Abstract
Several decades of research have sought to characterize tumor cell metabolism in the hopes that tumor-specific activities can be exploited to treat cancer. Having originated from Warburg's seminal observation of aerobic glycolysis in tumor cells, most of this attention has focused on glucose metabolism. However, since the 1950s cancer biologists have also recognized the importance of glutamine (Q) as a tumor nutrient. Glutamine contributes to essentially every core metabolic task of proliferating tumor cells: it participates in bioenergetics, supports cell defenses against oxidative stress, and complements glucose metabolism in the production of macromolecules. Interest in glutamine metabolism has been heightened further by the recent findings that c-myc controls glutamine uptake and degradation, and that glutamine itself exerts influence over a number of signaling pathways that contribute to tumor growth. These observations are stimulating a renewed effort to understand the regulation of glutamine metabolism in tumors and to develop strategies to target glutamine metabolism in cancer. Here we review the protean roles of glutamine in cancer, both in the direct support of tumor growth and in mediating some of the complex effects on whole-body metabolism that are characteristic of tumor progression.
Keywords: Glutamine, metabolism, cancer, Warburg effect, cachexia
Introduction
Tumors are metabolic entities. They draw nutrients from the bloodstream, consume them through biochemical pathways, and secrete waste products which then become substrates for metabolism elsewhere in the body. The metabolism of tumors has fascinated cancer biologists since Warburg's experiments in the 1920s demonstrated that ascites tumor cells from the mouse were capable of unexpectedly high rates of glucose consumption and lactate secretion in the presence of oxygen (the Warburg effect). Because this behavior was so different from the differentiated tissues he used in comparison, those early observations stimulated the hope that the metabolic idiosyncrasies of tumors could be exploited to benefit cancer patients (Warburg, 1925; Warburg, 1956). In terms of tumor imaging, this has turned out to be true: the utility of 18FDG-PET and 1H magnetic resonance spectroscopy in human cancer depend precisely on the ability of these modalities to detect glucose uptake and lactate production. Interest in the Warburg effect as an Achilles’ heel to be exploited in cancer treatment has been further stimulated by the demonstration that enhanced glucose metabolism is a common consequence of many of the mutations responsible for human cancer, and therefore may be a central process essential for tumor growth (Elstrom et al., 2004; Flier et al., 1987; Kroemer and Pouyssegur, 2008; Matoba et al., 2006; Osthus et al., 2000; Shim et al., 1997).
In terms of developing strategies to treat cancer, however, tumor metabolism has so far proven to be more of a Holy Grail than an Achilles’ heel. Part of the difficulty lies in the flexibility of metabolic systems and the panoply of nutrients to which tumors have access. Thus a complete picture of the metabolism of any tumor must consider the contribution of multiple nutrients simultaneously. Chief among the other nutrients available to tumors is glutamine, the most abundant amino acid in the plasma and the major carrier of nitrogen between organs. Glutamine is also second only to glucose in terms of persistent interest in its role in tumor cell metabolism, which now dates back more than 50 years (Eagle, 1955; Kvamme and Svenneby, 1960). In culture, tumor cells are avid glutamine consumers, metabolizing it at rates far in excess of any other amino acid (Eagle, 1955). The same is true in tumors implanted onto vascular pedicles in rats to allow precise measurements of the rates of amino acid extraction from the blood (Sauer and Dauchy, 1983; Sauer et al., 1982). These observations led to the notion that glutamine metabolism stood with the Warburg effect as a major component of the general metabolic phenotype of proliferating tumor cells (Kovacevic and McGivan, 1983).
Glutamine's importance in tumor cell metabolism derives from characteristics it shares with glucose. Both nutrients help to satisfy two important needs for proliferating tumor cells: bioenergetics (ATP production) and the provision of intermediates for macromolecular synthesis (DeBerardinis et al., 2008b). A number of excellent discussions on the utilization of glutamine in cancer have previously been presented (Medina et al., 1992; Souba, 1993). Surprisingly, however, only recently has it been reported that oncogenes influence glutamine metabolism as they do for glucose, and that tumor genetics can dictate cellular dependence on glutamine for survival. Furthermore, other studies have been uncovering diverse and unexpected roles for glutamine and its by-products in cell signaling, linking glutamine metabolism to cell survival and growth in ways beyond its roles in intermediary metabolism. Here we review glutamine's metabolic and non-metabolic functions in tumor cells, the integration of glucose and glutamine metabolism in tumor growth, and aspects of whole-body glutamine metabolism that may influence the morbidity and mortality of cancer patients.
Glutamine's roles in intermediary metabolism: functions and consequences
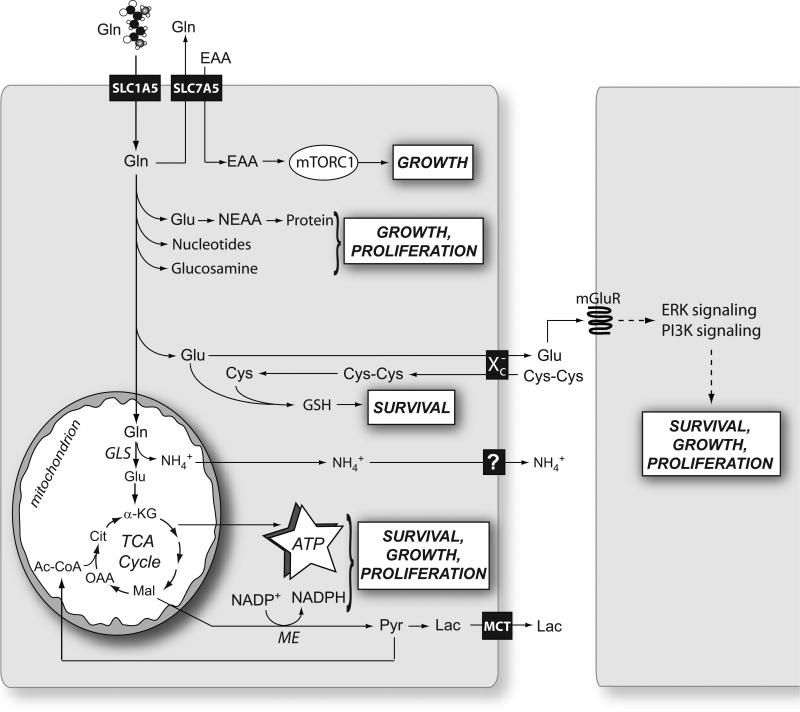
Glutamine has traditionally been viewed as a nonessential amino acid whose primary functions are to store nitrogen in the muscle and to traffic it between organs. Although it contributes only 4% of the amino acid in muscle protein, glutamine accounts for more than 20% of the free amino acid pool in plasma and more than 40% in muscle (Bergstrom et al., 1974; Kuhn et al., 1999). Mammals can synthesize glutamine in most tissues, but during periods of rapid growth or illness, the cellular demand for glutamine outstrips its supply and glutamine becomes essential (hence its designation as a “conditionally” essential amino acid). Proliferating cells display an intense appetite for glutamine, reflecting its incredible versatility as a nutrient and mediator of other processes (Figure 1).
Figure 1. Glutamine supports cell survival, growth and proliferation through metabolic and non-metabolic mechanisms.
