Abstract
B cells, the antibody-producing cells of the immune system, develop from hematopoietic stem cells (HSCs) through well-defined stages during which immunoglobulin (Ig) genes are rearranged to generate a clonal B cell receptor (BCR). Signaling through the BCR plays a role in the subsequent cell fate decisions leading to the generation of three distinct types of B cells: B1, marginal zone (MZ), and follicular (FO) B cells. Common lymphoid progenitors (CLPs) are descended from HSCs and, although recent observations suggest that CLPs may not be physiological T cell precursors, it is generally accepted that CLPs are obligate progenitors for B cells. In addition, a CLP-like progenitor of unknown significance that lacks expression of c-kit (kit-CLP) was recently identified in the mouse model. Here we show that CLPs, kit-CLPs and a population within the lin-Sca1+kit+flt3-HSC compartment generate mature B cell types in different proportions: CLPs and kit-CLPs show a stronger MZ/FO ratio than lin-Sca1+kit+flt3- cells, while kit-CLPs show a stronger B1 bias than any other progenitor population. Furthermore, expression of Sca1 on B cells depends on their progenitor origin as B cells derived from CLPs and kit-CLPs express more Sca1 than those derived from lin-Sca1+kit+flt3- cells. These observations indicate a role for progenitor origin in B cell fate choices and suggest the existence of CLP-independent B cell development.
Introduction
All lymphoid cells develop from hematopoietic stem cells (HSCs) in the bone marrow (BM). Current models hold that lymphoid commitment of HSCs (lin-Sca1+kit+flt3- or LSKF-) (1) is accompanied by a decrease in erythroid, megakaryocytic and myeloid potential (2,3), and a maintenance of lymphoid potential in multipotential progenitors (MPPs, lin-Sca1+kit+flt3+) (4,5) and subsequently in common lymphoid progenitors (CLPs, lin-Sca1lokitloflt3+IL7Rα+), which are predominantly lymphoid committed (6). This process is marked by a progressive induction of the expression of Rag genes (7). While CLPs possess B (7-10), T (7,10,11), NK (8) and dendritic cell (9,13) potential, recent observations suggest that CLPs may not be physiological T cell precursors (14-19), but that an earlier precursor seeds the thymus, although this controversy is not yet resolved (10,11). However, it is generally accepted that CLPs are obligate progenitors for B cell development (19,20). In addition, we and others have recently identified a population of lin-Sca1lokit-IL7Rα+Flt3+ cells with T, B and NK potential that is distinguished from CLPs by the absence of c-kit expression, lower proliferative capacity in vivo and in vitro, lower myeloid potential and lower expression of Rag genes and TdT (21,22). The role of this CLP-like progenitor, which we will term kit-CLP, is unclear, however.
Several major types of mature B cells are distinguished. B1 cells occur mainly in the pleural and peritoneal cavities and, in addition to producing antibodies in response to infection, also produce ‘natural’ IgM (25,26). B2 cells reside in the spleen, the blood and lymph nodes. The spleen contains two types of B2 cells: marginal zone (MZ) and follicular (FO) B cells (28,29). MZ B cells (IgDloIgMhiCD21hiCD23lo) reside in the region demarcating the white and red pulp, respond to type 2 thymus-independent antigens, such as multivalent polysaccharides, are recruited rapidly into antibody responses to blood-borne pathogens and play a critical role in their clearance. In contrast, FO B cells (IgDhiIgMloCD21loCD23hi) inhabit the follicles, circulate in the blood and produce high affinity antibodies for which they require T-cell help.
