic spine morphology and excitatory postsynaptic current (21-23). Morphine, but not etorphine and DAMGO, induces the collapse of dendritic spines and subsequently reduces the spine density in hippocampal neurons. In addition, when equivalent doses of agonists were administrated onto the mice, higher level of tolerance developed after morphine treatment (13, 14). These differences among agonists have been attributed to the different abilities of agonists to induce receptor internalization (24, 25). Although the hypothesis is still controversial, it provides a possible mechanism to correlate the agonist-selective signaling with agonist-selective physiological effects.
2.2 Agonist-selective ERK phosphorylation
ERK phosphorylation is almost the best-studied phenomenon of agonist-selective signaling. Two pathways can be used by GPCRs to mediate ERK phosphorylation, PKC/PKA pathway and β-arrestin pathway (26-28). The existence of the two pathways were demonstrated by blocking PKC/PKA activities with their specific inhibitors and by downregulating β-arrestin with siRNAs in the system of β2-adrenergic receptor (28) and parathyroid hormone receptor (29). The selection of agonists between the two pathways is indicated by abilities of agonists to activate the two pathways. For example, isoproterenol (β-adrenergic receptor agonist) induces ERK phosphorylation via both pathways, while ICI118551 (β2-adrenergic receptor inverse agonist) induces ERK phosphorylation in a β-arrestin-dependent manner completely (28, 30) and CCL19 uses only the PKC/PKA pathway in cells expressing the chemokine receptor CCR7 (10).
As mentioned above, modulating the subcellular location of phosphorylated ERK is a means by which agonist-selective signaling leads to the downstream effects. However, there are controversial observations on the influence of β-arrestin on the nucleus translocation of phosphorylated ERK. Overexpression of β-arrestin has been reported both to enhance (16, 31) and to suppress (32) the nucleus translocation of phosphorylated ERK. However, the abilities of β-arrestin to serve as a scaffold protein for ERK (26) and to translocate into nucleus by itself support the positive effect of β-arrestin on ERK translocation (33, 34).
2.3 Other agonist-selective signaling
There are other agonist-selective phenomena exist with GPCRs. 5-HT2 serotonin receptor mediates the formation of inositol phosphates via phospholipase C (PLC) and the release of arachidonic acid through phospholipase A2 (PLA2) (35, 36). Four signaling pathways (PLC activation, PLC desensitization, PLA2 activation and PLA2 desensitization) have been analyzed with a series of agonists of 5-HT2 serotonin receptor (37, 38). No correlation among their abilities to activate these four signaling pathways has been identified. Inositol phosphates influences the intracellular calcium level and thereby the activities of calmodulin and CaMKinases. The accumulation of arachidonic acid has critical effect on the functions of ion channels (39, 40). Therefore the different agonists of 5-HT2 serotonin receptor will have different effects on these cellular responses. Similar observations are obtained with D2–dopamine receptor. Quinpirole and propyldihydrexidine are both full agonist when used to activate adenylyl cyclase. However they have different abilities to inhibit the D2–dopamine receptor-mediated release of DA (41).
3. Mechanisms
The mechanism for agonist-selective signaling has also been studied for a long time. The accepted notion is that the conformational changes of receptor induced by agonists are different from each other (4). In addition, because the activation of different signaling pathways requires different conformational changes of receptor, agonists activate signaling pathways differentially. Then the agonist-selective signaling is observed. Although the crystal structures of G proteins, β-arrestin (42) and some GPCRs like rodposine (43) β2–adregneric receptor (44) and A2A–adensoine receptor (45) have been solved, the structural information of the receptor-agonist complex is not available so as to predict the conformational changes induced by different agonists. Thus various groups have attempted to categorize the signaling pathways. For example, modulation on adenylyl cyclase leads to the change of intracellular cAMP level, which subsequently influences the activities of phosphodiesterase and PKA. Because adenylyl cyclase modulation is normally mediated by Gα subunit, the ability of agonist to activate Gα will indicate its influence on phosphodiesterase and PKA.