After its import through surface transporters like SLC1A5, glutamine (Gln) is either exported in exchange with the import of essential amino acids (EAA) or consumed in various pathways that together support the basic metabolic functions needed for cell survival, growth and proliferation. In cancer cells, the mitochondrial enzyme glutaminase (GLS) appears to account for the largest fraction of net glutamine consumption. This enzyme produces NH4+, which is exported, perhaps through carrier-mediated mechanisms. Abbreviations: mTORC1, mammalian Target of Rapamycin Complex 1; Glu, glutamate; NEAA, nonessential amino acids; Cys, cysteine; Cys-Cys, cystine; GSH, glutathione; mGluR, metabotropic glutamate receptor; ERK, extracellular signal-regulated protein kinase; PI3K, phosphatidylinositol 3’-kinase; α-KG, α-ketoglutarate; Mal, malate; OAA, oxaloacetate; Ac-CoA, acetyl-CoA; Cit, citrate; Pyr, pyruvate; Lac, lactate; TCA, tricarboxylic acid; GLS, glutaminase; ME, malic enzyme.
The metabolic fates of glutamine can roughly be divided into reactions that use glutamine for its γ-nitrogen (nucleotide synthesis and hexosamine synthesis) and those that utilize either the α-nitrogen or the carbon skeleton. The reactions in the second category use glutamate, not glutamine, as the substrate. Although tumor cells tend to have large intracellular pools of glutamate, maintaining these pools rests on the ability to convert glutamine into glutamate, because glutamine is an abundant extracellular nutrient and glutamate is not. This process is largely due to the activity of phosphate-dependent glutaminase (GLS), a mitochondrial enzyme that is highly expressed in tumors and tumor cell lines. Classical experiments demonstrated that glutaminase activity correlates with tumor growth rates in vivo (Knox et al., 1969; Linder-Horowitz et al., 1969), and experimental models to limit glutaminase activity resulted in decreased growth rates of tumor cells and xenografts (Gao et al., 2009; Lobo et al., 2000). Thus, while not all the metabolic fates of glutamine require glutaminase activity, this enzyme is essential to the metabolic phenotype of growing tumors.
The following are the major aspects of glutamine-based intermediary metabolism and their relevance to tumor cell growth. They are summarized in Figure 1.
1. Nucleotide biosynthesis
Glutamine is a required nitrogen donor for the de novo synthesis of both purines and pyrimidines and is therefore essential for the net production of nucleotides during cell proliferation. The γ (amido) nitrogens of two glutamine molecules are added to the growing purine ring, and a third is used in the conversion of xanthine monophosphate to guanosine monophosphate. Other nitrogens are supplied by glycine and aspartate, but many of these are also initially derived from glutamine (the α-nitrogen). Pyrimidine rings contain one nitrogen from glutamine's amido group and one from aspartate, and an additional amido nitrogen is added to uridine triphosphate to form cytidine triphosphate. The importance of glutamine nitrogen in nucleotide biosynthesis probably explains why some transformed cells exhibit delayed transit through S-phase in low-glutamine conditions (Gaglio et al., 2009). However, the glutamine utilization rate exceeds nucleic acid synthesis by more than an order of magnitude in proliferating cells, and thus nitrogen donation to nucleotides accounts for only a small fraction of total glutamine consumption (Ardawi et al., 1989).
2. Hexosamine biosynthesis and glycolsylation reactions
The rate-limiting step in the formation of hexosamine is catalyzed by glutamine:fructose-6-phosphate amidotransferase (GFAT), which transfers glutamine's amido group to fructose-6-phosphate to form glucosamine-6-phosphate, a precursor for N-linked and O-linked glycolsylation reactions. These reactions are necessary to modify proteins and lipids for their participation in signal transduction, trafficking/secretion and other processes. Impairment of glucosamine-6-phosphate production is thus predicted to reduce cell growth and to interfere with cell signaling. Surprisingly, GFAT ativity could be suppressed by expressing an antisense GLS cDNA to decrease glutaminase acitivty in breast cancer cells. This resulted in disturbances of O-linked glycolsylation pathways, altering the glycosylation status of the transcription factor Sp-1 and increasing its transcriptional activity (Donadio et al., 2008). The mechanism by which loss of glutaminase activity influences glycolsylation is unclear, but the findings suggest that GFAT and perhaps other components of the glycosylation machinery are responsive to intracellular glutamine availability as determined by glutaminase.
3. Nonessential amino acids
Because of the high expression of glutaminase, tumor cells are poised to produce glutamate rapidly from glutamine, and a sizable fraction of their glutamate pool carries glutamine's α (amino) nitrogen. This nitrogen is then dispersed into various pools of nonessential amino acids (NEAA) through the activity of transaminases, particularly alanine aminotransferase and aspartate aminotransferase. These enzymes catalyze the reversible transfer of amino groups between glutamate and alanine or aspartate, respectively. Alanine is used in protein synthesis, but is also avidly secreted by tumor cells, carrying some of the excess carbon from glycolysis. Aspartate, in contrast, remains inside the cell and contributes to the synthesis of proteins and nucleotides and to electron transfer reactions through the malate-aspartate shuttle. In this shuttle, aspartate exits the mitochondria and is converted to oxaloacetate (OAA) by aspartate aminotransferase. OAA is then reduced to malate, using electrons donated by NADH generated in glycolysis. The malate then enters the mitochondria and donates the electrons to Complex I of the electron transport chain. Thus, the shuttle facilitates ongoing ATP production in both the mitochondria (via oxidative phosphorylation) and the cytosol (by re-supplying NAD+ for glycolysis).
4. Glutathione (GSH)
At a concentration of approximately 5 mM, GSH is the major thiol-containing endogenous antioxidant and serves as a redox buffer against various sources of oxidative stress. In tumors, maintaining a supply of GSH is critical for cell survival because it allows cells to resist the oxidative stress associated with rapid metabolism, DNA damaging agents, inflammation and other sources (Estrela et al., 2006). GSH is a tripeptide of glutamate, cysteine and glycine and its formation is highly dependent on glutamine. Not only does glutamine metabolism produce glutamate, but the glutamate pool is also necessary for cells to acquire cysteine, the limiting reagent for GSH production. This occurs by virtue of the Xc− antiporter, which exports glutamate and imports cystine, as shown in Figure 1. Cystine can then be converted to cysteine inside the cell and used in GSH synthesis. Because of the key role of the Xc− antiporter in maintaining glutathione levels, it has been suggested as a target for cancer therapy (Lo et al., 2008).
5. Respiratory substrate
Oxidation of glutamine's carbon backbone in the mitochondria is a major metabolic fate of glutamine and a primary source of energy for proliferating cells, including lymphocytes, enterocytes, fibroblasts and some cancer cell lines (DeBerardinis et al., 2008b; Miller, 1999; Reitzer et al., 1979). This requires conversion of glutamine to α-ketoglutarate, typically via glutaminase activity followed by conversion of glutamate to α-ketoglutarate by either transaminases or glutamate dehydrogenase. However, other processes such as the donation of glutamine's amido nitrogen to nucleotides or hexosamines could contribute a fraction of the glutamate pool as well. Overall, this contribution is small, but it might become more prominent during stages of the cell cycle characterized by transient increases in nucleotide biosynthesis or other activities. Thus, proliferating cells, which exhibit high fluxes through a number of biosynthetic pathways, are well-positioned to make use of glutamine's carbon skeleton as a respiratory substrate. Complete oxidation of glutamine carbon involves exit from the TCA cycle as malate, conversion to pyruvate and then acetyl-CoA, and finally re-entry into the cycle (Figure 1). It should be noted, however, that careful studies of tumor cell glutamine metabolism reveal that neither its nitrogen nor its carbon are used to completion in vitro. Rather, a high fraction of both of glutamine's nitrogens are secreted from glioblastoma cells as they proliferate (as ammonia, alanine and glutamate), and at least half of its carbon is secreted as lactate (DeBerardinis et al., 2007). This form of rapid glutamine utilization and secretion of glutamine by-products, similar to the Warburg effect in its apparent inefficiency, has been proposed to be an additional hallmark of tumor cell metabolism (Mazurek et al., 2005).