The mechanisms underlying the development of these different types of B cells are unclear. Studies in knockout mice where signaling through the B cell receptor (BCR, the clonal Ig expressed on the surface) was either enhanced or decreased suggested that BCR signal strength determines cell fate choices of transitional B cells, AA4.1+CD21-CD23-IgMhi derived from AA4.1+IgM+ immature B (iB) cells that migrate from the BM to the spleen and subsequently develop into mature splenic B cells (19,20,23,24). According to these, low BCR signal strength results in MZ B cells while intermediate signal strength results in FO B cells. High signal strength leads to the development of B1 B cells (27-32). In addition to BCR signal strength, BCR specificity has also been shown to play a role, as positive selection by autoantigens is required for the generation of MZ and B1 B cells in transgenic models (33-35). Additional mechanisms must play a role, however. Lymphopenia and impaired B cell development favor the generation or maintenance of MZ and B1 cells, likely through their enhanced capacity of homeostatic proliferation (27,28,36,37). Furthermore, plasticity exists among mature B cells as small resting lymph node B cells, the equivalent of recirculating FO B cells, can adopt a MZ phenotype after transfer into a lymphopenic host (38). In the spleen, evidence suggests a distinct differentiation pathway for MZ B cells. MZ B cells develop from AA4.1+CD21-CD23-IgMhi T1, into AA4.1+CD21+CD23+IgMhi T2 and finally through a AA4.1loCD21hiCD23+IgMhi MZ precursor stage into mature MZ B cells (39). Development of MZ B cells requires LFA1 and α4β1 integrins (40), as well as Notch2 (41), which interacts with DL1 expressed on MZ endothelial cells, an interaction that is enhanced by Fringe glycosyltransferases (42). Finally, it has been argued that B1 cells represent a distinct B cell lineage (43,44). A lin-B220-CD19+IgM- B1-specified pro-B cell has recently been identified, suggesting that B1 development branches off from B2 development at an early stage (45). B1 precursors have also been reported to be CD138- (46).
As at least two distinct lymphoid-specified progenitors at a similar stage of differentiation exist, CLPs and kit-CLPs, we explored the possibility that the origin of B cells within the hematopoietic stem and progenitor compartment might contribute to their cell fate choice in the mature B cell compartment. Therefore, we analyzed the B cell potential of CLPs and kit-CLPs in more detail, hypothesizing that both progenitors might show a differential bias towards distinct types of mature B cells. We observed, however, that the LSKF- compartment, which is highly enriched in repopulating HSCs, also contains progenitors for a FO-biased B cell development program that is independent from CLPs or kit-CLPs, which show a higher propensity to generate MZ B cells. Furthermore, B cells generated from CLPs and kit-CLPs expressed more Sca1 than those derived directly from the LSKF- compartment. CLPs and kit-CLPs differ in their B1 potential, however, as kit-CLPs produce relatively more B1 cells than CLPs. Taken together, our observations indicate that CLPs are not obligate B cell progenitors and that progenitor origin plays a role in the regulation of cell fate choices in the B cell lineage.
Material and Methods
Mice
The 4 to 8-week-old C57BL/6 (CD45.2) mice and B6.Ly5.2 (B6.Ly5SJL)(CD45.1) mice were purchased from the National Cancer Institute animal facility. CD45.1+ CD45.2+ C57BL/6 mice were generated at the Mount Sinai animal facility (New York, NY), by crossing C57BL/6 and B6.Ly5.2 (B6.Ly5SJL) mice. Rag1-/- (B6.129S7-Rag1tm1Mom/J) (CD45.2) mice were purchased from Jackson Laboratory. Experiments and animal care were performed in accordance with the Mount Sinai Institutional Animal Care and Use Committee.
Antibodies
FITC-conjugated anti- TER119, -B220, -Mac1, -Gr1, -CD4, -CD2, -CD3e, -CD8a, -CD19 and -CD21; APC-conjugated anti-c-Kit, PE-conjugated anti-CD25, biotin-conjugated anti-Sca1 and anti-CD86, APCCy7-conjugated anti-CD19, PerCP-conjugated streptavidin and PerCPCy5.5-conjugated CD45.2 were purchased from BD Pharmingen. PE-conjugated anti-Flt3, -CD21 and -Mac1; PECy7-conjugated anti- IL7Rα, -streptavidin, -CD23 and CD45.1; APC-conjugated anti-AA4.1; PECy5-conjugated anti-IgM, as well as Alexafluor750-conjugated anti-CD45.1 were from eBioscience. PE-conjugated anti-IgD and FITC-conjugated anti-CD45.1 were from Southern Biotech. Pacific blue-conjugated anti-Sca1 and anti-I-A/I-E were purchased from Biolegend.
Preparation of hematopoietic cells
BM cells were prepared by flushing the femura and tibia of mice with cold DMEM (Cellgro), containing 2% FBS and 100 ng/mL penicillin/streptomycin. Spleen cell suspensions were prepared by mincing the organs through nylon mesh. Red blood cells were lysed in 2mL 1X RBC lysis Buffer (eBioscience) for 5 minutes. Cells were washed and resuspended in an appropriate volume of PBS or DMEM for staining. Peritoneal cells were collected through a ventral midline incision with scissor into the abdomen of each mouse, followed by injection of 5 to 10 mL PBS. Peritoneal fluid was slowly withdrawn and transfer to a 15mL conical tube.