ERK phosphorylation mediated by GPCRs is one of the best-studied agonist-selective signaling phenomena as mentioned above. Because the two pathways used by GPCRs for ERK phosphorylation, PKA/PKC pathway and β-arrestin pathway, involve G protein and β-arrestin, respectively, we will discuss the relationship between G protein and β-arrestin in detail. The recruitment of β-arrestin has been reported to uncouple the G protein from receptor complex (26, 46). However, the competition between G protein and β-arrestin can take place at different time during the receptor signaling process, before agonist binding, before G protein activation and after G protein activation. Thus the receptor-G protein interaction vs the receptor-β-arrestin interaction will have impact on signaling pathways selected.
3.1 β-arrestin pathways
3.1.1 Introduction
β-arrestin is a member of the arrestin family that functions as cytosolic scaffold protein. Arrestin1 and arrestin4 (x-arrestin) are found exclusively in retinal rods and cones, while arrestin2 (β-arrestin1) and arrestin3 (β-arrestin2) are expressed ubiquitously. Arrestins are discovered originally in the desensitization of rhodopsin (47) and β2-adrenergic receptor (48). Recently, β-arrestin has been suggested to function as a scaffold protein which bridges the GPCRs and intracellular effectors (46). The signaling related to β-arrestin can be divided into G protein-dependent and G protein-independent.
3.1.2 G protein-dependent β-arrestin-related signaling
β-arrestin-related signaling pathways include β-arrestin recruitment to cell membrane, receptor internalization, β-arrestin-mediated ERK phosphorylation and β-arrestin-mediated receptor desensitization. The ability of agonist to activate one signaling pathway will indicates its influences on other pathways. The understanding on β-arrestin-related signaling pathways is based on the β-arrestin-mediated receptor desensitization. In the classical desensitization paradigm, the binding of agonist to GPCR leads to the receptor phosphorylation and the increases in the agonist-receptor affinity for the cytosolic protein β-arrestin. Translocation of β-arrestin to the receptor complex disrupts receptor-G protein interaction and terminates the activation of G protein-related signaling pathways (49). Instead of terminating GPCR signaling, several β-arrestin-mediated signaling pathways will be activated. The activation of β-arrestin-related signaling pathways requires the activation of G protein in this paradigm.
3.1.3 G protein-independent β-arrestin-related signaling
When using the G protein interaction deficient mutants of GPCRs, G protein-independent β-arrestin signaling has been identified. β2-adrenergic receptor mutants lacking G protein coupling mediates the ERK phosphorylation through a GRK5/6-β-arrestin pathway (28). The I35 mutant of OPRM1 (lacking the last five amino acids in the third intracellular loop to interact with G protein) still mediates etorphine-induced ERK phosphorylation in a β-arrestin dependent manner (50).
In addition, overexpression of β-arrestin enable the agonist that normally does not induce receptor internalization to induce receptor internalization (51), suggesting a basal affinity of β-arrestin for the receptor in the absence of receptor phosphorylation. Moreover, G protein-independent receptor phosphorylation has been observed on the Ser363 of rat δ-opioid receptor (52). Therefore, β-arrestin-related signaling can be initiated without G protein activation.
3.2 G protein and β-arrestin
Because of the coupling of G protein to receptor both in the absence and in the presence of agonist (50, 53), β-arrestin uncouples G protein from receptor complex before β-arrestin-related signaling pathways (both G protein-dependent and -independent) are activated.
If β-arrestin competes with G protein for the receptor prior to G protein activation, G protein-independent β-arrestin-related pathways will be activated. If the β-arrestin competes with G protein for the receptor after G protein activation, then G protein-dependent β-arrestin-related pathways will be activated. Otherwise, in the absence of any β-arrestin competition, G protein-related pathways, e.g. PKC/PKA pathway, will be activated. Thus the competition between G protein and β-arrestin determines the selection of the downstream pathways.
3.2.1 The binding site of G protein on GPCRs
The most critical regions of GPCRs for G protein coupling are the second intracellular loop (IL2), both N- and C-terminus of IL3 (NIL3 and CL3) (54). Moreover, the IL1 and the membrane-proximal 8–16 amino acids of IL4 also contribute to the coupling of G protein (55).
