SUMMARY
Doxorubicin (DOX) is one of the most widely used and successful antitumor drugs, but its cumulative and dose-dependent cardiac toxicity has been the major concern of oncologists in cancer therapeutic practice for decades. With the increasing population of cancer survivals, there is a growing need to develop preventive strategies and effective therapies against DOX-induced cardiotoxicity, in particular, the late onset cardiomyopathy. Although intensive investigations on the DOX-induced cardiotoxicity have been continued for decades, the underlying mechanisms responsible for DOX-induced cardiotoxicity have not been completely elucidated. A rapidly expanding body of evidence supports that cardiomyocyte death by apoptosis and necrosis is a primary mechanism of DOX-induced cardiomyopathy and other types of cell death, such as autophagy and senescence/aging, may participate in this process. In this review, we will focus on the current understanding of molecular mechanisms underlying DOX-induced cardiomyocyte death, including the major primary mechanism of excess production of reactive oxygen species (ROS) and other recently discovered ROS-independent mechanisms. Different sensitivity to DOX-induced cell death signals between adult and young cardiomyocytes will also be discussed.
Keywords: cardiomyocyte, doxorubicin, apoptosis, necrosis, autophagy
INTRODUCTION
The anthracyclines, primarily doxorubicin (DOX), but also including daunomycin, epirubicin and idarubicin, are among the most widely used and successful antitumor drugs. Cardiotoxicity is a major limiting factor in anticancer therapy (Singal and Iliskovic 1998; Yeh et al. 2004). DOX-induced cardiotoxicity may present as either acute or chronic cardiomyopathy. Acute cardiotoxicity is now rare, occurring after receiving high dose, and may present as acute tachyarrhythmias and acute heart failure while the chronic DOX-induced cardiac toxicity is dose-dependent. In this case, the patient may develop dilated cardiomyopathy many years after receiving the last doxorubicin treatment. Both acute and chronic DOX-induced cardiac toxicity may lead to cardiac dysfunction, cardiomyopathy, and eventually to severe heart failure and death (Wallace 2003; Yeh et al. 2004). Children and adolescents are particularly susceptible to the cardiotoxic effects of anthracycline chemotherapy, and there is no safe dose of anthracyclines in this population (Lipshultz et al. 1991; Von Hoff et al. 1977). About half of the young adult survivors of childhood cancer have received anthracyclines at some point in their treatment. Hence, the development of novel therapeutic strategies to improve the survivor outcome is important, particularly in children as a large number of pediatric cancer survivors are now expected to live in a cancer-free status for decades after cancer therapy.
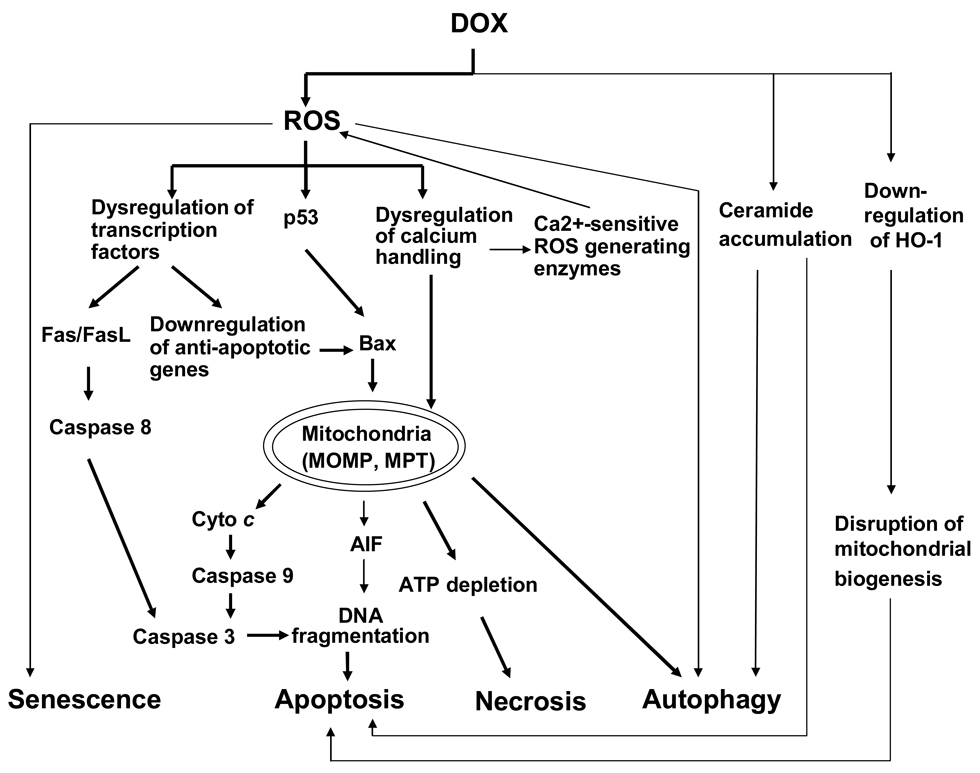
Intensive investigations on the DOX-induced cardiotoxicity have been continued for decades. The different lines of evidence have provided putative mechanisms, but the precise mechanism underlying DOX-induced cardiotoxicity is not completely elucidated. Most studies favor the free radical-induced oxidative stress playing a pivotal role, as it can be interpreted by the chemical structure of DOX possessing a tendency to generate reactive oxygen species (ROS) during drug metabolism (Iarussi et al. 2001; Neilan et al. 2007; Wallace 2003). Recent findings indicate that endothelial nitric oxide synthase (eNOS) reductase domain converts DOX to an unstable semiquinone intermediate that favors ROS generation (Neilan et al. 2007). Also, mitochondrial DNA lesions induced by ROS or directly by DOX further lead to respiratory chain failure and ROS liberation (Lebrecht and Walker 2007). Other contributors to DOX-induced cardiotoxicity include dysregulation of calcium handling, adrenergic dysfunction, and selective inhibition of cardiomyocyte-specific genes expression (Iarussi et al. 2001; Takemura and Fujiwara 2007). Most of these cellular events contribute to cardiomyocyte death, which is a primary mechanism for DOX-induced cardiomyopathy. Cell death is classified by the morphology of the affected cells: apoptosis, necrosis and autophagy. Most in vitro and in vivo studies during the past several decades have suggested that DOX-induced cardiac toxicity are associated with cardiomyocyte apoptosis and necrosis, other forms of cell death can also be related. In this review we focus on recent new findings on the possible mechanisms underlying DOX-induced cardiomyocyte death (Figure 1). Differences in sensitivity to DOX-induced cell death signals between adult and young cardiomyocytes will also be discussed.
Figure 1.
Potential signaling pathways involved in DOX-induced cardiomyocyte death, as described in the text. Mechanisms of cell death include apoptosis, necrosis, autophagy and senescence. Crosstalk between these different types of cardiomyocyte death may occur at multiple levels. Thick lines represent major mechanisms and thin lines represent alternative pathways.