Flow cytometry and cell sorting
BM, spleen, or peritoneal cells were stained with appropriate antibodies. Cells were sorted on three-laser FACSVantage SE with DiVa software (Becton Dickinson), a MoFLo (Cytomation) or a Influx (Cytopeia) flow cytometer. Doublets were excluded by plotting with forward scatter pulse area versus width. Purities > 95 % were routinely achieved, and were required to use the cells in adoptive transfer experiments. Analytical flow cytometry was performed on a three-laser LSR II or a Special Order 5-laser LSRII with Diva software (Becton Dickinson). On this instrument, all phycorerythrin-containing fluorochromes are excited by green 530 nm laser. Data were analyzed using FlowJo software. Biexponential transformation as implemented in Flowjo was used when > 8 % or dots fell on the axis. Lineage markers used for the exclusion of lineage positive cells were: CD3, CD4, CD8α, CD2, CD19, B220, Gr1. Mac1 and Ter119. Subpopulations were defined as follows: HSC: lin-Sca1+kit+flt3-; MPP: lin-Sca1+kit+flt3+; CLP: lin-Sca1lokitloflt3+IL7Rα+; kit-CLP: lin-Sca1lokit-IL7Rα+Flt3+; pro-B cells: AA4.1+B220+CD19+IgM-CD25-; pre-B cells: AA4.1+B220+CD19+IgM-CD25+; iB cells: B220+AA4.1+IgM+; T1 cells: CD21loCD23-IgM+AA4.1+; MZ B cells: CD21hiCD23loIgM+AA4.1-; FO B cells: CD21loCD23hiIgM+AA4.1-; B1 cells B220loMac1+, B2 cells: B220+Mac1-.
Reconstitution of Rag1-/- and C57BL/6 mice
Sorted LSKF- cells, MPPs, CLPs and kit-CLPs from BM of CD45.1+ C57BL/6 mice were injected in the tail vein of sublethally irradiated (700cG) CD45.2+ C57BL/6 mice. Donor-derived cells were distinguished by expression of CD45.1. For the competitive transplants, sorted LSKF- cells from CD45.1+ CD45.2+ C57BL/6 mice were mixed with sorted MPPs, CLPs and kit-CLPs from BM of CD45.1+ C57BL/6 mice and then were injected in the tail vein of sublethally irradiated (500cG) Rag1-/- mice. For reconstitution of lymphocyte-replete hosts, 5×105 iB cells from CD45.1+ C57BL/6 mice were injected in the tail vein of unconditioned CD45.2+ C57BL/6 hosts.
Immunohistochemistry
Spleens were removed and snap frozen in OCT compound (Tissue Tek) and stored at –80°C. Cryostat sections (7–8 μm) were air dried, fixed in 100% acetone (5 min at room temperature) and washed with PBS. Sections were stained with anti-IgM-Cy3 (Chemicon International) anti-MOMA-1-FITC (Serotec) and biotinylated anti-Sca1(BD Biosciences). Cy5-conjugated streptavidin (Jackson Immunoresearch) was used to detect biotinylated antibody. Slides were mounted with Vectashield containing DAPI (4,6-diamidino-2-phenylindole; Vector Laboratories). Images were acquired with a Leica DMRA2 fluorescence microscope (Wetzlar) and a digital Hamamatsu CCD camera and were analyzed with Openlab software (Improvision).