3.2.2 The binding site of β-arrestin on GPCRs
Receptor phosphorylation can increase the affinity of receptor for β-arrestin, therefore the phosphorylation site contributes to the binding of β-arrestin. Because majority of GPCRs have their phosphorylation sites on the carboxyl tail domain, the carboxyl tail domain have been shown to be critical for the binding of β-arrestin, as demonstrated with β-arrestin-dependent receptor internalization using various GPCR mutants (56). The carboxyl tail domain phosphorylation not only functions in recruiting β-arrestin, but also regulates the accessibility of β-arrestin to non-phosphorylated residues (57). These non-phosphorylated residues are in the IL2, NIL3 and CIL3, as demonstrated with dopamine receptors (58, 59), adrenergic receptors (60) and opioid receptors (61).
3.2.3 The competition of between G protein and β-arrestin
G protein and β-arrestin share similar binding sites on the GPCRs (IL2, NIL3 and CIL3), indicating the ability of β-arrestin to compete with the G protein for the receptor complex. However, it is also reasonable to propose that G protein can compete with the β-arrestin for the receptor complex when the affinity between G protein and the receptor is enhanced. However, because of the receptor internalization mediated by β-arrestin and the membrane location of G protein (62, 63), it is impossible for G protein to compete with the β-arrestin after the initiation of receptor endocytosis. Thus, increasing the binding affinity of G protein for receptor complex can only prevent β-arrestin to uncouple G protein from the receptor. For instance, modulating the expression level of G protein successfully decreases the G protein uncoupling mediated by β-arrestin (50).
3.2.4 Factors that influence the competition
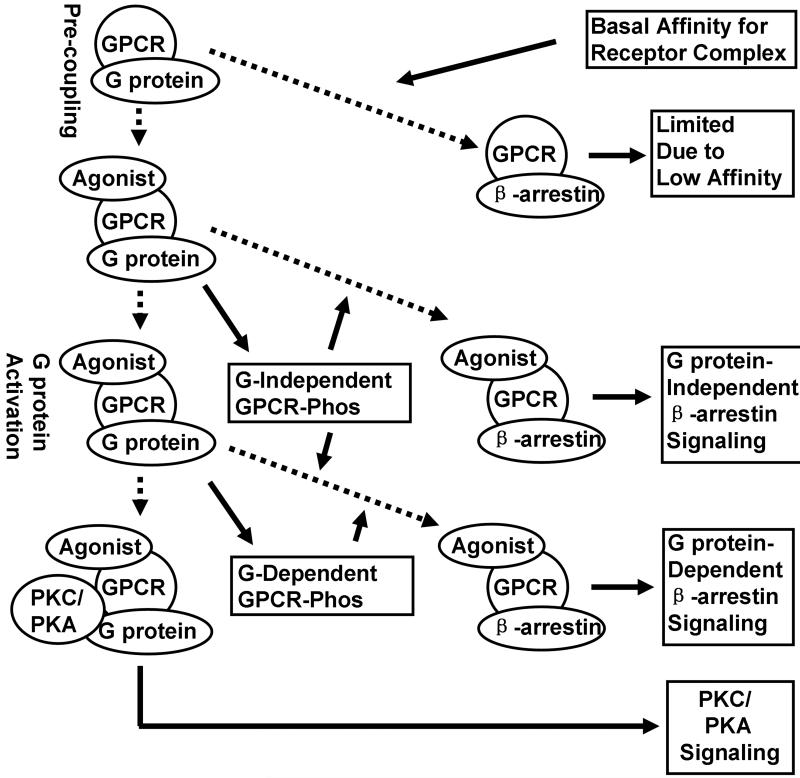
Based on the discussion above, we propose a new model for agonist signaling (Fig. 2). The G protein signaling (from G protein to PKC/PKA) can be considered as the major signaling of receptor. Although the competition between G protein and β-arrestin exists at any time point, it is only observed when the affinity of β-arrestin for receptor complex is high enough to uncouple G protein from the receptor. Thus several selections are observed during the signal transduction. As indicated in Fig. 2, the basal affinity of β-arrestin for the receptor enables it to compete with G protein even before agonist binding. However, because the affinity is relative low, nearly no GPCR signaling is mediated by β-arrestin at that time. After agonist binding, G protein-independent receptor phosphorylation (52) can initiate the G protein-independent β-arrestin signaling. After G protein activation, the receptor phosphorylation (both G protein-dependent and -independent), the phosphorylation-induced uncoupling of G protein from receptor complex (64, 65) and G protein dissociation (66) can initiate the G protein-dependent β-arrestin signaling.