APOPTOSIS
Apoptosis is a highly conserved, tightly regulated, and energy-dependent active form of cell death. It is crucial for normal development and cell homeostasis. The typical morphological changes are cell shrinkage, DNA fragmentation, chromatin condensation, and package of the cell into a form called “apoptotic body” that allows for its removal by phagocytosis. Apoptosis starts from two canonical signaling pathways, including extrinsic and intrinsic pathways. In the extrinsic pathway, the binding of death ligands (FasL, TNFα, TRAIL) with their receptors induces recruitment and activation of caspase 8, which subsequently activates downstream effector caspases such as caspase 3. The intrinsic pathway is mediated by mitochondrial cytochrome c release. This process is regulated by the members of the Bcl-2 family, which includes three groups: anti-apoptotic members Bcl-2, Bcl-XL, and Mcl-1, pro-apoptotic members Bax and Bak, and BH3 only proteins such as Bad, Bid, Nix and BNip3 that enhance apoptosis via inhibition of anti-apoptotic Bcl-2 proteins or activation of pro-apoptotic Bax and Bak (Shi and Wei 2007). Activation of BH3-only proteins by stress stimuli promotes Bax/Bak translocation from the cytosol to the outer membrane of mitochondria, resulting in increased mitochondrial outer membrane permeabilization, leading to protein release from the intermembrane space to the cytoplasm, including apoptogenic molecule cytochrome c. In the cytosol, cytochrome c forms a complex with the adaptor protein apoptosis protease activator protein-1 (Apaf-1), dATP, and caspase 9. The result is the formation of a structure known as the apoptosome, which in turn activates caspase 9. Both extrinsic and intrinsic apoptotic pathways converge on the activation of downstream executioners, caspases 3, 6, and 7.
These two apoptotic signaling pathways are evolutionally conserved, but the precise molecular events involved in the regulation of caspase enzymatic cascades are often specific to cell type and death stimulus. The mechanism of DOX-induced cardiomyocyte apoptosis has been extensively studied in both acute and chronic cardiotoxicity (Arola et al. 2000; Bennink et al. 2004; Fisher et al. 2005; Kawamura et al. 2004; Kotamraju et al. 2000; Wang et al. 2001). These studies have shown that multiple pathways are involved (Table 1). It is worth noting that the studies of DOX-induced cardiotoxicity utilized a wide variety of treatments (i.e., single vs. multiple doses, differences in total dose, differences in timing of assays, etc.). The underlying mechanisms reported in these studies may vary from experimental conditions, species differences, in vitro versus in vivo studies, and so on.
Table 1.
Potential mechanisms and therapeutic targets involved in DOX-induced cardiomyocyte apoptosis
| Mechanisms | Cell type / Animal | References |
|---|---|---|
| Increase in [Ca2+]i,mitochondrial dysfunction | Adult rat cardiomyocytes,mouse, rat | Childs et al. 2002; Kim et al. 2006; Kluza et al. 2004 |
| Dysregulation of apoptosis-related proteins: p53, Akt, ERKs,Bcl-2 family, etc | H9c2, mouse,rat cardiomyocytes | Fan et al. 2008; Kawamura et al. 2004; Liu et al. 2008; Zhu et al. 2009 |
| Dysregulation of transcriptionfactors/coactivators: GATA-4,CARP, NF-κB, NFAT, p300, etc | H9c2, mouse, rat,neonatal rat cardiomyocytes | Aihara et al. 2000; Aries et al. 2004; Jeyaseelan et al. 1997; Kalivendi et al. 2005; Kim et al. 2003; Kim et al. 2007; Li et al. 2007; Wang et al. 2002 |
| Caspase 12 mediated SR-apoptotic pathway | Rat | Jang et al. 2004 |
| Heme oxygenase-1 down-regulation | H9c2, mouse | Bernuzzi et al. 2009; Piantadosi et al. 2008; Suliman et al. 2007 |
| Ceramide accumulation | Neonatal rat cardiomyocytes | Armstrong 2004; Parra et al. 2008 |
| Reduced ARC expression | Neonatal rat cardiomyocytes | An et al. 2009 |
| Toll-like receptor-2 | Mouse | Nozaki et al. 2004 |
| eNOS (NOS3) | Mouse | Neilan et al. 2007 |
Intrinsic apoptotic pathway
DOX treatment increases oxidative stress and disrupts cytosolic calcium homeostasis. ROS increases intracellular calcium levels by promoting the release of calcium from the sarcoplasmic reticulum (SR) via opening of the ryanodine receptor and by impairing calcium clearance systems in cardiomyocytes (Camello-Almaraz et al. 2006; Gen et al. 2001; Kim et al. 2006; Zeng et al. 2008; Zima and Blatter 2006). The increased intracellular calcium in turn induces ROS production through calcium-sensitive ROS generating enzymes (Kim et al. 2006). In cardiomyocytes, the mitochondria are located near calcium-release sites on the SR and can capture a large quantity of the released calcium. Due to the significantly raised oxidative stress, mitochondrial calcium level increases beyond a threshold. This mitochondrial calcium overload triggers mitochondrial permeability transition (MPT), resulting in a loss of mitochondrial membrane potential, mitochondrial swelling, and outer membrane rupture, and consequently, release of cytochrome c and apoptosis inducing factor (AIF) from mitochondria (Camello-Almaraz et al. 2006; Childs et al. 2002; Deniaud et al. 2008).
Numerous studies have shown that DOX-induced cardiomyocyte apoptosis is associated with increased expression and activation of p53 tumor suppressor protein (L'Ecuyer et al. 2006; Liu et al. 2004; Liu et al. 2008). DNA lesions induced by ROS or directly by DOX activated ERK1/2, followed by increased phosphorylation of p53, the latter further up-regulated p53 downstream genes such as Bax. As a result, the intrinsic apoptosis pathway was activated. Pifithrin-α, an inhibitor of p53, did attenuate the increased protein levels of Bax and effectively inhibited DOX-induced apoptosis in H9c2 cells, neonatal rat cardiomyocytes, and mouse hearts (Liu et al. 2004; Liu et al. 2008). Inhibition of DOX-induced cardiomyocyte apoptosis was also observed in p53 knockout mice (Shizukuda et al. 2005) and in adult mouse hearts expressing cardiomyocyte-restricted dominant-interfering p53 (Zhu et al. 2009). P53 may also mediate DOX-induced cardiotoxicity through other pathways independent of cardiomyocyte apoptosis. For example, p53-mediated inhibition of mammalian target of rapamycin signaling may contribute to cardiac mass reduction and dysfunction observed in acute doxorubicin cardiotoxicity (Zhu et al. 2009).
In cardiomyocytes, transcriptional factor GATA-4, a critical regulator in heart development, has been shown to be a pivotal survival factor for the postnatal period. GATA-4 transcriptionally regulates the apoptotic pathway via activating the anti-apoptotic gene Bcl-XL, thus preserving mitochondrial function and integrity. An early event observed in the DOX cardiotoxicity was GATA-4 depletion, which sequentially caused cardiomyocyte apoptosis (Aries et al. 2004; Kim et al. 2003). DOX-induced inhibition of Akt phosphorylation was suggested to be involved in the underlying mechanism, which increased active GSK3β, a negative regulator of GATA-4 in the nucleus (Suliman et al. 2007). Moreover, the cardiac p300 mRNA, a transcriptional coactivator required for the maintenance of the differentiated phenotype of cardiac myocytes, was depleted in mouse hearts after DOX treatment, but the overexpression of p300 protein in cardiomyocytes could prevent DOX-induced apoptosis and cardiac dysfunction. It was believed to be due to the up-regulation of Bcl-2 and Mdm2 (Kawamura et al. 2004). Other studies reported that DOX caused p38 MAPK activation and p300 degradation via hyperphosphorylation (Lou et al. 2005; Poizat et al. 2005).
Apoptosis Repressor with a caspase recruitment domain (ARC) is an endogenous inhibitor of apoptosis and is restricted primarily to terminally differentiated cells such as skeletal myocytes, neurons and cardiomyocytes. It disables apoptotic pathways through preventing Bax translocation to the mitochondrion or binding to components of the extrinsic pathway such as Fas, FADD, and caspase 8 to prevent the formation of death inducing signaling complex (DISC) (Mercier et al. 2005). Down-regulation of ARC mRNA and protein expression levels was observed in neonatal rat cardiomyocytes and mouse hearts upon DOX exposure. P53-dependent transcriptional down-regulation or p53-induced ubiquitin E3 ligase Mdm2 may be linked to the ARC decrease. In contrast, enforced ARC expression markedly attenuated the DOX-induced cardiomyocyte apoptosis, prevented activation of the mitochondrial death pathway, and subsequent cardiomyocyte death (An et al. 2009).