IgH rearrangement in B1 B cells
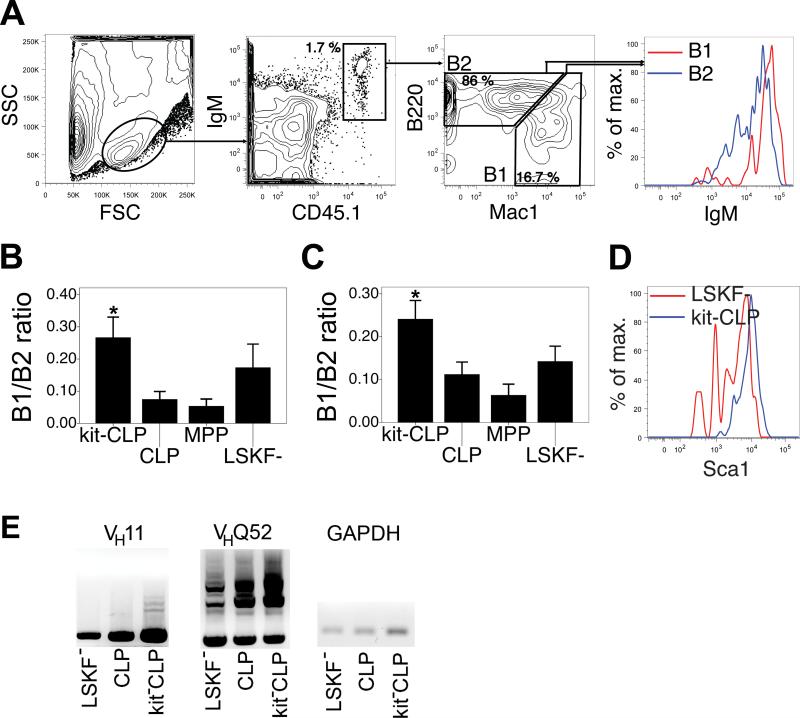
Donor-derived B1 B cells were sorted 4 weeks after transplantation of 5,000 LSKF-, CLPs and kit-CLPs cells into sublethally irradiated Rag1-/- mice. mRNA was extracted using RNeasy microkit (Qiagen) following the manufacturer's instructions. cDNA was prepared using SuperScript III Synthesis System for RT-PCR (Invitrogen) according to the manufacturer's instructions. The two gene segments predominantly expressed by B1 B cells, VH11 and VHQ52 were assessed through a nested PCR. DNA amplification was carried out using Advantage cDNA polymerase mix (Clontech), following the protocol described by Montecino-Rodriguez et al. (45).
Proliferation assay and expression of activation markers CD86 and MHCII
Donor-derived splenic B cells were sorted 3 weeks after transplantation of 3,000 LSKF- cells, CLPs and kit-CLPs cells into sublethally irradiated Rag1-/- mice. MZ and FO B cells were plated in 96-well plates containing RPMI medium (Cellgro), supplemented with 50 μM 2-Mercaptoethanol, 10% FBS, 2mM glutamine (GIBCO), 10mM Hepes (Cellgro). The cells were stimulated with 10μ□̃mL LPS (Sigma) for 48 hours, pulsed with 1 μCi/well 3H-Thymidine and harvested into a 96-well filter plate. The 3H-thymidine incorporation was detected as counts per minute using the liquid scintillator & luminescence counter 1450 Microbeta TRILUX (PerkinElmer). Unstimulated cells were used as a control. Each population was plated in triplicate. For the expression of activation markers, cells were stimulated with 10μ□̃mL LPS or F(ab’)2 for 24 hours and stained for CD86 and MHCII, immediately after been harvested from the recipient mice (t0), and after stimulation in vitro.
Ca2+ flux
Intracellular Ca2+ levels were measured using the Ca2+ indicator Indo-I acetoxymethil ester dye (Invitrogen). Splenic B cells were obtained 4 weeks after transplantation of 3000 LSKF-, MPPs, CLPs and kit-CLPs CD45.1 cells into sublethally irradiated Rag1-/- mice. Before staining, 106 CD45.2+ wt spleen cells were added, which served as internal control and were distinguished from donor-derived cells by the expression of the CD45 allelic variants. Cells were resuspended in 500 μL of Hanks’ Balanced Salt Solutions (HBSS, GIBCO) containing 10% FBS and incubated with 5 μL of Indo-I 1μM at 32 °C for 40 minutes. Cells were then washed with HBSS and incubated with anti-CD16/CD32 for 5 minutes, and with antibodies against AA4.1, CD21, CD23, CD19, CD45.1 and CD45.2 for 15 minutes at 4 °C. Cells were washed with HBSS and warmed to 37°C just before the analysis. Indo-1-loaded cells were then examined with a FACSVantage SE flow cytometer equipped with a UV laser and appropriate filters for the 405- and 485-nm wavelengths. After the establishment of a stable baseline, the cells were simulated with 10 μg/mL of F(ab)2 goat anti-mouse IgM μ chain (Jackson laboratories) and monitored for another 5 minutes. The change in intracellular Ca2+ levels was determined through the ratio of emission signals of Indo-1 at 405 nm and 485 nm, representing the ratio of free to bound Ca2+. The kinetic analysis was performed using FlowJo software.