Figure 2. Schematic diagram of the competition between G protein and β-arrestin.
“G” and “Phos” were used to represent G protein and phosphorylation of GPCR, respectively.
Receptor phosphorylation can be mediated by GRKs (67). GRK1, 4 and 7 are expressed specific regions, while GRK2, 3, 5 and 6, are ubiquitously expressed as reviewed (68). GRK2/3 are cytosolic protein and their activation requires the membrane targeting and free Gβγ subunits (69, 70). In contrast, GRK5/6 are membrane-associated proteins (68). Thus the ability of GPCR agonists to activate different GRKs may also contribute to the competition between G protein and β-arrestin.
3.3 Interactions with lipids
As seven-transmembrane receptors, the functions of GPCRs associate tightly with cell membrane, where the protein-lipid interaction is numerous.
3.3.1 Lipid raft microdomains
Although the concept of lipid raft is still controversial, lipid raft (or the cholesterol-rich microdomain) regulates the signaling of several GPCRs, such as gonadotropin-releasing hormone receptor, type-1 cannabinoid receptor, α1-adrenergic receptor, NK1 receptor, and opioid receptors (71). The lipid raft is enriched with cholesterol, sphingomyelin, gangliosides and various signaling proteins, including heterotrimeric G proteins, adenylyl cyclase (AC) and Src kinases (71, 72). It provides an environment in which GPCRs interact freely with a selective set of signaling molecules to execute their functions (50, 71). As lipid raft markers, G proteins especially the Gα subunits’ location in the lipid raft microdomains is determined by the palmitoylation and myrisitoylation on their N-termini (72, 73). Through the precoupling of G protein, GPCR can be anchored in the lipid raft (50). If β-arrestin uncouples G protein from receptor complex, the GPCR may diffuse to non-raft fractions preceding the endocytosis of the receptor (50). In addition, the enriched signaling molecules in the lipid raft will favor the signaling mediated by G protein, but not that mediated by β-arrestin, because the majority of β-arrestin signaling (ERK phosphorylation) is dependent on its scaffold function. Therefore the size of lipid raft microdomain will influence the competition between G protein and β-arrestin.
3.3.2 Cholesterol
Cholesterol is an essential component for lipid raft: the regulatory roles of lipid raft on GPCR signaling are identified by disrupting lipid raft with cholesterol lowering chemicals (71, 72). However, cholesterol also participates in other aspects of GPCRs’ functions. Cholesterol not only stabilizes the crystallization of β2-adrenerfic receptor, but also has been found in the interface of two β2-adrenerfic receptor molecules (44). Thus cholesterol (not the cholesterol in lipid raft because it locates between two receptors) has been suggested to facilitate the dimerization of GPCRs. Because the intracellular interface seems to be too small for GPCR monomer to interact with the heterotrimeric G protein molecule (74), therefore the dimerization of GPCRs facilitates the interaction between receptor and G protein. Cholesterol, therefore, may facilitate receptor-G protein interaction by contributing to the stability of receptor dimers. Considering the possible interaction between monomer GPCR and G protein (75), cholesterol may also participate in the direct interaction between G protein and receptor.
3.3.3 Palmitoylation
Receptor palmitoylation is also involved in regulating the agonist-selective signaling. Cholesterol identified in the crystal structure of β2-adrenerfic receptor interacts with the palmitic acid covalently linked to the receptor (44). Because G proteins are also highly palmitoylated in their N-terminuses (63, 76), cholesterol molecules may bridge the receptor and G proteins by interacting with the palmitic acids linked to these two proteins in a manner similar to how cholesterol bridges the two β2-adrenergic receptors.
In addition, palmitoylation targets the receptors to the correct membrane location (77), where GPCRs interact with G protein. Palmitoylation-dependent receptor-G protein interaction is observed with both β2-adrenergic receptor (78) and M2 muscarinic acetylcholine receptor (79), whereas negative responses have also been observed with the α2A-adrenergic receptor (80) and dopamine D1 receptor (81) .