In addition, a recent study has shown that DOX-induced up-regulation of Ser/Thr phosphatase PP1 may be involved in dephosphorylation of Akt and Bad, resulting in caspase 3 activation (Fan et al. 2008). DOX-induced ceramide generation may also contribute to cardiomyocyte apoptosis through mitochondrial fragmentation, mitochondrial outer membrane permeabilization, and cytochrome c release (Armstrong 2004; Parra et al. 2008).
Extrinsic apoptotic pathway
Although cardiomyocytes are usually resistant to Fas-induced apoptosis, studies indicate that cardiomyocyte apoptosis in DOX-induced cardiomyopathy can be executed through a Fas-mediated pathway (Nakamura et al. 2000). Cardiac-targeted expression of soluble Fas (sFas), a competitive inhibitor of FasL, could attenuate DOX-induced cardiotoxicity partly by inhibiting cardiomyocyte apoptosis and reducing ROS and peroxynitrite formation in mice (Niu et al. 2009). Other studies showed that DOX treatment of rat cardiomyocytes increased mitochondrial ROS production, activated the calcium/calcineurin signaling pathway, and further activated nuclear factor-activated T cell-4 (NFAT4), leading to up-regulation of Fas/FasL (Kalivendi et al. 2005). Interestingly, NFAT5, a novel member of NFAT family, was degraded by proteolysis in cultured rat neonatal cardiomyocytes after DOX exposure. As the result, the neonatal cardiomyocytes became more susceptible to cell damage (Ito et al. 2007). Transcription factor NF-κB was activated by ROS in DOX-treated neonatal rat cardiomyocytes and myocardium and exerted a pro-apoptotic effect via direct activation of apoptotic genes, including FasL, Fas, c-Myc and p53 (Kim et al. 2007; Li et al. 2007; Wang et al. 2002). ROS down-regulated expression of FLIP, a FLICE/caspase-8 inhibitory protein, and thereby at least in part, sensitized Fas-mediated apoptosis (Nitobe et al. 2003). In addition, an innate immune system has been implicated in the regulation of apoptotic pathway. Studies reported that Toll-like receptor-2 (TLR-2) functions as a novel “death receptor” that employs the apoptotic apparatus such as FADD and caspase 8 without a conventional cytoplasmic death domain (Aliprantis et al. 2000). In a study, fewer TUNEL-positive nuclei and less caspase-3 activity in myocardium were observed in TLR-2-knockout mice than that in wild type mice after DOX treatment. This could partly involve the inhibition of NF-κB activation and reduction of proinflammatory cytokine (e.g. TNF-α) in TLR-2-knockout mice (Nozaki et al. 2004).
Other mechanisms
Endoplasmic/sarcoplasmic reticulum (ER/SR)-mediated apoptotic pathway was reported to mediate cardiac apoptosis induced by DOX (Jang et al. 2004). Caspase-12, an essential caspase to initiate SR-mediated apoptosis and is located in the SR, was activated by calpain in DOX-treated rat hearts. As shown by recent studies, heme oxygenase-1 (HO-1) expression was down-modulated in H9c2 cells exposed to DOX (Bernuzzi et al. 2009), and other in vivo studies indicate that the HO-1/Akt/Nrf2 pathway mediates cardiac mitochondrial biogenesis and down-regulation of HO-1 by DOX disrupts cardiac mitochondrial biogenesis, which promotes intrinsic apoptosis (Piantadosi et al. 2008; Suliman et al. 2007). Other potential mechanisms involved in DOX-induced cardiomyocyte apoptosis include: dysregulaiton of a phosphodiesterase 3A/inducible cAMP early repressor feedback loop (Yan et al. 2007), activation of the endocannabinoid system (Mukhopadhyay et al. 2007), activation of volume-sensitive chloride channels and subsequent apoptotic volume decrease (D'Anglemont de Tassigny et al. 2004), oxidative stress induced up-regulation of lectin-like oxidized LDL receptor-1 (LOX-1) (Spallarossa et al. 2005).
NECROSIS
Necrosis is typically described as early rupture of the plasma membrane and swelling of cytoplasmic organelles, in particular the mitochondria, and is often described as an uncontrolled, energy-independent process. However, recent studies have shown that necrotic cell death can be well controlled and programmed (Diwan et al. 2009; Dorn 2009). Numerous studies showed that cardiac expression of proinflammatory cytokine, inflammatory cell infiltration, and necrosis are increased in DOX treated mouse hearts (Ikegami et al. 2007; Li et al. 2006; Riad et al. 2009). Oxidative stress is implicated in necrotic cardiomyocyte death. The use of free radical scavengers protected cardiomyocytes from anthracycline induced necrosis (Ikegami et al. 2007). The rationale is that increased ROS leads to mitochondrial calcium overloading, promotes MPT pore opening, causes mitochondrial swelling and ATP depletion, and hence triggers necrotic cell death (Dorn 2009; Gustafsson and Gottlieb 2008). For that reason, disturbance of mitochondrial calcium homeostasis may exert a critical factor in the accumulative and irreversible cardiomyopathy associated with long-term DOX treatment. DOX also induces mitochondrial DNA damage, mitochondrial respiration mutilation, mitochondrial dysfunction, and ATP depletion. All these events contribute to necrosis (Lebrecht and Walker 2007; Solem et al. 1996; Wallace 2003; Wallace 2007; Zhou et al. 2001). In addition, ROS-induced lipid peroxidation may also contribute to cardiomyocyte necrosis (Casey et al. 2007). Furthermore, degradation of titin, the largest myofilament protein, was enhanced in the early stage of DOX treatment by activation of the calcium-dependent proteases calpains, which may represent an important proximal step that leads to accelerated myofilament degradation and necrosis (Lim et al. 2004).
AUTOPHAGY
Autophagy, first described in the 1960s in mammalian cells, is a highly regulated dynamic process involving cytosolic proteins and organelle degradation through engulfment into double-membraned vesicles called autophagosomes, which then fuse with lysosomes and subsequently degrade the contents. Autophagy plays important roles in cell growth and development, organelle biogenesis and turnover, and in controlling the precise balance between protein synthesis and degradation. Many excellent reviews have discussed some aspects of the molecular mechanism of autophagy (Rubinsztein et al. 2005; Schmid and Munz 2007; Tsujimoto and Shimizu 2005; Yorimitsu and Klionsky 2005). Autophagy normally occurs in the myocardium, represents the most prevalent renewal mechanism of cellular constituents, and is substantially enhanced in pathological conditions, including cardiac hypertrophy, cardiomyopathy, and heart failure. Studies indicate that autophagy serves as a double-edged sword in the heart under stress; on one hand, it functions by removing protein aggregates and damaged organelles as a pro-survival pathway maintaining energy homeostasis, while on the other hand, intense enhancement of autophagy can lead to cell death (De Meyer and Martinet 2008; Gustafsson and Gottlieb 2009; Matsui et al. 2008; Rothermel and Hill 2008; Shimomura et al. 2001; Terman and Brunk 2005).