Statistical analysis
For multiple comparisons, one-way ANOVA, as implemented in SSPS software was used. If P < 0.5 pairwise comparison of groups using unpaired t-test was performed. Error bars represent standard error of the mean (SEM).
Results
Different lymphoid progenitors generate MZ and FO B cell in different ratios
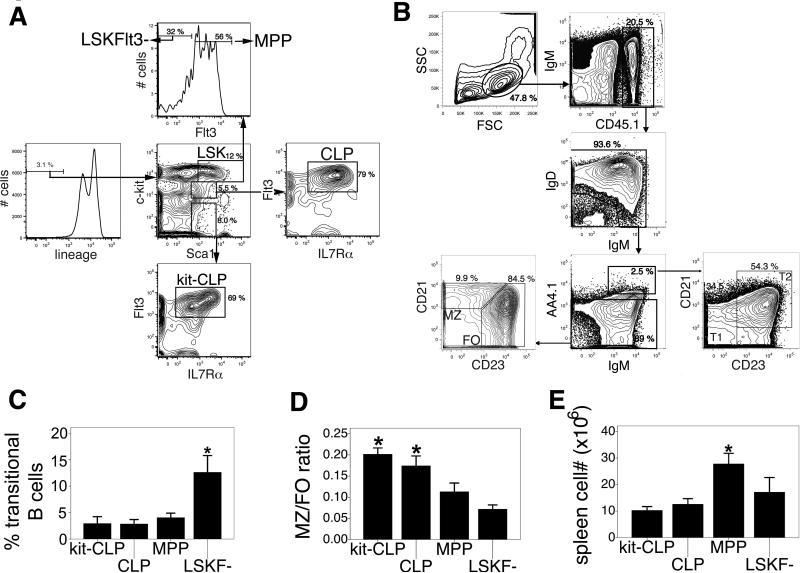
To analyze their B cell potential, we injected 3,000 CLPs or kit-CLPs purified from CD45.1+ congenic C57BL/6 mice (see Fig. 1A for representative sort gates) into sublethally irradiated CD45.2+ C57BL/6 mice. Previous studies have shown that both Il7Rα- and Il7Rα+ lin-Sca1lokit- cells have similar B cell potential (21). In the current study, we defined kit-CLPs as lin-Sca1lokit- cells with similar Flt3 and IL7Rα expression as CLPs, but lacking expression of c-kit. As in preliminary experiments differentiation into distinguishable MZ and FO B cells occurred between two and three weeks after transfer (see Fig. 1B for representative analysis gates), we measured the output of mature B cells in the spleen after 3 weeks. Similar data as those presented below were obtained 4 weeks after transfer, however. Because we assumed that both LSKF- cells (containing short-term and long-term HSCs) and MPPs (1-5) would be universal B cell precursors that fully reconstitute the mature B cell compartment, we used these populations as controls (See Fig. 1A for representative sort gates). As AA4.1 expression decreases progressively during the maturation of B cells in the spleen (20,23,39), we distinguished mature B cells from transitional B cells using this marker. The different expression profiles of CD21/CD23 on what we label AA4.1- (with distinguishable FO and MZ fractions, Fig. 1B) vs AA4.1+ (with T1 and T2, and no MZ fraction, Fig. 1B) indicates that this gating is accurate.
Fig. 1. B cell potential of progenitor populations in sublethally irradiated C57BL/6 recipients.
(A) Representative sort gates for the isolation of CLPs, kit-CLPs, MPPs, and LSKF- cells. (B) Representative analysis gates for splenic FO and MZ B cells and AA4.1+ transitional B cells. (C) Fraction of donor-derived transitional B cells in the spleen of sublethally irradiated C57BL/6 mice transplanted with CLPs, kit-CLPs, MPPs, or LSKF- cells (n = 4 * significantly different from kit-CLPs, CLPs and MPPs). (D) Ratio of MZ to FO B cells in donor-derived cells 3 weeks after transfer of 3,000 CLPs, kit-CLPs, MPPs, or LSKF- cells into sublethally irradiated C57BL/6 mice (n = 6, * significantly different from LSKF- cells). (E) Splenic B cell number 3 weeks after transfer of 3,000 CLPs, kit-CLPs, MPPs, or LSKF- cells into sublethally irradiated C57BL/6 mice (n = 6; * significantly different from kit-CLPs, CLPs and LSKF- cells).