6. Reducing equivalents
One of the benefits of converting glutamine to pyruvate is the reduction of NADP+ to NADPH by malic enzyme (Figure 1). NADPH is a required electron donor for reductive steps in lipid synthesis, in nucleotide metabolism, and in maintaining GSH in its reduced state. Therefore proliferating cells must produce a large supply of it. Although cells contain numerous potential sources of NADPH, in glioblastoma cells the malic enzyme flux was estimated to be high enough to supply all of the reductive power needed for lipid synthesis (DeBerardinis et al., 2007).
7. Ammoniagenesis
Glutaminase activity generates free ammonia, a potentially toxic metabolite. In glioblastoma cells, the rate at which ammonia is secreted into the extracellular space is about 75% the rate of glutamine disappearance from the medium (our unpublished observations), consistent with a high fraction of glutamine being metabolized in the mitochondria by glutaminase. Without a mechanism to dispose of ammonia rapidly, intracellular ammonia concentrations would reach several hundred mmoles/L within a few hours. It is not known how tumor cells dispose of ammonia during rapid glutamine catabolism. The traditional view held that passive diffusion of the gaseous form (NH3) across the lipid bilayer accounted for essentially all ammonia transport. This simple model no longer holds for some tissues with a high demand for ammonia transport. In the kidney, where ammonia metabolism is a key mediator of acid-base homeostasis, a number of protein transporters exist to traffic ammonia as NH3 and/or NH4+. These systems include ion channels, aquaporins and Rh glycoproteins (Weiner and Hamm, 2007), some of which are over-expressed in tumors. Although the exact mechanism of tumor cell ammonia secretion has not been established, the process carries therapeutic potential. Blocking ammonia secretion would presumably either suppress net glutamine consumption or cause toxic intracellular accumulation of ammonia, both of which might impair cell survival and growth.
Glutamine's roles in cell signaling and gene expression
Beyond its roles in intermediary metabolism, glutamine exerts other effects that support cell survival and growth. Reflecting the importance of glutamine to anabolic metabolism, cells have developed glutamine-dependent mechanisms to control growth, including the modulation of signal transduction pathways. A recent study revealed the necessity of glutamine for the well-known stimulatory effect of essential amino acids on the mammalian target of rapamycin (mTOR) pathway (Nicklin et al., 2009). In the study, stimulation of mTOR compelx-1 (mTORC1) in HeLa cells required bidirectional transport of glutamine: the cells imported glutamine through the Na+-dependent transporter SLC1A5 and then exported it through the Na+-independent transporter SLC7A5 (Figure 1). The latter step was accompanied by import of essential amino acids and subsequent activation of mTORC1, stimulating cell growth while suppressing autophagy. It was the sequential import and export of glutamine itself and not the by-products of glutamine metabolism that facilitated the uptake of essential amino acids, because addition of glutamate or α-ketoglutarate failed to rescue mTORC1 activation in the absence of glutamine. Thus, this mechanism of control relies on glutamine abundance exceeding the cells’ metabolic needs, suggesting that glutamine excess is a signal to promote cell growth and suppress catabolism.
Other data has identified a role for glutamine in extracellular signal-regulated protein kinase (ERK) signaling pathways. This has been best characterized in intestinal epithelial cells, which consume glutamine as their major bioenergetic substrate and require glutamine both for proliferation and survival. Addition of glutamine was sufficient to stimulate ERK signaling within a few minutes in porcine intestinal epithelial cells, and it enhanced 3H-thymidine incorporation (Rhoads et al., 1997). In rat intestinal epithelial cells, glutamine was comparable to serum in preventing apoptosis, and it stimulated a sustained activation of ERK signaling (Larson et al., 2007). In those cells, inhibitors of the ERK pathway eliminated the protective effect of glutamine supplementation. It was not clear from these studies whether glutamine import alone was required for the effects, or whether the cells needed to metabolize glutamine to activate ERK signaling.
Glutamate also influences signaling and tumor growth if it is secreted by tumor cells. In the central nervous system, the rate of glutamate secretion by glioma cells correlated with the ability of those cells to elicit regional neuronal cell death and to form tumors in the rat striatum. Both these effects were due to glutamate signaling through ionotropic receptors, because they were blocked by NMDA receptor antagonists (Takano et al., 2001). Glutamate also has non-ionotropic signaling properties through its effects on metabotropic glutamate receptors (mGluRs) (Figure 1). These G-protein coupled receptors are widely expressed in both neuronal and non-neuronal tissues, implying that they have signaling duties beyond their traditional function in synaptogenesis (Nicoletti et al., 2007; Skerry and Genever, 2001). Activation of mGluR isoforms leads to stimulation of ERK and phosphatidylinositol 3’-kinase (PI3K) signaling, supporting cell survival, growth and proliferation. This may translate into a role for glutamate signaling in cancer. The isoform mGluR1 is expressed in human melanoma cells but not in melanocytes or benign nevi, and overexpression of mGluR1 in mouse melanocytes caused hyperproliferation and occasional transformation into melanoma (Pollock et al., 2003). In mice with doxycycline-repressible mGluR1 expression in melanocytes, the growth of existing melanomas could be impaired by administering doxycycline, and this was correlated with a reduction in activated ERK in the tumors (Ohtani et al., 2008). Human glioblastomas also express mGluRs, and a small molecule inhibitor against mGluR2 and 3 inactivated ERK and PI3K signaling in glioma cell lines, inhibited cell proliferation, and suppressed the growth of tumors in both the brain and subcutaneous tissue (Arcella et al., 2005). Thus these receptors might be useful targets in cancer therapy. Brain tumors may have access to sufficient extracellular glutamate to signal through these receptors without requiring glutamate secretion by the tumor itself. However, the glutamate concentration in the plasma is quite low (~ 50 μM). For melanomas and other tumors outside the central nervous system, mGluR signaling may require that the tumor secrete glutamate produced from glutamine metabolism. This could result in autocrine or paracrine effects on cell growth.
Consistent with glutamine's effects on cell signaling, a number of reports have demonstrated that it also influences gene expression (Brasse-Lagnel et al., 2009). In cell lines, addition of glutamine increases expression of the pro-proliferation factors c-jun and c-myc within a few minutes (Kandil et al., 1995; Rhoads et al., 1997) and promotes cell survival through negative effects on growth-inhibitory and pro-apoptotis factors like CHOP, GADD45, Fas and ATF5 (Abcouwer et al., 1999; Huang et al., 1999; Watatani et al., 2007; Yeo et al., 2006). In Ehrich ascites tumor cells, glutaminase knockdown led to enhanced phosphorylation, DNA binding, and transcriptional activity of Sp1 (Segura et al., 2005). In HepG2 hepatoma cells, glutamine was required for the induction of manganese superoxide dismutase (MnSOD) expression that accompanied the depletion of essential amino acids (Aiken et al., 2008). Glutamine's involvement in MnSOD expression was blocked by inhibiting the TCA cycle, ERK1/2 or mTOR, suggesting that an integration between mitochondrial glutamine metabolism and signal transduction facilitates the effect.
Abundant evidence suggests that glutamine also modulates immune responses, although it is unclear exactly how these changes are achieved. Conceivably glutamine could exert its effects via redox homeostasis, bioenergetics, nitrogen balance or other functions (Eliasen et al., 2006; Roth, 2007). During radiation-induced oxidative stress in the rat abdomen, pre-treatment of the animals with glutamine significantly decreased tissue inflammation and expression of NF-κB, suggesting that in this case glutamine was used to buffer the redox capacity (Erbil et al., 2005). NF-κB seems to be a key mediator linking glutamine availability to stress responses, since other reports also demonstrated an inverse correlation between glutamine abundance and NF-κB-mediated gene expression (Bobrovnikova-Marjon et al., 2004; Hubert-Buron et al., 2006). The role of glutamine as an immunomodulator in cancer has not been explored at the molecular level, but this is an area worth further study. If the avid consumption of glutamine by tumors reduces its availability for neighboring cells, this could modulate local NF-κB signaling and expression of inflammatory mediators in the stroma.