Crosstalk among the autophagic apoptotic and necrotic pathways has been frequently reported. Bcl-2 family has been implicated in the crosstalk between apoptosis and autophagy (Hoyer-Hansen et al. 2007; Levine et al. 2008; Maiuri et al. 2007; Nishida et al. 2008; Shimizu et al. 2004; Tsujimoto and Shimizu 2005). ROS-induced increase in intracellular calcium not only triggers apoptosis and necrosis, but also induces autophagy by activation of calmodulin-dependent kinase kinase and AMP-activated protein kinase (Hoyer-Hansen et al. 2007). Other apoptosis-related proteins such as p53 have also been shown to play a role in autophagy (Maiuri et al. 2007). Another recent study suggests that activation of poly (ADP-ribose) polymerase 1 (PARP-1), one of the major nuclear targets of caspases, is involved in autophagy which might be cytoprotective during the response to DNA damage (Munoz-Gamez et al. 2009).
Mitochondria function as the crossroads for autophagic, apoptotic, and necrotic pathways. Under conditions with mild stress, autophagy is induced to degrade and recycle cytoplasmic components. With increasing stresses, apoptosis begins to occur because of cytochrome c release from mitochondria. Under extreme stress, the mitochondrial permeability transition occurs in all mitochondria, the intracellular supply of ATP is exhausted, and necrosis occurs because of ATP depletion. Excessive autophagy induced by severe stimuli can also damage cytosol and organelles, especially mitochondria and ER, and release lysosomal enzymes or other cell death-inducing factors, thereby leading to apoptotic and necrotic cell death (Nishida et al. 2008; Nishida et al. 2009).
Three types of cell death may converge in dying cells on many levels of different pathways, such as oxidative stress, dysregulation of calcium homeostasis, mitochondrial damage, DNA damage, and induction of pro-apoptotic proteins. All of these occur in DOX-induced cardiotoxicity. Although no evidence of autophagy in DOX-induced cardiotoxocity has been reported so far, it is possible that DOX is able to induce cardiomyocyte autophagy, which might be protective or detrimental depending on the stress levels, in particular the dosage of DOX.
SENESCENCE (AGING)
Senescence, which is characterized by progressive accumulation of macromolecular damage, growth arrest of normal somatic cells, and reduction in function, mainly affects long-lived postmitotic cells such as neurons and cardiac myocytes. It is a risk factor for cardiac dysfunction and heart diseases (Terman et al. 2006). The molecular and cellular pathways controlling senescence include telomere shortening, accumulation of DNA and chromosomal damage, as well as the expression of cell cycle inhibitors p16INK4a and p53 (Bergmann et al. 2008; Kajstura et al. 2006). The known factors involved in senescence of cardiomyocytes include oxidative stress, altered gene expression/mutations, inflammation, reduced cellular protection and repair, altered cellular metabolism, altered protein degradation machinery and autophagy machinery, and others (Bernhard and Laufer 2008). Cardiomyocyte senescence may play a role in DOX-induced latent myocardial toxicity many years after the last treatment. A recent study showed that cultured neonatal rat cardiomyocytes treated with DOX exhibited characteristic changes similar to cardiomyocytes of aged rats. These changes included increased positive staining for senescence-associated β-galactosidase and cell cycle inhibitor expression and decreased cardiac troponin I phosphorylation and telomerase activity. Oxidative stress and p53 acetylation might be involved in this process (Maejima et al. 2008).
DIFFERENT SENSITIVITY TO DOX-INDUCED CARDIOTOXICITY BETWEEN YOUNG, ADULT AND OLD HEARTS
As suggested by clinical studies, children and adolescents are particularly susceptible to the cardiotoxic effects of anthracycline chemotherapy (Von Hoff et al. 1977). The possible rationale was due to the loss of myocytes and impaired cardiac growth resulting in inadequate left ventricular mass and cardiomyopathy a year or more after cessation of chemotherapy (Lipshultz et al. 1991). Cardiomyocyte atrophy and myofiber disarray may also contribute to cardiac dysfunction observed in DOX treated juvenile mice (Zhu et al., 2008). Another effect of age is the alteration of doxorubicin pharmacokinetics in the old age group, and the effect was particularly evident in the heart (Cusack et al. 2003). A study showed that the age was highly correlated with drug distribution clearance. A reduction of the distribution clearance in heart tissue contributed to DOX-induced cardiotoxicity, and was attributed to the decline in regional blood flow with age (Li and Gwilt 2003).
The alteration of cardiac transcriptional activities in response to DOX may contribute to more severe DOX cardiotoxicity in neonatal hearts, as some cardiac transcriptional factors may present at a higher level or a higher degree of sensitivity to DOX in neonatal hearts than in adult hearts (Aihara et al. 2000; Jeyaseelan et al. 1997). CARP, also called cardiac ankyrin repeat protein, is present at the earliest stages of cardiac morphogenesis and gradually decreases from neonatal to adult hearts. It may function as a transcriptional regulator of cardiac muscle genes which are important for the growth and/or morphogenesis of myocardium (Aihara et al. 2000; Jeyaseelan et al. 1997; Zou et al. 1997). The early, rapid, and high repression of CARP gene transcription by DOX was observed in neonatal hearts, and the underlying mechanism may involve oxidative stress and subsequent activation of H7-sensitive serine/threonine kinase (Aihara et al. 2000).
The different susceptibility to DOX may also be due to differences in expression levels of apoptotic signaling molecules between children and adult hearts. Some studies have suggested the decrease of apoptotic potential in postmitotic cells such as skeletal muscle cells (Burgess et al. 1999), neuronal cells (Yakovlev et al. 2001) as well as cardiomyocytes (Sanchis et al. 2003). Reduced expression levels of Apaf-1, caspases, and some pro-apoptotic members of the Bcl-2 family, may contribute to the reduced apoptotic potential in postmitotic cells (Bahi et al. 2006; Burgess et al. 1999; Sanchis et al. 2003; Yakovlev et al. 2001). Recent in vitro studies further support that different pathways may be involved in DOX-induced cell death in adult and immature cardiomyocytes (Bahi et al. 2006; Konorev et al. 2008). The intrinsic apoptotic pathway was more active in immature cardiac cells compared to adult cardiac cells, which may explain why the higher sensitivity to DOX-induced injury is seen in immature hearts. Another in vivo study also described down-regulation of intrinsic apoptotic pathway-related proteins in mouse brain, skeletal muscle, and heart from neonate to adult. The expression levels of Bim, Apaf-1 and caspase 3 were dramatically decreased during postnatal development in the brain, skeletal muscle and heart, which is consistent with the observation that the TUNEL positive cells presented a significant reduction in adult brain, skeletal muscle, and heart compared with the neonate (Madden et al. 2007).
It is worth noting that the majority in vivo DOX studies with animals heavily rely on acute or chronic drug administration in young adults (including mouse and rat between the ages of 5–22 weeks) but not in neonates. Given the unique properties of cardiomyocytes during postnatal development, it is therefore important to understand the molecular events involved in cardiomyocyte apoptosis in this age group.
POTENTIAL STRATEGIES TO REDUCE DOX-INDUCED CARDIOMYOCYTE DEATH
As generation of ROS has been considered a primary mechanism of DOX-induced cardiotoxicity, clinical approaches designed to attenuate the DOX-induced cardiotoxicity consist of anti-oxidation, iron chelator, and free radical scavenger (Table 2). Carvedilol, an adrenergic blocking agent with potent anti-oxidant activity, has been found to be protective against doxorubicin-induced ROS generation and apoptosis (Armstrong 2004; Machado et al. 2008; Spallarossa et al. 2004). Dexrazoxane, the only cardioprotective drug currently available clinically, is an intracellular iron chelator which has been proved to protect myocardial mitochondria from genetic and functional lesions induced by DOX via removing iron from its complex with DOX, and thereby reducing the formation of hydroxyl radicals and superoxide (Armstrong 2004; Lebrecht et al. 2007b). However, the effect of oxidative stress in clinical cardiotoxicity is increasingly questioned. Application with anti-oxidants, such as vitamin E and N-acetylcysteine, did not provide visible protection in long-term experimental and clinical trials (Gianni et al. 2008).