4. Future Direction
Although the agonist-selective signaling has been reported extensively, several questions need further investigation. The agonist-selective signaling has been suggested to result from the different conformational changes of receptor. That is, agonists induce different kinds of conformational changes of receptor that are required for the activation of different downstream signaling pathways. The correlation among agonists, conformational changes and signaling pathways needs to be identified. The crystal structure of agonist-binding receptor will provide information for the correlation.
In addition, by comparing the different agonists of one particular GPCR, the understanding of agonist-selective signaling will be further improved. Although the number of signaling pathways is large, it is not necessary to test all the signaling pathways. For example, the ability of agonist to induce receptor phosphorylation can indicate the abilities of agonists to induceβ-arrestin recruitment and to active β-arrestin-related signaling. Thus, the examination of β-arrestin-dependent pathway signaling will reflect the degree of receptor phosphorylation and subsequent cellular events triggered by receptor phosphorylation.
However, understanding the agonist-selective signaling should not be the final goal, the downstream effects, especially the downstream effects in vivo, of agonist-selective signaling has significant implication. As discussed, the regulations on gene expression, posttranscriptional modification and cellular location of the activated proteins may form the basis for the agonist-selective signaling and the agonist-selective physiological responses in vivo. Subsequent agonist-selective physiological responses in vivo stemming from agonist-selective signaling would affect the eventual outcome of GPCR activation. Being viable drug targets, agonist selective signaling in GPCRs will have significant impact in future drug development.
Acknowledgement
This research was supported in part by National Institutes of Health grants DA007339, DA016674, DA000564 and DA011806. H.H.L. and P.Y.L. are recipients of K05-DA70544 and K05-DA00513, respectively.
Abbreviations
- DAMGO
[D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- ERK
extracellular signal-regulated kinase
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- IL
intracellular loop
- PLA2
phospholipase A2
- PLC
phospholipase C
- PKC
protein kinase C
Reference
- [1].Ariens EJ. Affinity and intrinsic activity in the theory of competitive inhibition. I. Problems and theory. Arch Int Pharmacodyn Ther. 1954;99:32–49. [PubMed] [Google Scholar]
- [2].Furchgott R. The use of haloalkylamines in the differentiation of receptors and in the determination of dissciationof receptor-agonist complexes. Adv Drug Res. 1966;3:21–55. Harper NJ and Simmonds AB eds. [Google Scholar]
- [3].Kenakin T. Agonist-receptor efficacy. I: Mechanisms of efficacy and receptor promiscuity. Trends Pharmacol Sci. 1995;16:188–92. doi: 10.1016/s0165-6147(00)89020-3. [DOI] [PubMed] [Google Scholar]
- [4].Kenakin T. Pharmacological proteus? Trends Pharmacol Sci. 1995;16:256–8. doi: 10.1016/s0165-6147(00)89037-9. [DOI] [PubMed] [Google Scholar]
- [5].Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- [6].Kenakin T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol Sci. 1995;16:232–8. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- [7].Law PY, Loh HH. Regulation of opioid receptor activities. J Pharmacol Exp Ther. 1999;289:607–24. [PubMed] [Google Scholar]
- [8].Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- [9].Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci U S A. 1998;95:7157–62. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kohout TA, Nicholas SL, Perry SJ, Reinhart G, Junger S, Struthers RS. Differential desensitization, receptor phosphorylation, beta-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J Biol Chem. 2004;279:23214–22. doi: 10.1074/jbc.M402125200. [DOI] [PubMed] [Google Scholar]
- [11].Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271:19021–4. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- [12].Zhao H, Loh HH, Law PY. Adenylyl cyclase superactivation induced by long-term treatment with opioid agonist is dependent on receptor localized within lipid rafts and is independent of receptor internalization. Mol Pharmacol. 2006;69:1421–32. doi: 10.1124/mol.105.020024. [DOI] [PubMed] [Google Scholar]