Integrating glutamine and glucose metabolism in tumor cell growth
Because tumor cells are exposed to many nutrients simultaneously, achieving a comprehensive view of tumor metabolism requires understanding how cells integrate these pathways into an over-arching metabolic phenotype. Consequently, discussions about glutamine cannot ignore the rapid glucose utilization that also accompanies cell proliferation. In fact the rates of glucose and glutamine consumption far outpace the utilization of other nutrients available to the cell. Presumably this type of metabolism supports both bioenergetics and the production of precursor pools while sparing other energy-rich substrates like fatty acids and essential amino acids for direct incorporation into macromolecules (DeBerardinis et al., 2006).
Warburg's work and many studies since then have focused on the production of lactate from glucose, on the low relative rate of glucose oxidation, and on the high apparent contribution of glycolysis to overall energetics in tumor cells. These have led to the generalization that tumors do not or cannot engage in oxidative metabolism, and that aerobic glycolysis (i.e. conversion of glucose to lactate in the presence of ample oxygen) is a sine qua non for the tumor metabolic phenotype. A few points should be made about these assumptions. First, metabolism of glucose to lactate is not limited to tumor cells, but is a common feature of rapid cell proliferation (Brand, 1985; Wang et al., 1976). The potential advantages of this form of metabolism for proliferating cells have been reviewed (DeBerardinis et al., 2008a). Second, while many tumor cell lines do exhibit high glycolytic rates, the contribution of glycolysis to total cellular ATP content varies widely, from over 50% as Warburg found to less than 5% in other cells (Zu and Guppy, 2004). Thus oxidative phosphorylation is not universally impaired in tumor cells. Third, mitochondrial metabolism directly contributes to cell growth because many macromolecular precursors are produced in the TCA cycle (DeBerardinis et al., 2008b). Even if the glycolytic rate is high enough to support most of the cell's need for ATP synthesis, growth requires that the cells produce lipids, proteins and nucleic acids, and building blocks for these molecules come from the TCA cycle. Therefore, the aerobic glycolysis discovered by Warburg is only one piece of the puzzle of anabolic metabolism in tumor cells.
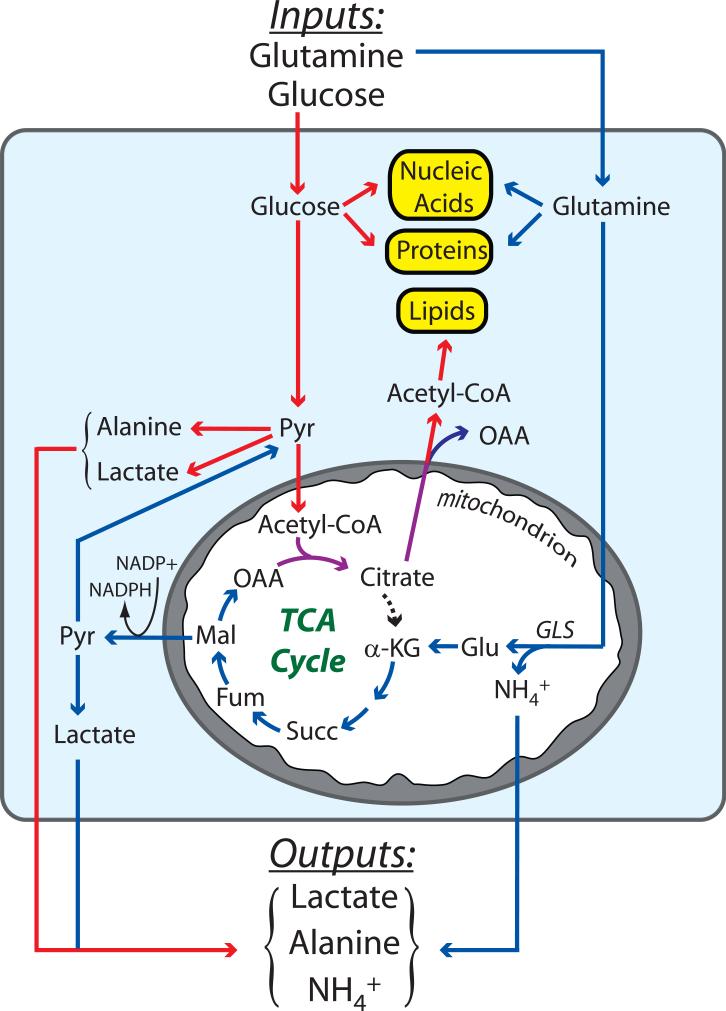
Our growing understanding of glutamine metabolism promises to help fill in this picture, as the concurrent consumption of glucose and glutamine has obvious theoretical advantages (Figure 2). The simultaneous metabolism of these two nutrients would support bioenergetics in cells exhibiting the Warburg effect by delivering glutamine-derived α-KG to the TCA cycle for oxidation. Moreover, the entry of α-KG offsets the export of intermediates used in biosynthetic pathways. The process of replenishing TCA cycle intermediates during cell growth (anaplerosis) is a keystone of biomass production and is much less active in quiescent cells. In rapidly proliferating cultured glioblastoma cells, for example, most of the acetyl-CoA pool comes from glucose, while essentially all of the anaplerotic carbon (i.e. the oxaloacetate) comes from glutamine (DeBerardinis et al., 2007). This results in citrate molecules that contain carbon from both glucose and glutamine (purple arrows in Figure 2). After this citrate leaves the TCA cycle, the two glucose-derived carbons are released as acetyl-CoA and used in lipid synthesis. Protein and nucleic acid synthesis also require precursor molecules derived from glucose and glutamine metabolism. Thus during cell proliferation, glucose and glutamine are the two major nutrient inputs, and the primary outputs are biomass (nucleic acids, proteins and lipids) and the by-products secreted as a result of this type of metabolism: lactate, alanine and ammonia (Figure 2).
Figure 2. Cooperativity between glucose and glutamine metabolism in growing tumors.
The major nutrients consumed by tumors are glutamine and glucose, which provide precursors for nucleic acids, proteins and lipids, the three classes of macromolecules needed to produce daughter cells. The metabolism of glutamine (blue arrows) and glucose (red arrows) are complementary, converging on the production of citrate (purple arrows). Glutamine metabolism produces oxaloacetate (OAA) and NADPH, both of which are required to convert glucose carbon into macromolecules. Glutamine metabolism also supplements the pyruvate pool, which is predominantly formed from glucose. As a consequence of the rapid metabolism of these two nutrients, lactate, alanine and NH4+ are secreted by the tumor. Abbreviations: GLS, glutaminase; Glu, glutamate; α-KG, α-ketoglutarate; Succ, succinate; Fum, fumarate; Mal, malate; Pyr, pyruvate; Lac, lactate; TCA, tricarboxylic acid.