Table 2.
Potential strategies to reduce DOX-induced cardiomyopathy
| Category | Molecules | References |
|---|---|---|
| Anti-oxidants and free radical scavengers | Dexrazoxane (Only cardioprotective drug currently available clinically) | Hensley et al. 2009; Lebrecht et al. 2007b |
| Carvedilol (Clinically approved drug) | Armstrong 2004; Machado et al. 2008; Spallarossa et al. 2004 | |
| Melatonin | Liu et al. 2002 | |
| C-phycocyanin | Khan et al. 2006 | |
| Rosmarinic acid | Kim et al. 2005 | |
| Flavonoid | Bast et al. 2007; Bruynzeel et al. 2007 | |
| Resveratrol (RVT) | Tatlidede et al. 2009 | |
| Statin | Riad et al. 2009 | |
| Inhibition of increased [Ca2+] | Carnitine | Mijares and Lopez 2001 |
| Inhibitors of ceramide production | Carnitine | Armstrong 2004 |
| Inhibitors of NF-κB | Plantainoside D | Kim et al. 2007 |
| Pyrrolidine dithiocarbamate | Li et al. 2007 | |
| Inhibitors of p53 | Pifithrin-α | Chua et al. 2006; Liu et al. 2004 |
| Preservation of p-Akt | Heat shock protein 20 | Fan et al. 2008 |
| Modified DOX | Liposomal doxorubicin(DaunoXome, etc) | Rigacci et al. 2007; Yildirim et al. 2008 |
| Prodrugs and derivativesof DOX | Doxazolidine Carbamates,DOXO-EMCH, etc | Burkhart et al. 2006; Kratz et al. 2007; Lebrecht et al. 2007a |
| Others | Thrombopoietin | Li et al. 2006 |
| SNAP (exogenous NO donor) | Maejima et al. 2005 |
Other potential approaches to increased tumor response and decreased cardiotocicity include application of liposomal anthracyclines, prodrugs and derivatives of DOX (Burkhart et al. 2006; Kratz et al. 2007; Lebrecht et al. 2007a; Rigacci et al. 2007; Yildirim et al. 2008). According to a recent study, another promising method is to find candidates with the ability to form diffusible metabolites that eliminate excess anthracycline and prevent accumulation in the heart (Salvatorelli et al. 2009). Results from basic research have provided an increasing number of potential therapeutic targets for the development of new strategies of cardioprotection against DOX-induced cardiotoxicity (Table 1 and Table 2). The research in our laboratory has indicated that ROCK1 (Rho-associated coiled-coil containing protein kinase-1) is a key mediator of cardiomyocyte apoptosis (Chang et al. 2006; Shi and Wei 2007). ROCK1 may also mediate DOX-induced cardiotoxicity in adult mouse hearts (Shi et al, unpublished observations). Continuous efforts in elucidating the pathogenic mechanisms, as well as identifying new therapeutic targets, will certainly be helpful for the development of more effective therapies.
CONCLUSIONS AND FUTURE DIRECTIONS
Cardiac dysfunction is the most severe side effect from DOX treatment. Considerable data indicate that cardiomyocyte death through apoptosis, necrosis, and other forms is a primary contributor to the progression of DOX-induced cardiomyopathy. Excessive oxidative stress, damage to nuclear DNA, changes in calcium handling and cellular contractility, suppression of transcription factors that regulate cell survival and sarcomere protein synthesis, and disruption of sarcomere stability are identified as contributors to the mechanisms of cardiomyocyte death. Numerous studies evaluating DOX-induced cardiomyocyte death were performed in vitro or in vivo with a time window of hours or days after exposure to DOX at high concentrations. Future studies using long-term animal models should be performed to evaluate the contribution of different types of cardiomyocyte death to the chronic and delayed DOX-induced cardiotoxicity associated with clinically relevant doses of the drugs. In addition, in order to draw a comprehensive picture for DOX-induced cardiotoxicity, more information is needed to compare the relative importance of each cell death type and other mechanisms of cardiomyocyte injury, particularly, how these different mechanisms interact during the development of cardiomyopathy.
As mentioned above, the mechanisms for the late-onset anthracycline cardiac toxicity in children remain under-explored. The postnatal hearts contain pluripotent stem cells, which are capable of giving rise to functional cardiomyocytes (Davani et al. 2005). Like other undifferentiated cells, cardiac stem cells could be more sensitive to DOX and their death will limit the regenerative capacity of heart. It is possible that DOX-induced loss of cardiomyocytes, together with early damage of cardiac stem cells in pediatric patients, can cause permanent cardiotoxicity among those long-term cancer survivors. Future research will continually validate the essential mechanisms and develop therapeutic strategies to prevent premature cardiomyocyte death in pediatric patients who need anthracycline treatment.
Acknowledgements
This work was supported by National Institutes of Health grants (HL072897 and HL085098 (project 2) to L.W.) and the Showalter Trust (89829 to J.S.).
REFERENCES
- Aihara Y, Kurabayashi M, Tanaka T, et al. Doxorubicin represses CARP gene transcription through the generation of oxidative stress in neonatal rat cardiac myocytes: possible role of serine/threonine kinase-dependent pathways. J Mol Cell Cardiol. 2000;32:1401–1414. doi: 10.1006/jmcc.2000.1173. [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Yang RB, Weiss DS, et al. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 2000;19:3325–3336. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Li P, Li J, et al. ARC is a critical cardiomyocyte survival switch in doxorubicin cardiotoxicity. J Mol Med. 2009;87:401–410. doi: 10.1007/s00109-008-0434-z. [DOI] [PubMed] [Google Scholar]
- Aries A, Paradis P, Lefebvre C, et al. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci U S A. 2004;101:6975–6980. doi: 10.1073/pnas.0401833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong SC. Anti-oxidants and apoptosis: attenuation of doxorubicin induced cardiomyopathy by carvedilol. J Mol Cell Cardiol. 2004;37:817–821. doi: 10.1016/j.yjmcc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Arola OJ, Saraste A, Pulkki K, et al. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60:1789–1792. [PubMed] [Google Scholar]
- Bahi N, Zhang J, Llovera M, et al. Switch from caspase-dependent to caspase-independent death during heart development: essential role of endonuclease G in ischemia-induced DNA processing of differentiated cardiomyocytes. J Biol Chem. 2006;281:22943–22952. doi: 10.1074/jbc.M601025200. [DOI] [PubMed] [Google Scholar]
- Bast A, Haenen GR, Bruynzeel AM, et al. Protection by flavonoids against anthracycline cardiotoxicity: from chemistry to clinical trials. Cardiovasc Toxicol. 2007;7:154–159. doi: 10.1007/s12012-007-0018-0. [DOI] [PubMed] [Google Scholar]
- Bennink RJ, Van Den Hoff MJ, Van Hemert FJ, et al. Annexin V imaging of acute doxorubicin cardiotoxicity (apoptosis) in rats. J Nucl Med. 2004;45:842–848. [PubMed] [Google Scholar]
- Bergmann MW, Zelarayan L, Gehrke C. Treatment-sensitive premature renal and heart senescence in hypertension. Hypertension. 2008;52:61–62. doi: 10.1161/HYPERTENSIONAHA.107.108563. [DOI] [PubMed] [Google Scholar]
- Bernhard D, Laufer G. The aging cardiomyocyte: a mini-review. Gerontology. 2008;54:24–31. doi: 10.1159/000113503. [DOI] [PubMed] [Google Scholar]