- [13].Duttaroy A, Yoburn BC. The effect of intrinsic efficacy on opioid tolerance. Anesthesiology. 1995;82:1226–36. doi: 10.1097/00000542-199505000-00018. [DOI] [PubMed] [Google Scholar]
- [14].Paronis CA, Holtzman SG. Development of tolerance to the analgesic activity of mu agonists after continuous infusion of morphine, meperidine or fentanyl in rats. J Pharmacol Exp Ther. 1992;262:1–9. [PubMed] [Google Scholar]
- [15].Li LY, Chang KJ. The stimulatory effect of opioids on mitogen-activated protein kinase in Chinese hamster ovary cells transfected to express mu-opioid receptors. Mol Pharmacol. 1996;50:599–602. [PubMed] [Google Scholar]
- [16].Zheng H, Loh HH, Law PY. {beta}-Arrestin-Dependent {micro}-Opioid Receptor-Activated Extracellular Signal-Regulated Kinases (ERKs) Translocate to Nucleus in Contrast to G Protein-Dependent ERK Activation. Mol Pharmacol. 2008;73:178–90. doi: 10.1124/mol.107.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chu J, Zheng H, Loh HH, Law PY. Morphine-induced mu-opioid receptor rapid desensitization is independent of receptor phosphorylation and beta-arrestins. Cell Signal. 2008;20:1616–24. doi: 10.1016/j.cellsig.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. Agonist-selective mechanisms of mu-opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol. 2006;70:676–85. doi: 10.1124/mol.106.022376. [DOI] [PubMed] [Google Scholar]
- [19].Narita M, Suzuki M, Narita M, Niikura K, Nakamura A, Miyatake M, Yajima Y, Suzuki T. mu-Opioid receptor internalization-dependent and -independent mechanisms of the development of tolerance to mu-opioid receptor agonists: Comparison between etorphine and morphine. Neuroscience. 2006;138:609–19. doi: 10.1016/j.neuroscience.2005.11.046. [DOI] [PubMed] [Google Scholar]
- [20].Zheng H, Zeng Y, Zhang X, Chu J, Loh HH, Law PY. {micro}-Opioid Receptor Agonists Differentially Regulate the Expression of miR-190 and NeuroD. Mol Pharmacol. 2009 doi: 10.1124/mol.109.060848. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liao D, Grigoriants OO, Loh HH, Law PY. Agonist-dependent postsynaptic effects of opioids on miniature excitatory postsynaptic currents in cultured hippocampal neurons. J Neurophysiol. 2007;97:1485–94. doi: 10.1152/jn.00790.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liao D, Grigoriants OO, Wang W, Wiens K, Loh HH, Law PY. Distinct effects of individual opioids on the morphology of spines depend upon the internalization of mu opioid receptors. Mol Cell Neurosci. 2007;35:456–69. doi: 10.1016/j.mcn.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liao D, Lin H, Law PY, Loh HH. Mu-opioid receptors modulate the stability of dendritic spines. Proc Natl Acad Sci U S A. 2005;102:1725–30. doi: 10.1073/pnas.0406797102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, Beyer A, Grecksch G, Hollt V. Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol. 2005;67:280–7. doi: 10.1124/mol.104.004994. [DOI] [PubMed] [Google Scholar]
- [25].Lin H, Higgins P, Loh HH, Law PY, Liao D. Bidirectional Effects of Fentanyl on Dendritic Spines and AMPA Receptors Depend Upon the Internalization of Mu Opioid Receptors. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–22. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- [27].DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- [28].Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281:1261–73. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- [29].Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–64. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- [30].Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Pineyro G. Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci U S A. 2003;100:11406–11. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kobayashi H, Narita Y, Nishida M, Kurose H. Beta-arrestin2 enhances beta2-adrenergic receptor-mediated nuclear translocation of ERK. Cell Signal. 2005;17:1248–53. doi: 10.1016/j.cellsig.2004.12.014. [DOI] [PubMed] [Google Scholar]
- [32].Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem. 2002;277:9429–36. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- [33].Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, Zhang M, Bao G, Wang F, Zhang X, Yang R, Fan F, Chen X, Pei G, Ma L. A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell. 2005;123:833–47. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- [34].Ma L, Pei G. beta-arrestin signaling and regulation of transcription. J Cell Sci. 2007;120:213–8. doi: 10.1242/jcs.03338. [DOI] [PubMed] [Google Scholar]