Among donor-derived splenic B cells, kit-CLPs and CLPs had generated a larger fraction of mature B cells (defined as donor-derived CD45.1+AA4.1- cells expressing IgM and/or IgD, Fig. 1B) and a smaller fraction of transitional B cells (defined as CD45.1+AA4.1+IgMhi cells, Fig. 1B) than MPPs and, in particular, than LSKF- cells (Fig. 1C). This is a reflection of the developmental hierarchy of the injected populations, as earlier progenitors, LSKF- cells and MPPs, take longer to generate fully mature progeny (1-5,11). Nevertheless, up to 80 % of LSKF--derived B cells had a mature AA4.1- phenotype 3 weeks after transfer (Fig. 1C). There was a striking difference in ratio of mature MZ to FO B cells generated by these progenitors (see Fig. 1B for representative analysis gates). The MZ/FO ratio in the progeny of kit-CLPs and CLPs was threefold higher than in the progeny of LSKF- cells, while MPPs were in between these two extremes (Fig. 1D). Thus, CLPs, kit-CLPs on one hand and LSKF- cells on the other hand have qualitatively different potentials with the respect to the generation of mature B cells: CLPs and kit-CLPs have a relatively stronger propensity to generate MZ B cells than LSKF- cells, which are highly FO biased. A MZ fate is favored in conditions of lymphopenia (28,36-38), suggesting that the observed difference in B cell potential might be due to different degrees of lymphopenia in the host. However, as the injected populations competed with endogenous B cell development, their progeny encountered the same average degree of lymphopenia upon arrival in the spleen, thus eliminating bias introduced by varying levels of B cell reconstitution. Furthermore, splenic B cell number was similar in recipients of CLPs, kit-CLPs or LSKF- cells (Fig. 1E). Therefore, the difference in MZ/FO ratio among B cells derived from CLPs, kit-CLPs and LSKF- cells cannot be explained by different degrees of lymphopenia to which the cells are exposed. Nonetheless, it cannot be excluded that the lower MZ/FO ratio of MPP-derived B cells might be due to the fact that, because of the high proliferative capacity of MPPs, recipients of MPPs had the highest number splenic B cells. Thus, our observations suggest that distinct progenitor populations display cell-intrinsic qualitative differences in their capacity to generate mature B cell subtypes.
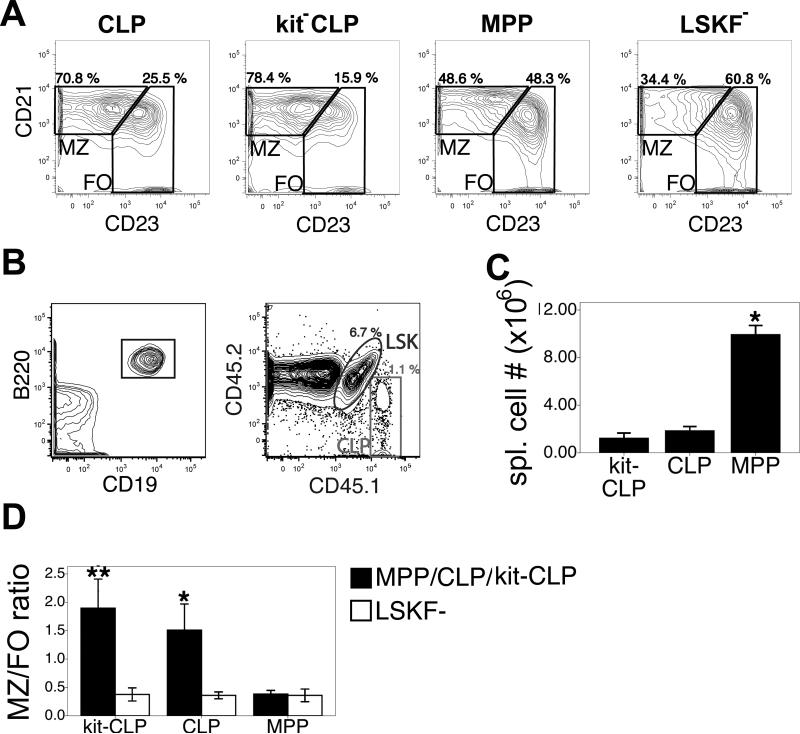
To find further support for this notion, we injected 1,500 CD45.1+ MPPs, CLPs, kit-CLPs or LSKF- cells into sublethally irradiated Rag1-/- hosts (CD45.2+), which have no T or B cells, and therefore provide an extremely lymphopenic environment to developing B cells (47). As shown in the representative example in Fig. 2A, even in such a lymphopenic environment, LSKF- cells generated relatively fewer MZ B cells than CLPs and kit-CLPs, while MPPs were in between these two extremes, confirming the higher MZ/FO differentiation ratio of CLPs and kit-CLPs compared to cells in the LSKF- population. In order to determine if different progenitor populations exhibit cell-intrinsic differences in their ability to generate specific