The importance of glucose and glutamine metabolism to tumor cell biology is underscored by the fact that mutations in tumor suppressors and oncogenes allow cells to by-pass the normal, growth factor-dependent handling of these two nutrients. This has been extensively studied for glucose metabolism. As early as the 1980s it was demonstrated that over-expression of Ras or Myc was sufficient to drive glucose uptake in fibroblasts (Flier et al., 1987). Subsequently, mutations in many other tumor suppressors and oncogenes have been implicated as drivers of the Warburg effect in tumor cells (reviewed in DeBerardinis, 2008). While much less is known about the regulation of glutamine metabolism, several reports have recently implicated c-Myc as a major player. Enhanced c-Myc activity was sufficient to drive glutamine metabolism and to impair cell survival in low-glutamine conditions (Wise et al., 2008; Yuneva et al., 2007). c-Myc regulates glutamine metabolism in part by stimulating the expression of surface transporters (Wise et al., 2008). Interestingly, c-Myc also indirectly regulates the protein expression of glutaminase through effects on the microRNAs miR23a and miR23b. Normally, these microRNAs bind to the GLS 3’-UTR and prevent translation of the message. However, c-Myc suppresses miR-23a/b expression, and thus enhanced c-Myc activity de-repressed glutaminase translation and facilitated glutamine oxidation in the mitochondria (Gao et al., 2009).
The simplest mechanism to explain the enhanced utilization of both glutamine and glucose by tumor cells is that metabolism of the two nutrients is co-regulated. However, recent findings suggest that they can be regulated by independent signaling pathways within the same cells. In a glioblastoma cell line with genomic c-myc amplification, inhibition of Akt signaling led to a decrease in glycolysis but had no effect on glutamine metabolism, which was only inhibited when c-Myc was suppressed to normal levels (Wise et al., 2008). This raises the possibility that the complex metabolic phenotype observed in tumor cells is the result of multiple different signaling inputs, presumably through multiple mutations. This notion is consistent with the observation that serial transduction of human fibroblasts leads to step-wise changes in the cells’ metabolic profile and dependence on particular pathways to sustain ATP supply (Ramanathan et al., 2005).
Tumors often display regional heterogeneity in oxygen availability, and this can significantly influence intermediary metabolism independently of tumor genetics. Stabilization of the transcription factor hypoxia-inducible factor-1α (HIF-1α) in hypoxic cells enhances the expression of glucose transporters, glycolytic enzymes, and inhibitory kinases for the pyruvate dehydrogenase complex, all of which serve to increase the production of lactate from glucose (Kim et al., 2006; Papandreou et al., 2006; Semenza, 2003). Little is known about the effects of hypoxia on glutamine metabolism. Presumably in order to survive, cells would at least need to maintain some of the proximal steps of glutamine metabolism to satisfy homeostatic requirements for amino acid and nucleotide synthesis. One study showed that hypoxia could stimulate the import of glutamine in neuroblastoma cells, although downstream metabolism was not examined (Soh et al., 2007). On the other hand, rat pheochromocytoma cells displayed increased rates of endogenous glutamine synthesis under hypoxia, perhaps reflecting a need for glutamine that exceeded transport capacity (Kobayashi and Millhorn, 2001). Further work is needed to determine how hypoxia influences glutamine metabolism and whether glutamine influences cell survival during hypoxic stress.
Glucose and glutamine metabolism also have overlapping functions in redox homeostasis, since both can produce NADPH and glutamine has the additional role of supporting GSH biosynthesis. This is an important consideration during cell proliferation, because NADPH is required for biosynthetic reactions and because some production of reactive oxygen species is inevitable during rapid nutrient metabolism. Glutamine withdrawal led to decreased GSH pools in fibroblasts with enhanced c-Myc activity, and to frank oxidative damage in hybridoma cells (Guerin et al., 2006; Yuneva et al., 2007). In neither case, however, was the redox stress sufficient to explain the loss of cell viability. On the other hand, in P-493 B lymphoma cells and PC3 prostate cancer cells the loss of cell proliferation and viability triggered by glutamine deprivation or GLS knockdown could be partially reversed with antioxidants. Thus, while impaired glutamine metabolism limits the availability of GSH, this effect is not always responsible for the death of glutamine-dependent cells. It is likely that tumor cells differ in their use of glucose and glutamine to maintain redox balance, and exploiting these differences may be therapeutically useful, especially during therapies that induce oxidative stress.
Tumor growth and whole-body metabolism: is the tumor the tail that wags the dog?
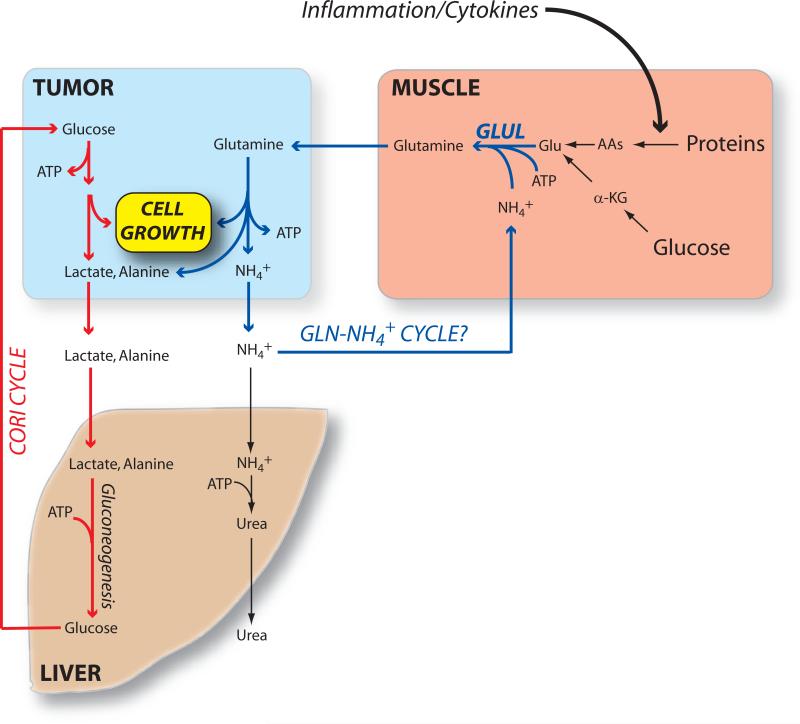
The aggressive metabolism of glucose and glutamine by tumors begs the question of how cancer affects metabolism in the rest of the body. Two issues are particularly relevant to this discussion. First, how do tumors acquire the nutrients they need to grow? And second, what is the fate of the waste products secreted by tumor cells? Both these issues relate to the phenomenon of declining nutrition observed in patients with cancer or other chronic health problems. Cachexia, the progressive loss of muscle and adipose tissue mass, is a well-known and clinically important dimension of cancer, affecting more than half of all patients. It consists of a combination of complex changes in muscle and liver metabolism culminating in weight loss. Changes in appetite and taste sensation may accompany cachexia and can be exacerbated by chemotherapy, but these are not sufficient to explain the phenotype. Weight loss often predates the diagnosis and treatment of cancer, and normalizing the caloric intake usually does not reverse the loss of lean mass. These observations imply that fundamental changes in whole-body metabolism, including an increase in energy expenditure, are at the root of the problem. The clinical importance of cachexia is emphasized by the finding that it is the principal cause of death in one-third or more of cancer patients (NCI Website, 2009). Many aspects of the cachectic phenotype are stimulated (directly or indirectly) by the tumor, and evidence suggests that the tumor derives benefits from altered metabolism in the liver and muscle. Two aspects of cachexia are discussed here: the Cori cycle and muscle proteolysis, each of which has implications for the metabolism of growing tumors (Figure 3).
Figure 3. Proposed inter-organ metabolic cycles in cachectic cancer patients.
As summarized in Figure 2, growth of the tumor involves consumption of glucose and glutamine with secretion of lactate, alanine and ammonia. Some of the lactate may be taken up by well-oxygenated regions of the tumor and used as a respiratory fuel. Other lactate and alanine are delivered to the liver and used to produce glucose, which can then return to the tumor (the Cori cycle). Meanwhile, the ammonia can be disposed through the urea cycle, or possibly delivered to the muscle for incorporation into new glutamine molecules produced during protein catabolism and glucose metabolism. Both the Cori cycle and the putative glutamine-ammonia cycle deliver energy to the tumor, but cost energy in the other organs involved, driving up whole-body energy expenditure as is typically observed in cancer cachexia. Glu, glutamate; α-KG, α-ketoglutarate; AAs, amino acids; GLUL, glutamate-ammonia ligase (i.e. glutamine synthetase).