- [35].Felder CC, Kanterman RY, Ma AL, Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci U S A. 1990;87:2187–91. doi: 10.1073/pnas.87.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- [37].Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ. mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J Biol Chem. 1998;273:23534–41. doi: 10.1074/jbc.273.36.23534. [DOI] [PubMed] [Google Scholar]
- [38].Stout BD, Clarke WP, Berg KA. Rapid desensitization of the serotonin(2C) receptor system: effector pathway and agonist dependence. J Pharmacol Exp Ther. 2002;302:957–62. doi: 10.1124/jpet.302.3.957. [DOI] [PubMed] [Google Scholar]
- [39].Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. 2008;14:461–9. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- [40].Meves H. Arachidonic acid and ion channels: an update. Br J Pharmacol. 2008;155:4–16. doi: 10.1038/bjp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mottola DM, Kilts JD, Lewis MM, Connery HS, Walker QD, Jones SR, Booth RG, Hyslop DK, Piercey M, Wightman RM, Lawler CP, Nichols DE, Mailman RB. Functional selectivity of dopamine receptor agonists. I. Selective activation of postsynaptic dopamine D2 receptors linked to adenylate cyclase. J Pharmacol Exp Ther. 2002;301:1166–78. doi: 10.1124/jpet.301.3.1166. [DOI] [PubMed] [Google Scholar]
- [42].Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C. Crystal structure of beta-arrestin at 1.9 A: possible mechanism of receptor binding and membrane Translocation. Structure. 2001;9:869–80. doi: 10.1016/s0969-2126(01)00644-x. [DOI] [PubMed] [Google Scholar]
- [43].Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–45. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- [44].Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–65. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jaakola V-P, Griffith MT, Hanson MA, Cherezov V, Chien EYT, Lane JR, IJzerman AP, Stevens RC. The 2.6 angstrom crystal structure of a human A2A adensoine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lefkowitz RJ, Whalen EJ. beta-arrestins: traffic cops of cell signaling. Curr Opin Cell Biol. 2004;16:162–8. doi: 10.1016/j.ceb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- [47].Wilden U, Wust E, Weyand I, Kuhn H. Rapid affinity purification of retinal arrestin (48 kDa protein) via its light-dependent binding to phosphorylated rhodopsin. FEBS Lett. 1986;207:292–5. doi: 10.1016/0014-5793(86)81507-1. [DOI] [PubMed] [Google Scholar]
- [48].Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–50. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- [49].Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–80. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- [50].Zheng H, Chu J, Qiu Y, Loh HH, Law PY. Agonist-selective signaling is determined by the receptor location within the membrane domains. Proc Natl Acad Sci U S A. 2008;105:9421–6. doi: 10.1073/pnas.0802253105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zuo Z. The role of opioid receptor internalization and beta-arrestins in the development of opioid tolerance. Anesth Analg. 2005;101:728–34. doi: 10.1213/01.ANE.0000160588.32007.AD. table of contents. [DOI] [PubMed] [Google Scholar]
- [52].Bradbury FA, Zelnik JC, Traynor JR. G protein independent phosphorylation and internalization of the delta-opioid receptor. J Neurochem. 2009;109:1526–35. doi: 10.1111/j.1471-4159.2009.06082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gales C, Van Durm JJ, Schaak S, Pontier S, Percherancier Y, Audet M, Paris H, Bouvier M. Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol. 2006;13:778–86. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- [54].Wess J. G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. Faseb J. 1997;11:346–54. [PubMed] [Google Scholar]
- [55].Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- [56].Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Pierce KL, Lefkowitz RJ. Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci. 2001;2:727–33. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- [58].Macey TA, Liu Y, Gurevich VV, Neve KA. Dopamine D1 receptor interaction with arrestin3 in neostriatal neurons. J Neurochem. 2005;93:128–34. doi: 10.1111/j.1471-4159.2004.02998.x. [DOI] [PubMed] [Google Scholar]