mature B cells, we performed competitive transplants, in which 1,500 CD45.1+ MPPs, CLPs or kit-CLPs were injected together with 1,500 CD45.1+CD45.2+ LSKF- in sublethally irradiated Rag1-/- mice. (CD45.2, see Fig. 2B for a representative example of analysis gates of donor populations in the spleen). Since competing populations were exposed to the same environment, any difference in B cell development potential will be intrinsic to the respective progenitor populations. After three weeks, MPPs had generated tenfold more B cells than any of the other cell populations, so that recipients of MPPs + LSKF- cells were significantly less lymphopenic than recipients of either CLPs + LSKF- cells or kit-CLPs + LSKF- cells (Fig. 2C). The MZ/FO ratio among mature AA4.1- splenic B cells was 4- and 6-fold higher in CLP and kit-CLP-derived cells, respectively, compared to competing LSKF--derived cells (Fig. 2D). Despite varying degrees of lymphopenia in host mice (Fig. 2C), the MZ/FO ratio of LSKF--derived cells was equally low (Fig. 2D). Therefore, the differences in MZ/FO ratio between CLPs or kit-CLPs on one hand and LSKF- cells on the other hand must be cell intrinsic and cannot be explained by varying degrees of host lymphopenia. We observed several differences using Rag1-/- compared to C57BL/6 mice as hosts, however. In all progenitor populations the MZ/FO ratio was higher in Rag1-/- hosts than in C57BL/6 hosts (Fig. 1D, Fig. 2D). Furthermore, in contrast to experiments where sublethally irradiated C57LB/6 mice were used as recipients (Fig. 1), the MZ/FO ratio was slightly, though significantly (P = 0.017, paired t-test) higher in the progeny of kit-CLPs than of CLPs in Rag1-/- hosts (Fig. 2D). Furthermore, the MZ/FO ratio of kit-CLPs and CLPs in Rag1-/- hosts was ninefold higher than in C57BL/6 hosts. In contrast, the MZ/FO ratio of LSKF- cells was only fivefold higher than in C57BL/6 hosts. These observations indicate that lymphopenia affects the propensity to generate MZ B cells more strongly in CLPs and, in particular, in kit-CLPs than in LSKF- cells.
Fig. 2. B cell potential of progenitor populations in sublethally irradiated Rag1-/- recipients.
(A) Representative example of MZ/FO ratios in the spleens 3 weeks after transplantation of Rag1-/- mice with 1,500 CD45.1+ CLPs, kit-CLPs, MPPs or LSKF- cells. (B) Representative example of analysis gates to identify splenic B cells derived from competing populations (CD45.1+ CLPs and CD45.1+CD45.2+ LSKF- cells) in sublethally irradiated Rag1-/- hosts. (C) Splenic B cell number 3 weeks after transfer of 1,500 CD45.1+ CLPs, kit-CLPs or MPPs together with 1,500 CD45.1+CD45.2+ LSKF- cells into sublethally irradiated Rag1-/- mice (n = 3; * significantly different from mice receiving kit-CLPs, CLPs together with LSKF- cells). (D) Ratio of MZ to FO B cells in donor-derived cells 3 weeks after transfer 1,500 CD45.1+ CLPs, kit-CLPs or MPPs together with 1,500 CD45.1+CD45.2+ LSKF- cells into sublethally irradiated Rag1-/- mice (n = 3; * significantly different from MPPs and LSKF- cells, ** significantly different from CLPs, MPPs and LSKF- cells).