In the Cori cycle, lactate and alanine released from glucose-consuming tissues like the muscle and brain are returned to the liver and used as precursors for gluconeogenesis. As early as the 1950s, Hiatt demonstrated that a high fraction of glucose produced by the liver of tumor-bearing mice had been generated from 3-carbon units released by the tumor, suggesting a Cori-like cycle between the tumor and the liver (Hiatt, 1957). Similar experiments in humans also documented higher rates of Cori cycle metabolism in individuals with cancer of various types (Holroyde et al., 1984; Reichard GA, 1963). The Cori cycle would thus be a way to minimize the apparent energetic inefficiency of the Warburg effect, since the “wasted” 3-carbon units could ultimately be returned to the tumor as glucose. However, while the cycle produces energy in the tumor (two ATPs per glucose used), this is more than offset by energy expenditure in the liver (6-12 ATP equivalents per glucose formed). Thus the Cori cycle imposes an energetic burden on whole-body metabolism. Increased flux through this pathway has been postulated to be one of the drivers of energy dissipation in cachectic cancer patients (Tisdale, 2009). Secretion of lactate by hypoxic cells within tumors can also have local effects on metabolism. If this lactate can reach better oxygenated cells within the tumor, it can be taken up and used as a respiratory fuel in the mitochondria. Sonveaux et al. identified the cell surface monocarboxylate transporter MCT1 as the enabler of such a metabolic symbiosis in xenografts. MCT1 allowed normoxic cells to take up lactate, presumably sparing glucose for the more hypoxic regions of the tumor. Inhibiting MCT1 significantly reduced tumor growth and increased the effect of radiation therapy (Sonveaux et al., 2008).
Cancer also induces a negative nitrogen balance in the host characterized by loss of protein and amino acids from the muscle, eventually leading to loss of muscle mass. This observation predates even Warburg's work by several decades and has been credited to Müller (Parry-Billings et al., 1991), who compared the host response in cancer to that of a prolonged febrile illness. While the inciting agents that promote muscle catabolism have not been fully elucidated, many studies have implicated inflammatory mediators like transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), and various cytokines, consistent with Müller's hypothesis (Tisdale, 2005). Clearly glutamine metabolism is tied to cachexia, since more than 90% of the body's glutamine stores are in the muscle, and glutamine is the major amino acid exported from the muscle during catabolic stress. Newsholme suggested that the demand for glutamine by rapidly proliferating cells like lymphocytes or tumor cells might be the trigger for cachexia (Newsholme et al., 1985). Evidence in support of this hypothesis includes the observation that implantation of tumor tissue in rodents rapidly triggers an increase in muscle glutamine output and a drop in muscle glutamine stores (Chen et al., 1993; Parry-Billings et al., 1991). Similar changes have been suggested to occur with prolonged tumor burden in humans (Souba, 1993).
The process of muscle nitrogen loss during cachexia involves the transfer of amino acid nitrogen first to glutamate and then to glutamine, which is secreted into the bloodstream (Figure 3). Production of glutamine from glutamate is catalyzed by glutamine synthetase (also known as glutamate-ammonia ligase, GLUL). Tumor burden is associated with increased expression of the GLUL mRNA and activity in the muscle of mammals and birds, implicating this enzyme as an evolutionarily conserved mediator of cachexia (Chen et al., 1993; Matsuno and Satoh, 1986; Quesada et al., 1988). Glutamine synthesis consumes energy and thus would tend to increase energy expenditure in the muscle. It also requires ammonia. Interestingly, myocytes can take up and metabolize ammonia, and this is one of the mechanisms by which ammonia is cleared during exercise in humans (Bangsbo et al., 1996). The source of muscle ammonia during glutamine secretion in cachexia is unknown, but presumably comes from an alteration in inter-organ ammonia handling. This could be similar to the changes that accompany chronic liver disease, when the muscle takes up excess ammonia and uses it to produce glutamine as a detoxification strategy (Olde Damink et al., 2009). Thus, it is possible that some of the ammonia secreted by tumors as a result of their avid glutamine consumption is trafficked to the muscle to re-create glutamine from the intracellular glutamate pool (Figure 3). While speculative, such a system would be analogous to glucose handling in the tumor-liver Cori cycle.
The question of whether cancer patients with cachexia would benefit from glutamine supplementation has generated a large amount of interest and debate. A theoretical concern is that increasing glutamine availability might stimulate tumor growth, but it is not clear whether well-perfused tumors ever experience glutamine deprivation in vivo, and one study in sarcoma-bearing rats found no enhancement of tumor growth when the animals’ diet was supplemented with glutamine (Klimberg et al., 1990). A large number of clinical human trials have been performed to assess glutamine's utility in improving muscle mass or in limiting chemotherapeutic toxicity, alone or in combination with other dietary agents, but there is still no consensus as to whether glutamine is generally helpful.
In principle, one could also attempt to intervene at the level of glutamine release from the muscle. It is tempting to speculate that if muscle export is the major source of glutamine for the tumor, then suppressing either proteolysis or glutamine synthesis in the muscle might suppress tumor growth by reducing the supply of an essential nutrient. Transcription of the GLUL mRNA seems to be the pivotal level of regulation in this process. In various cell lines, GLUL expression is stimulated by glucocorticoids, which have also been implicated in the development of cancer cachexia (Gaunitz et al., 2002; Wang and Watford, 2007). Perhaps targeting this system in the muscle could be of some benefit.
Conclusions and more questions
A number of landmark reports over the last decade cemented the intimate connections between oncogenes and glucose metabolism in tumors, and demonstrated that the reliance of transformed cells on the Warburg effect could be exploited experimentally to suppress tumor growth. The next wave of interest in tumor metabolism will likely focus increased attention on cellular glutamine handling, and should re-assess the question of whether the appetite for glutamine displayed by tumors can be used against them. Glutamine's diverse contributions to intermediary metabolism, cell signaling, gene expression and cachexia argue in favor of attempting to develop rational strategies to limit tumor glutamine uptake and to image glutamine metabolism in tumors. Presumably such maneuvers would be particularly useful in tumors containing enhanced c-Myc activity.
There are also a number of fundamental biological questions that deserve further study. For example, how do cells manage to allocate glutamine appropriately into the numerous pathways that use it? Are there mechanisms to channel glutamine towards the enzymes and cellular compartments that most need it, and are these systems responsive to stresses that change cellular patterns of glutamine use? The observation that some cells require glutamine export to activate mTOR signaling begs the question of how these cells can sense when glutamine is abundant enough to permit its secretion. It will also be interesting to determine how signals related to glutamine abundance/deprivation are transmitted to the nucleus to influence gene expression. A deeper understanding of these issues should help explain why glutamine pervades so many aspects of cell biology, and should support future efforts to manipulate glutamine metabolism in cancer and other diseases.
Acknowledgements
The authors thank Roland Knoblauch and Andrew Mullen for critically reading the manuscript. RJD is supported by grants from the NIH (DK072565) and the American Cancer Society (ACS-IRG-02-196).