- [59].Lan H, Liu Y, Bell MI, Gurevich VV, Neve KA. A dopamine D2 receptor mutant capable of G protein-mediated signaling but deficient in arrestin binding. Mol Pharmacol. 2009;75:113–23. doi: 10.1124/mol.108.050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].DeGraff JL, Gurevich VV, Benovic JL. The third intracellular loop of alpha 2-adrenergic receptors determines subtype specificity of arrestin interaction. J Biol Chem. 2002;277:43247–52. doi: 10.1074/jbc.M207495200. [DOI] [PubMed] [Google Scholar]
- [61].Cen B, Xiong Y, Ma L, Pei G. Direct and differential interaction of beta-arrestins with the intracellular domains of different opioid receptors. Mol Pharmacol. 2001;59:758–64. doi: 10.1124/mol.59.4.758. [DOI] [PubMed] [Google Scholar]
- [62].Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- [63].Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–9. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- [64].Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- [65].Okamoto T, Murayama Y, Hayashi Y, Inagaki M, Ogata E, Nishimoto I. Identification of a Gs activator region of the beta 2-adrenergic receptor that is autoregulated via protein kinase A-dependent phosphorylation. Cell. 1991;67:723–30. doi: 10.1016/0092-8674(91)90067-9. [DOI] [PubMed] [Google Scholar]
- [66].Digby GJ, Lober RM, Sethi PR, Lambert NA. Some G protein heterotrimers physically dissociate in living cells. Proc Natl Acad Sci U S A. 2006;103:17789–94. doi: 10.1073/pnas.0607116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- [68].Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F., Jr. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–22. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- [69].Aragay AM, Ruiz-Gomez A, Penela P, Sarnago S, Elorza A, Jimenez-Sainz MC, Mayor F., Jr. G protein-coupled receptor kinase 2 (GRK2): mechanisms of regulation and physiological functions. FEBS Lett. 1998;430:37–40. doi: 10.1016/s0014-5793(98)00495-5. [DOI] [PubMed] [Google Scholar]
- [70].Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowitz RJ. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257:1264–7. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- [71].Chini B, Parenti M. G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J Mol Endocrinol. 2004;32:325–38. doi: 10.1677/jme.0.0320325. [DOI] [PubMed] [Google Scholar]
- [72].Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci U S A. 2003;100:5813–8. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Martosella J, Zolotarjova N, Liu H, Moyer SC, Perkins PD, Boyes BE. High recovery HPLC separation of lipid rafts for membrane proteome analysis. J Proteome Res. 2006;5:1301–12. doi: 10.1021/pr060051g. [DOI] [PubMed] [Google Scholar]
- [74].Milligan G, Canals M, Pediani JD, Ellis J, Lopez-Gimenez JF. The role of GPCR dimerisation/oligomerisation in receptor signalling. Ernst Schering Found Symp Proc. 2006:145–61. doi: 10.1007/2789_2006_007. [DOI] [PubMed] [Google Scholar]
- [75].Chabre M, le Maire M. Monomeric G-protein-coupled receptor as a functional unit. Biochemistry. 2005;44:9395–403. doi: 10.1021/bi050720o. [DOI] [PubMed] [Google Scholar]
- [76].Ross EM. Protein modification. Palmitoylation in G-protein signaling pathways. Curr Biol. 1995;5:107–9. doi: 10.1016/s0960-9822(95)00026-1. [DOI] [PubMed] [Google Scholar]
- [77].Escriba PV, Wedegaertner PB, Goni FM, Vogler O. Lipid-protein interactions in GPCR-associated signaling. Biochim Biophys Acta. 2007;1768:836–52. doi: 10.1016/j.bbamem.2006.09.001. [DOI] [PubMed] [Google Scholar]
- [78].O’Dowd BF, Hnatowich M, Caron MG, Lefkowitz RJ, Bouvier M. Palmitoylation of the human beta 2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J Biol Chem. 1989;264:7564–9. [PubMed] [Google Scholar]
- [79].Hayashi MK, Haga T. Palmitoylation of muscarinic acetylcholine receptor m2 subtypes: reduction in their ability to activate G proteins by mutation of a putative palmitoylation site, cysteine 457, in the carboxyl-terminal tail. Arch Biochem Biophys. 1997;340:376–82. doi: 10.1006/abbi.1997.9906. [DOI] [PubMed] [Google Scholar]
- [80].Kennedy ME, Limbird LE. Mutations of the alpha 2A-adrenergic receptor that eliminate detectable palmitoylation do not perturb receptor-G-protein coupling. J Biol Chem. 1993;268:8003–11. [PubMed] [Google Scholar]
- [81].Jin H, Xie Z, George SR, O’Dowd BF. Palmitoylation occurs at cysteine 347 and cysteine 351 of the dopamine D(1) receptor. Eur J Pharmacol. 1999;386:305–12. doi: 10.1016/s0014-2999(99)00727-x. [DOI] [PubMed] [Google Scholar]