- Bernuzzi F, Recalcati S, Alberghini A, et al. Reactive oxygen species-independent apoptosis in doxorubicin-treated H9c2 cardiomyocytes: role for heme oxygenase-1 down-modulation. Chem Biol Interact. 2009;177:12–20. doi: 10.1016/j.cbi.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Bruynzeel AM, Abou El Hassan MA, Torun E, et al. Caspase-dependent and -independent suppression of apoptosis by monoHER in Doxorubicin treated cells. Br J Cancer. 2007;96:450–456. doi: 10.1038/sj.bjc.6603598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess DH, Svensson M, Dandrea T, et al. Human skeletal muscle cytosols are refractory to cytochrome c-dependent activation of type-II caspases and lack APAF-1. Cell Death Differ. 1999;6:256–261. doi: 10.1038/sj.cdd.4400489. [DOI] [PubMed] [Google Scholar]
- Burkhart DJ, Barthel BL, Post GC, et al. Design, synthesis, and preliminary evaluation of doxazolidine carbamates as prodrugs activated by carboxylesterases. J Med Chem. 2006;49:7002–7012. doi: 10.1021/jm060597e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, et al. Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Physiol Cell Physiol. 2006;291:C1082–C1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- Casey TM, Arthur PG, Bogoyevitch MA. Necrotic death without mitochondrial dysfunction-delayed death of cardiac myocytes following oxidative stress. Biochim Biophys Acta. 2007;1773:342–351. doi: 10.1016/j.bbamcr.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Chang J, Xie M, Shah VR, et al. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci U S A. 2006;103:14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs AC, Phaneuf SL, Dirks AJ, et al. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002;62:4592–4598. [PubMed] [Google Scholar]
- Chua CC, Liu X, Gao J, et al. Multiple actions of pifithrin-alpha on doxorubicin-induced apoptosis in rat myoblastic H9c2 cells. Am J Physiol Heart Circ Physiol. 2006;290:H2606–H2613. doi: 10.1152/ajpheart.01138.2005. [DOI] [PubMed] [Google Scholar]
- Cusack BJ, Musser B, Gambliel H, et al. Effect of dexrazoxane on doxorubicin pharmacokinetics in young and old rats. Cancer Chemother Pharmacol. 2003;51:139–146. doi: 10.1007/s00280-002-0544-1. [DOI] [PubMed] [Google Scholar]
- D'Anglemont De Tassigny A, Souktani R, Henry P, et al. Volume-sensitive chloride channels (ICI,vol) mediate doxorubicin-induced apoptosis through apoptotic volume decrease in cardiomyocytes. Fundam Clin Pharmacol. 2004;18:531–538. doi: 10.1111/j.1472-8206.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- Davani S, Deschaseaux F, Chalmers D, et al. Can stem cells mend a broken heart? Cardiovasc Res. 2005;65:305–316. doi: 10.1016/j.cardiores.2004.10.037. [DOI] [PubMed] [Google Scholar]
- De Meyer GR, Martinet W. Autophagy in the cardiovascular system. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbamcr.2008.12.011. Doi:10.1016/j.bbamcr.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Deniaud A, Sharaf El Dein O, Maillier E, et al. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- Diwan A, Matkovich SJ, Yuan Q, et al. Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. J Clin Invest. 2009;119:203–212. doi: 10.1172/JCI36445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW., 2nd Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res. 2009;81:465–473. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan GC, Zhou X, Wang X, et al. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res. 2008;103:1270–1279. doi: 10.1161/CIRCRESAHA.108.182832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PW, Salloum F, Das A, et al. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- Gen W, Tani M, Takeshita J, et al. Mechanisms of Ca2+ overload induced by extracellular H2O2 in quiescent isolated rat cardiomyocytes. Basic Res Cardiol. 2001;96:623–629. doi: 10.1007/s003950170014. [DOI] [PubMed] [Google Scholar]
- Gianni L, Herman EH, Lipshultz SE, et al. Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol. 2008;26:3777–3784. doi: 10.1200/JCO.2007.14.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res. 2008;77:334–343. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- Gustafsson AB, Gottlieb RA. Autophagy in ischemic heart disease. Circ Res. 2009;104:150–158. doi: 10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley ML, Hagerty KL, Kewalramani T, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009;27:127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Bastholm L, Szyniarowski P, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Iarussi D, Indolfi P, Casale F, et al. Recent advances in the prevention of anthracycline cardiotoxicity in childhood. Curr Med Chem. 2001;8:1649–1660. doi: 10.2174/0929867013371888. [DOI] [PubMed] [Google Scholar]
- Ikegami E, Fukazawa R, Kanbe M, et al. Edaravone, a potent free radical scavenger, prevents anthracycline-induced myocardial cell death. Circ J. 2007;71:1815–1820. doi: 10.1253/circj.71.1815. [DOI] [PubMed] [Google Scholar]
- Ito T, Fujio Y, Takahashi K, et al. Degradation of NFAT5, a transcriptional regulator of osmotic stress-related genes, is a critical event for doxorubicin-induced cytotoxicity in cardiac myocytes. J Biol Chem. 2007;282:1152–1160. doi: 10.1074/jbc.M609547200. [DOI] [PubMed] [Google Scholar]
- Jang YM, Kendaiah S, Drew B, et al. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett. 2004;577:483–490. doi: 10.1016/j.febslet.2004.10.053. [DOI] [PubMed] [Google Scholar]
- Jeyaseelan R, Poizat C, Baker RK, et al. A novel cardiac-restricted target for doxorubicin. CARP, a nuclear modulator of gene expression in cardiac progenitor cells and cardiomyocytes. J Biol Chem. 1997;272:22800–22808. doi: 10.1074/jbc.272.36.22800. [DOI] [PubMed] [Google Scholar]
- Kajstura J, Rota M, Urbanek K, et al. The telomere-telomerase axis and the heart. Antioxid Redox Signal. 2006;8:2125–2141. doi: 10.1089/ars.2006.8.2125. [DOI] [PubMed] [Google Scholar]
- Kalivendi SV, Konorev EA, Cunningham S, et al. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem J. 2005;389:527–539. doi: 10.1042/BJ20050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Hasegawa K, Morimoto T, et al. Expression of p300 protects cardiac myocytes from apoptosis in vivo. Biochem Biophys Res Commun. 2004;315:733–738. doi: 10.1016/j.bbrc.2004.01.105. [DOI] [PubMed] [Google Scholar]
- Khan M, Varadharaj S, Shobha JC, et al. C-phycocyanin ameliorates doxorubicin-induced oxidative stress and apoptosis in adult rat cardiomyocytes. J Cardiovasc Pharmacol. 2006;47:9–20. doi: 10.1097/01.fjc.0000191520.48404.27. [DOI] [PubMed] [Google Scholar]
- Kim DS, Kim HR, Woo ER, et al. Inhibitory effects of rosmarinic acid on adriamycin-induced apoptosis in H9c2 cardiac muscle cells by inhibiting reactive oxygen species and the activations of c-Jun N-terminal kinase and extracellular signal-regulated kinase. Biochem Pharmacol. 2005;70:1066–1078. doi: 10.1016/j.bcp.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Kim DS, Woo ER, Chae SW, et al. Plantainoside D protects adriamycin-induced apoptosis in H9c2 cardiac muscle cells via the inhibition of ROS generation and NF-kappaB activation. Life Sci. 2007;80:314–323. doi: 10.1016/j.lfs.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Kim SY, Kim SJ, Kim BJ, et al. Doxorubicin-induced reactive oxygen species generation and intracellular Ca2+ increase are reciprocally modulated in rat cardiomyocytes. Exp Mol Med. 2006;38:535–545. doi: 10.1038/emm.2006.63. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ma AG, Kitta K, et al. Anthracycline-induced suppression of GATA-4 transcription factor: implication in the regulation of cardiac myocyte apoptosis. Mol Pharmacol. 2003;63:368–377. doi: 10.1124/mol.63.2.368. [DOI] [PubMed] [Google Scholar]