Support: NIDDK (DK072565) and American Cancer Society (ACS-IRG-02-196)
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- Abcouwer SF, Schwarz C, Meguid RA. Glutamine deprivation induces the expression of GADD45 and GADD153 primarily by mRNA stabilization. J Biol Chem. 1999;274:28645–51. doi: 10.1074/jbc.274.40.28645. [DOI] [PubMed] [Google Scholar]
- Aiken KJ, Bickford JS, Kilberg MS, Nick HS. Metabolic regulation of manganese superoxide dismutase expression via essential amino acid deprivation. J Biol Chem. 2008;283:10252–63. doi: 10.1074/jbc.M709944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcella A, Carpinelli G, Battaglia G, D'Onofrio M, Santoro F, Ngomba RT, et al. Pharmacological blockade of group II metabotropic glutamate receptors reduces the growth of glioma cells in vivo. Neuro Oncol. 2005;7:236–45. doi: 10.1215/S1152851704000961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardawi MS, Majzoub MF, Masoud IM, Newsholme EA. Enzymic and metabolic adaptations in the gastrocnemius, plantaris and soleus muscles of hypocaloric rats. Biochem J. 1989;261:219–25. doi: 10.1042/bj2610219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Kiens B, Richter EA. Ammonia uptake in inactive muscles during exercise in humans. Am J Physiol. 1996;270:E101–6. doi: 10.1152/ajpendo.1996.270.1.E101. [DOI] [PubMed] [Google Scholar]
- Bergstrom J, Furst P, Noree LO, Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974;36:693–7. doi: 10.1152/jappl.1974.36.6.693. [DOI] [PubMed] [Google Scholar]
- Bobrovnikova-Marjon EV, Marjon PL, Barbash O, Vander Jagt DL, Abcouwer SF. Expression of angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 is highly responsive to ambient glutamine availability: role of nuclear factor-kappaB and activating protein-1. Cancer Res. 2004;64:4858–69. doi: 10.1158/0008-5472.CAN-04-0682. [DOI] [PubMed] [Google Scholar]
- Brand K. Glutamine and glucose metabolism during thymocyte proliferation. Pathways of glutamine and glutamate metabolism. Biochem J. 1985;228:353–61. doi: 10.1042/bj2280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasse-Lagnel C, Lavoinne A, Husson A. Control of mammalian gene expression by amino acids, especially glutamine. FEBS J. 2009;276:1826–44. doi: 10.1111/j.1742-4658.2009.06920.x. [DOI] [PubMed] [Google Scholar]
- Chen MK, Espat NJ, Bland KI, Copeland EM, 3rd, Souba WW. Influence of progressive tumor growth on glutamine metabolism in skeletal muscle and kidney. Ann Surg. 1993;217:655–66. doi: 10.1097/00000658-199306000-00007. discussion 666-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ. Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med. 2008;10:767–77. doi: 10.1097/GIM.0b013e31818b0d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008a;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Thompson CB. Phosphatidylinositol 3-kinase-dependent modulation of carnitine palmitoyltransferase 1A expression regulates lipid metabolism during hematopoietic cell growth. J Biol Chem. 2006;281:37372–80. doi: 10.1074/jbc.M608372200. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–50. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008b;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadio AC, Lobo C, Tosina M, de la Rosa V, Martin-Rufian M, Campos-Sandoval JA, et al. Antisense glutaminase inhibition modifies the O-GlcNAc pattern and flux through the hexosamine pathway in breast cancer cells. J Cell Biochem. 2008;103:800–11. doi: 10.1002/jcb.21449. [DOI] [PubMed] [Google Scholar]
- Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122:501–14. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- Eliasen MM, Winkler W, Jordan V, Pokar M, Marchetti M, Roth E, et al. Adaptive cellular mechanisms in response to glutamine-starvation. Front Biosci. 2006;11:3199–211. doi: 10.2741/2043. [DOI] [PubMed] [Google Scholar]
- Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–9. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- Erbil Y, Oztezcan S, Giris M, Barbaros U, Olgac V, Bilge H, et al. The effect of glutamine on radiation-induced organ damage. Life Sci. 2005;78:376–82. doi: 10.1016/j.lfs.2005.04.068. [DOI] [PubMed] [Google Scholar]
- Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43:143–81. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- Flier JS, Mueckler MM, Usher P, Lodish HF. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987;235:1492–5. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- Gaglio D, Soldati C, Vanoni M, Alberghina L, Chiaradonna F. Glutamine deprivation induces abortive s-phase rescued by deoxyribonucleotides in k-ras transformed fibroblasts. PLoS ONE. 2009;4:e4715. doi: 10.1371/journal.pone.0004715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009 doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunitz F, Heise K, Schumann R, Gebhardt R. Glucocorticoid induced expression of glutamine synthetase in hepatoma cells. Biochem Biophys Res Commun. 2002;296:1026–32. doi: 10.1016/s0006-291x(02)02044-2. [DOI] [PubMed] [Google Scholar]
- Guerin PJ, Furtak T, Eng K, Gauthier ER. Oxidative stress is not required for the induction of apoptosis upon glutamine starvation of Sp2/0-Ag14 hybridoma cells. Eur J Cell Biol. 2006;85:355–65. doi: 10.1016/j.ejcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hiatt HH. Glycolytic activity in vivo of the mouse Ehrlich ascites tumor. Cancer Res. 1957;17:240–4. [PubMed] [Google Scholar]
- Holroyde CP, Skutches CL, Boden G, Reichard GA. Glucose metabolism in cachectic patients with colorectal cancer. Cancer Res. 1984;44:5910–3. [PubMed] [Google Scholar]
- Huang Q, Lau SS, Monks TJ. Induction of gadd153 mRNA by nutrient deprivation is overcome by glutamine. Biochem J. 1999;341(Pt 1):225–31. [PMC free article] [PubMed] [Google Scholar]
- Hubert-Buron A, Leblond J, Jacquot A, Ducrotte P, Dechelotte P, Coeffier M. Glutamine pretreatment reduces IL-8 production in human intestinal epithelial cells by limiting IkappaBalpha ubiquitination. J Nutr. 2006;136:1461–5. doi: 10.1093/jn/136.6.1461. [DOI] [PubMed] [Google Scholar]
- Kandil HM, Argenzio RA, Chen W, Berschneider HM, Stiles AD, Westwick JK, et al. L-glutamine and L-asparagine stimulate ODC activity and proliferation in a porcine jejunal enterocyte line. Am J Physiol. 1995;269:G591–9. doi: 10.1152/ajpgi.1995.269.4.G591. [DOI] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–85. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Klimberg VS, Souba WW, Salloum RM, Plumley DA, Cohen FS, Dolson DJ, et al. Glutamine-enriched diets support muscle glutamine metabolism without stimulating tumor growth. J Surg Res. 1990;48:319–23. doi: 10.1016/0022-4804(90)90066-b. [DOI] [PubMed] [Google Scholar]
- Knox WE, Horowitz ML, Friedell GH. The proportionality of glutaminase content to growth rate and morphology of rat neoplasms. Cancer Res. 1969;29:669–80. [PubMed] [Google Scholar]
- Kobayashi S, Millhorn DE. Hypoxia regulates glutamate metabolism and membrane transport in rat PC12 cells. J Neurochem. 2001;76:1935–48. doi: 10.1046/j.1471-4159.2001.00214.x. [DOI] [PubMed] [Google Scholar]
- Kovacevic Z, McGivan JD. Mitochondrial metabolism of glutamine and glutamate and its physiological significance. Physiol Rev. 1983;63:547–605. doi: 10.1152/physrev.1983.63.2.547. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles’ heel. Cancer Cell. 2008;13:472–82. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Kuhn KS, Schuhmann K, Stehle P, Darmaun D, Furst P. Determination of glutamine in muscle protein facilitates accurate assessment of proteolysis and de novo synthesis-derived endogenous glutamine production. Am J Clin Nutr. 1999;70:484–9. doi: 10.1093/ajcn/70.4.484. [DOI] [PubMed] [Google Scholar]