- Kluza J, Marchetti P, Gallego MA, et al. Mitochondrial proliferation during apoptosis induced by anticancer agents: effects of doxorubicin and mitoxantrone on cancer and cardiac cells. Oncogene. 2004;23:7018–7030. doi: 10.1038/sj.onc.1207936. [DOI] [PubMed] [Google Scholar]
- Konorev EA, Vanamala S, Kalyanaraman B. Differences in doxorubicin-induced apoptotic signaling in adult and immature cardiomyocytes. Free Radic Biol Med. 2008;45:1723–1728. doi: 10.1016/j.freeradbiomed.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotamraju S, Konorev EA, Joseph J, et al. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. Role of reactive oxygen and nitrogen species. J Biol Chem. 2000;275:33585–33592. doi: 10.1074/jbc.M003890200. [DOI] [PubMed] [Google Scholar]
- Kratz F, Ehling G, Kauffmann HM, et al. Acute and repeat-dose toxicity studies of the (6-maleimidocaproyl)hydrazone derivative of doxorubicin (DOXO-EMCH), an albumin-binding prodrug of the anticancer agent doxorubicin. Hum Exp Toxicol. 2007;26:19–35. doi: 10.1177/0960327107073825. [DOI] [PubMed] [Google Scholar]
- L'Ecuyer T, Sanjeev S, Thomas R, et al. DNA damage is an early event in doxorubicin-induced cardiac myocyte death. Am J Physiol Heart Circ Physiol. 2006;291:H1273–H1280. doi: 10.1152/ajpheart.00738.2005. [DOI] [PubMed] [Google Scholar]
- Lebrecht D, Geist A, Ketelsen UP, et al. The 6-maleimidocaproyl hydrazone derivative of doxorubicin (DOXO-EMCH) is superior to free doxorubicin with respect to cardiotoxicity and mitochondrial damage. Int J Cancer. 2007a;120:927–934. doi: 10.1002/ijc.22409. [DOI] [PubMed] [Google Scholar]
- Lebrecht D, Geist A, Ketelsen UP, et al. Dexrazoxane prevents doxorubicin-induced long-term cardiotoxicity and protects myocardial mitochondria from genetic and functional lesions in rats. Br J Pharmacol. 2007b;151:771–778. doi: 10.1038/sj.bjp.0707294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrecht D, Walker UA. Role of mtDNA lesions in anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7:108–113. doi: 10.1007/s12012-007-0009-1. [DOI] [PubMed] [Google Scholar]
- Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Gu H, Sun B. Protective effects of pyrrolidine dithiocarbamate on myocardium apoptosis induced by adriamycin in rats. Int J Cardiol. 2007;114:159–165. doi: 10.1016/j.ijcard.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Li J, Gwilt PR. The effect of age on the early disposition of doxorubicin. Cancer Chemother Pharmacol. 2003;51:395–402. doi: 10.1007/s00280-002-0554-z. [DOI] [PubMed] [Google Scholar]
- Li K, Sung RY, Huang WZ, et al. Thrombopoietin protects against in vitro and in vivo cardiotoxicity induced by doxorubicin. Circulation. 2006;113:2211–2220. doi: 10.1161/CIRCULATIONAHA.105.560250. [DOI] [PubMed] [Google Scholar]
- Lim CC, Zuppinger C, Guo X, et al. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem. 2004;279:8290–8299. doi: 10.1074/jbc.M308033200. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Colan SD, Gelber RD, et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- Liu J, Mao W, Ding B, et al. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295:H1956–H1965. doi: 10.1152/ajpheart.00407.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen Z, Chua CC, et al. Melatonin as an effective protector against doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2002;283:H254–H263. doi: 10.1152/ajpheart.01023.2001. [DOI] [PubMed] [Google Scholar]
- Liu X, Chua CC, Gao J, et al. Pifithrin-alpha protects against doxorubicin-induced apoptosis and acute cardiotoxicity in mice. Am J Physiol Heart Circ Physiol. 2004;286:H933–H939. doi: 10.1152/ajpheart.00759.2003. [DOI] [PubMed] [Google Scholar]
- Lou H, Danelisen I, Singal PK. Involvement of mitogen-activated protein kinases in adriamycin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;288:H1925–H1930. doi: 10.1152/ajpheart.01054.2004. [DOI] [PubMed] [Google Scholar]
- Machado V, Cabral A, Monteiro P, et al. Carvedilol as a protector against the cardiotoxicity induced by anthracyclines (doxorubicin) Rev Port Cardiol. 2008;27:1277–1296. [PubMed] [Google Scholar]
- Madden SD, Donovan M, Cotter TG. Key apoptosis regulating proteins are down-regulated during postnatal tissue development. Int J Dev Biol. 2007;51:415–423. doi: 10.1387/ijdb.062263sm. [DOI] [PubMed] [Google Scholar]
- Maejima Y, Adachi S, Ito H, et al. Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging Cell. 2008;7:125–136. doi: 10.1111/j.1474-9726.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Maejima Y, Adachi S, Morikawa K, et al. Nitric oxide inhibits myocardial apoptosis by preventing caspase-3 activity via S-nitrosylation. J Mol Cell Cardiol. 2005;38:163–174. doi: 10.1016/j.yjmcc.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, et al. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Kyoi S, Takagi H, et al. Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy. 2008;4:409–415. doi: 10.4161/auto.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier I, Vuolo M, Madan R, et al. ARC, an apoptosis suppressor limited to terminally differentiated cells, is induced in human breast cancer and confers chemo- and radiation-resistance. Cell Death Differ. 2005;12:682–686. doi: 10.1038/sj.cdd.4401631. [DOI] [PubMed] [Google Scholar]
- Mijares A, Lopez JR. L-carnitine prevents increase in diastolic [CA2+] induced by doxorubicin in cardiac cells. Eur J Pharmacol. 2001;425:117–120. doi: 10.1016/s0014-2999(01)01158-x. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Batkai S, Rajesh M, et al. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol. 2007;50:528–536. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Gamez JA, Rodriguez-Vargas JM, Quiles-Perez R, et al. PARP-1 is involved in autophagy induced by DNA damage. Autophagy. 2009;5:61–74. doi: 10.4161/auto.5.1.7272. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ueda Y, Juan Y, et al. Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: In vivo study. Circulation. 2000;102:572–578. doi: 10.1161/01.cir.102.5.572. [DOI] [PubMed] [Google Scholar]
- Neilan TG, Blake SL, Ichinose F, et al. Disruption of nitric oxide synthase 3 protects against the cardiac injury, dysfunction, and mortality induced by doxorubicin. Circulation. 2007;116:506–514. doi: 10.1161/CIRCULATIONAHA.106.652339. [DOI] [PubMed] [Google Scholar]
- Nishida K, Kyoi S, Yamaguchi O, et al. The role of autophagy in the heart. Cell Death Differ. 2009;16:31–38. doi: 10.1038/cdd.2008.163. [DOI] [PubMed] [Google Scholar]
- Nishida K, Yamaguchi O, Otsu K. Crosstalk between autophagy and apoptosis in heart disease. Circ Res. 2008;103:343–351. doi: 10.1161/CIRCRESAHA.108.175448. [DOI] [PubMed] [Google Scholar]
- Nitobe J, Yamaguchi S, Okuyama M, et al. Reactive oxygen species regulate FLICE inhibitory protein (FLIP) and susceptibility to Fas-mediated apoptosis in cardiac myocytes. Cardiovasc Res. 2003;57:119–128. doi: 10.1016/s0008-6363(02)00646-6. [DOI] [PubMed] [Google Scholar]