- Kvamme E, Svenneby G. Effect of anaerobiosis and addition of keto acids on glutamine utilization by Ehrlich ascites-tumor cells. Biochim Biophys Acta. 1960;42:187–8. doi: 10.1016/0006-3002(60)90779-4. [DOI] [PubMed] [Google Scholar]
- Larson SD, Li J, Chung DH, Evers BM. Molecular mechanisms contributing to glutamine-mediated intestinal cell survival. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1262–71. doi: 10.1152/ajpgi.00254.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder-Horowitz M, Knox WE, Morris HP. Glutaminase activities and growth rates of rat hepatomas. Cancer Res. 1969;29:1195–9. [PubMed] [Google Scholar]
- Lo M, Wang YZ, Gout PW. The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- Lobo C, Ruiz-Bellido MA, Aledo JC, Marquez J, Nunez De Castro I, Alonso FJ. Inhibition of glutaminase expression by antisense mRNA decreases growth and tumourigenicity of tumour cells. Biochem J. 2000;348(Pt 2):257–61. [PMC free article] [PubMed] [Google Scholar]
- Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, et al. p53 regulates mitochondrial respiration. Science. 2006;312:1650–3. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- Matsuno T, Satoh T. Glutamine metabolism in the avian host bearing transplantable hepatomatous growth induced by MC-29 virus. Int J Biochem. 1986;18:187–9. doi: 10.1016/0020-711x(86)90155-2. [DOI] [PubMed] [Google Scholar]
- Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–8. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Medina MA, Sanchez-Jimenez F, Marquez J, Rodriguez Quesada A, Nunez de Castro I. Relevance of glutamine metabolism to tumor cell growth. Mol Cell Biochem. 1992;113:1–15. doi: 10.1007/BF00230880. [DOI] [PubMed] [Google Scholar]
- Miller AL. Therapeutic considerations of L-glutamine: a review of the literature. Altern Med Rev. 1999;4:239–48. [PubMed] [Google Scholar]
- NCI Website 2009 http://www.cancer.gov/cancertopics/pdq/supportivecare/nutrition/
- Newsholme EA, Crabtree B, Ardawi MS. Glutamine metabolism in lymphocytes: its biochemical, physiological and clinical importance. Q J Exp Physiol. 1985;70:473–89. doi: 10.1113/expphysiol.1985.sp002935. [DOI] [PubMed] [Google Scholar]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–34. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F, Arcella A, Iacovelli L, Battaglia G, Giangaspero F, Melchiorri D. Metabotropic glutamate receptors: new targets for the control of tumor growth? Trends Pharmacol Sci. 2007;28:206–13. doi: 10.1016/j.tips.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Ohtani Y, Harada T, Funasaka Y, Nakao K, Takahara C, Abdel-Daim M, et al. Metabotropic glutamate receptor subtype-1 is essential for in vivo growth of melanoma. Oncogene. 2008;27:7162–70. doi: 10.1038/onc.2008.329. [DOI] [PubMed] [Google Scholar]
- Olde Damink SW, Jalan R, Dejong CH. Interorgan ammonia trafficking in liver disease. Metab Brain Dis. 2009;24:169–81. doi: 10.1007/s11011-008-9122-5. [DOI] [PubMed] [Google Scholar]
- Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000;275:21797–800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–97. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Parry-Billings M, Leighton B, Dimitriadis GD, Curi R, Bond J, Bevan S, et al. The effect of tumour bearing on skeletal muscle glutamine metabolism. Int J Biochem. 1991;23:933–7. doi: 10.1016/0020-711x(91)90082-x. [DOI] [PubMed] [Google Scholar]
- Pollock PM, Cohen-Solal K, Sood R, Namkoong J, Martino JJ, Koganti A, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–12. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- Quesada AR, Medina MA, Marquez J, Sanchez-Jimenez FM, Nunez de Castro I. Contribution by host tissues to circulating glutamine in mice inoculated with Ehrlich ascites tumor cells. Cancer Res. 1988;48:1551–3. [PubMed] [Google Scholar]
- Ramanathan A, Wang C, Schreiber SL. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc Natl Acad Sci U S A. 2005;102:5992–7. doi: 10.1073/pnas.0502267102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard GAMN, Hochella NJ, Patterson AL, Weinhouse S. Quantitative estimation of teh Cori cycle in the human. J Biol. Chem. 1963;238:498–501. [Google Scholar]
- Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–76. [PubMed] [Google Scholar]
- Rhoads JM, Argenzio RA, Chen W, Rippe RA, Westwick JK, Cox AD, et al. L-glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am J Physiol. 1997;272:G943–53. doi: 10.1152/ajpgi.1997.272.5.G943. [DOI] [PubMed] [Google Scholar]
- Roth E. Immune and cell modulation by amino acids. Clin Nutr. 2007;26:535–44. doi: 10.1016/j.clnu.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Sauer LA, Dauchy RT. Ketone body, glucose, lactic acid, and amino acid utilization by tumors in vivo in fasted rats. Cancer Res. 1983;43:3497–503. [PubMed] [Google Scholar]
- Sauer LA, Stayman JW, 3rd, Dauchy RT. Amino acid, glucose, and lactic acid utilization in vivo by rat tumors. Cancer Res. 1982;42:4090–7. [PubMed] [Google Scholar]
- Segura JA, Donadio AC, Lobo C, Mates JM, Marquez J, Alonso FJ. Inhibition of glutaminase expression increases Sp1 phosphorylation and Sp1/Sp3 transcriptional activity in Ehrlich tumor cells. Cancer Lett. 2005;218:91–8. doi: 10.1016/j.canlet.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A. 1997;94:6658–63. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerry TM, Genever PG. Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci. 2001;22:174–81. doi: 10.1016/s0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- Soh H, Wasa M, Fukuzawa M. Hypoxia upregulates amino acid transport in a human neuroblastoma cell line. J Pediatr Surg. 2007;42:608–12. doi: 10.1016/j.jpedsurg.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–42. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souba WW. Glutamine and cancer. Ann Surg. 1993;218:715–28. doi: 10.1097/00000658-199312000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7:1010–5. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ. Molecular pathways leading to cancer cachexia. Physiology (Bethesda) 2005;20:340–8. doi: 10.1152/physiol.00019.2005. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- Wang T, Marquardt C, Foker J. Aerobic glycolysis during lymphocyte proliferation. Nature. 1976;261:702–5. doi: 10.1038/261702a0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Watford M. Glutamine, insulin and glucocorticoids regulate glutamine synthetase expression in C2C12 myotubes, Hep G2 hepatoma cells and 3T3 L1 adipocytes. Biochim Biophys Acta. 2007;1770:594–600. doi: 10.1016/j.bbagen.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. Uber den stoffwechsel der carcinomzelle. Klin. Wochenschr. 1925;4:534–536. [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–70. [PubMed] [Google Scholar]
- Watatani Y, Kimura N, Shimizu YI, Akiyama I, Tonaki D, Hirose H, et al. Amino acid limitation induces expression of ATF5 mRNA at the post-transcriptional level. Life Sci. 2007;80:879–85. doi: 10.1016/j.lfs.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol. 2007;69:317–40. doi: 10.1146/annurev.physiol.69.040705.142215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–7. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo JH, Lo JC, Nissom PM, Wong VV. Glutamine or glucose starvation in hybridoma cultures induces death receptor and mitochondrial apoptotic pathways. Biotechnol Lett. 2006;28:1445–52. doi: 10.1007/s10529-006-9110-y. [DOI] [PubMed] [Google Scholar]
- Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu XL, Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun. 2004;313:459–65. doi: 10.1016/j.bbrc.2003.11.136. [DOI] [PubMed] [Google Scholar]