- Niu J, Azfer A, Wang K, et al. Cardiac-targeted expression of soluble fas attenuates doxorubicin-induced cardiotoxicity in mice. J Pharmacol Exp Ther. 2009;328:740–748. doi: 10.1124/jpet.108.146423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki N, Shishido T, Takeishi Y, et al. Modulation of doxorubicin-induced cardiac dysfunction in toll-like receptor-2-knockout mice. Circulation. 2004;110:2869–2874. doi: 10.1161/01.CIR.0000146889.46519.27. [DOI] [PubMed] [Google Scholar]
- Parra V, Eisner V, Chiong M, et al. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res. 2008;77:387–397. doi: 10.1093/cvr/cvm029. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Carraway MS, Babiker A, et al. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poizat C, Puri PL, Bai Y, et al. Phosphorylation-dependent degradation of p300 by doxorubicin-activated p38 mitogen-activated protein kinase in cardiac cells. Mol Cell Biol. 2005;25:2673–2687. doi: 10.1128/MCB.25.7.2673-2687.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad A, Bien S, Westermann D, et al. Pretreatment with statin attenuates the cardiotoxicity of Doxorubicin in mice. Cancer Res. 2009;69:695–699. doi: 10.1158/0008-5472.CAN-08-3076. [DOI] [PubMed] [Google Scholar]
- Rigacci L, Mappa S, Nassi L, et al. Liposome-encapsulated doxorubicin in combination with cyclophosphamide, vincristine, prednisone and rituximab in patients with lymphoma and concurrent cardiac diseases or pre-treated with anthracyclines. Hematol Oncol. 2007;25:198–203. doi: 10.1002/hon.827. [DOI] [PubMed] [Google Scholar]
- Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Circ Res. 2008;103:1363–1369. doi: 10.1161/CIRCRESAHA.108.186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Difiglia M, Heintz N, et al. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- Salvatorelli E, Menna P, Lusini M, et al. Doxorubicinolone formation and efflux: a salvage pathway against epirubicin accumulation in human heart. J Pharmacol Exp Ther. 2009;329:175–184. doi: 10.1124/jpet.108.149260. [DOI] [PubMed] [Google Scholar]
- Sanchis D, Mayorga M, Ballester M, et al. Lack of Apaf-1 expression confers resistance to cytochrome c-driven apoptosis in cardiomyocytes. Cell Death Differ. 2003;10:977–986. doi: 10.1038/sj.cdd.4401267. [DOI] [PubMed] [Google Scholar]
- Schmid D, Munz C. Innate and adaptive immunity through autophagy. Immunity. 2007;27:11–21. doi: 10.1016/j.immuni.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Wei L. Rho kinase in the regulation of cell death and survival. Arch Immunol Ther Exp (Warsz) 2007;55:61–75. doi: 10.1007/s00005-007-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Kanaseki T, Mizushima N, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- Shimomura H, Terasaki F, Hayashi T, et al. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn Circ J. 2001;65:965–968. doi: 10.1253/jcj.65.965. [DOI] [PubMed] [Google Scholar]
- Shizukuda Y, Matoba S, Mian OY, et al. Targeted disruption of p53 attenuates doxorubicin-induced cardiac toxicity in mice. Mol Cell Biochem. 2005;273:25–32. doi: 10.1007/s11010-005-5905-8. [DOI] [PubMed] [Google Scholar]
- Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- Solem LE, Heller LJ, Wallace KB. Dose-dependent increase in sensitivity to calcium-induced mitochondrial dysfunction and cardiomyocyte cell injury by doxorubicin. J Mol Cell Cardiol. 1996;28:1023–1032. doi: 10.1006/jmcc.1996.0095. [DOI] [PubMed] [Google Scholar]
- Spallarossa P, Fabbi P, Manca V, et al. Doxorubicin-induced expression of LOX-1 in H9c2 cardiac muscle cells and its role in apoptosis. Biochem Biophys Res Commun. 2005;335:188–196. doi: 10.1016/j.bbrc.2005.07.064. [DOI] [PubMed] [Google Scholar]
- Spallarossa P, Garibaldi S, Altieri P, et al. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J Mol Cell Cardiol. 2004;37:837–846. doi: 10.1016/j.yjmcc.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Suliman HB, Carraway MS, Ali AS, et al. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–3741. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Tatlidede E, Sehirli O, Velioglu-Ogunc A, et al. Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radic Res. 2009;43:195–205. doi: 10.1080/10715760802673008. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovasc Res. 2005;68:355–365. doi: 10.1016/j.cardiores.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Terman A, Gustafsson B, Brunk UT. The lysosomal-mitochondrial axis theory of postmitotic aging and cell death. Chem Biol Interact. 2006;163:29–37. doi: 10.1016/j.cbi.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;2(12 Suppl):1528–1534. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, Rozencweig M, Layard M, et al. Daunomycin-induced cardiotoxicity in children and adults. A review of 110 cases. Am J Med. 1977;62:200–208. doi: 10.1016/0002-9343(77)90315-1. [DOI] [PubMed] [Google Scholar]
- Wallace KB. Doxorubicin-induced cardiac mitochondrionopathy. Pharmacol Toxicol. 2003;93:105–115. doi: 10.1034/j.1600-0773.2003.930301.x. [DOI] [PubMed] [Google Scholar]
- Wallace KB. Adriamycin-induced interference with cardiac mitochondrial calcium homeostasis. Cardiovasc Toxicol. 2007;7:101–107. doi: 10.1007/s12012-007-0008-2. [DOI] [PubMed] [Google Scholar]
- Wang GW, Klein JB, Kang YJ. Metallothionein inhibits doxorubicin-induced mitochondrial cytochrome c release and caspase-3 activation in cardiomyocytes. J Pharmacol Exp Ther. 2001;298:461–468. [PubMed] [Google Scholar]
- Wang S, Kotamraju S, Konorev E, et al. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J. 2002;367:729–740. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev AG, Ota K, Wang G, et al. Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J Neurosci. 2001;21:7439–7446. doi: 10.1523/JNEUROSCI.21-19-07439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Ding B, Shishido T, et al. Activation of extracellular signal-regulated kinase 5 reduces cardiac apoptosis and dysfunction via inhibition of a phosphodiesterase 3A/inducible cAMP early repressor feedback loop. Circ Res. 2007;100:510–519. doi: 10.1161/01.RES.0000259045.49371.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh ET, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- Yildirim Y, Gultekin E, Avci ME, et al. Cardiac safety profile of pegylated liposomal doxorubicin reaching or exceeding lifetime cumulative doses of 550 mg/m2 in patients with recurrent ovarian and peritoneal cancer. Int J Gynecol Cancer. 2008;18:223–227. doi: 10.1111/j.1525-1438.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;2(12 Suppl):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Zhou Q, Yao F, et al. Endothelin-1 regulates cardiac L-type calcium channels via NAD(P)H oxidase-derived superoxide. J Pharmacol Exp Ther. 2008;326:732–738. doi: 10.1124/jpet.108.140301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Starkov A, Froberg MK, et al. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res. 2001;61:771–777. [PubMed] [Google Scholar]
- Zhu W, Shou W, Payne RM, et al. A mouse model for juvenile doxorubicin-induced cardiac dysfunction. Pediatr Res. 2008;64:488–494. doi: 10.1203/PDR.0b013e318184d732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Soonpaa MH, Chen H. Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation. 2009;119:99–106. doi: 10.1161/CIRCULATIONAHA.108.799700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Zou Y, Evans S, Chen J, et al. CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2-5 homeobox gene pathway. Development. 1997;124:793–804. doi: 10.1242/dev.124.4.793. [DOI] [PubMed] [Google Scholar]