Collectively, these results demonstrate that cells within the LSKF- population can give rise to a B cell development program that is less biased to the development of MZ B cells and is less affected by lymphopenia than the program that emanates from CLPs and kit-CLPs.
B cells derived from LSKF- cells and from CLPs or kit- CLPs differ in Sca1 expression
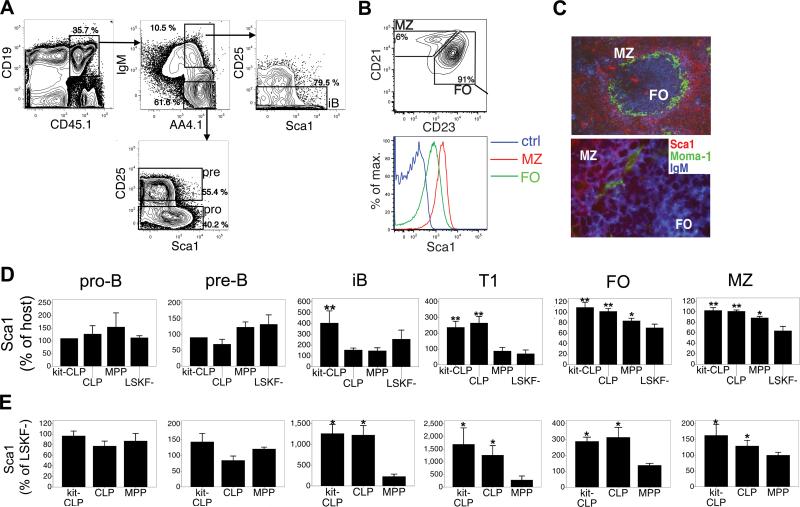
Next, we looked for markers that might distinguish B cells derived from LSKF- cells, CLPs and kit-CLPs. Both in competitive transplants in Rag1-/- hosts and in non-competitive transplants in sublethally irradiated C57BL/6 mice, we observed that B cells generated from kit-CLPs and CLPs expressed more Sca1, a marker of hematopoietic stem and progenitor cells and of activated T cells (48), than those derived from LSKF- cells. In wt mice (not shown) and in donor-derived B-lineage cells (Fig. 3A, shown for sublethally irradiated C57BL/6 mice), Sca1 is expressed at low levels in pro-B cells (CD19+IgM-AA4.1+CD25-), is mostly absent in pre-B cells (CD19+IgM-AA4.1+CD25+), and reappears in a fraction of iB cells after they lost CD25 expression. Mature splenic B cells express a wide range of Sca1 and Sca1 expression was higher on MZ than on FO B cells, possibly a reflection of the activated state of MZ B cells (Fig. 3B). By fluorescence microscopy, Sca1 marked the MZ, although Sca1+ B cells could be observed in the follicles (Fig. 3C). For reasons that are unclear, however, the difference in Sca1 expression between MZ and FO B cells appeared more pronounced when analyzed by fluorescence microscopy than by flow cytometry.
Fig. 3. Expression of Sca1.
(A) Analysis gates for donor derived pro-B, pre-B and iB cells and Sca1 expression on these fractions. Representative example of sublethally irradiated C57BL/6 mice transplanted with 3,000 MPPs. (B) Expression of Sca1 on MZ and FO B cells in C57LB/6 mice. (C) Fluorescence microscopy of the spleen after staining for Sca1, MOMA-1 (which detects matrix metallophilic macrophages that separate the follicles from the MZ) and IgM (upper 100x, lower 630x). (D) Expression of Sca1 on donor-derived B lineage cells 3 weeks after transfer of 3,000 of CLPs, kit-CLPs, MPPs, or LSKF- cells into sublethally irradiated C57BL/6 mice. Sca1 expression calculated as the ratio between the geometric mean of Sca1 fluorescence of kit-CLPs, CLPs, MPPs or LSKF- -derived cells and that of host cells of the same phenotype in each recipient. (n = 6, * significantly different from LSKF- cells, ** significantly different from MPPs and LSKF- cells). (E) Expression of Sca1 on donor-derived B lineage cells 3 weeks after transfer 1,500 CD45.1+ CLPs, kit-CLPs or MPPs together with 1,500 CD45.1+CD45.2+ LSKF- cells into sublethally irradiated Rag1-/- mice. Sca1 expression calculated as the ratio between the geometric mean of Sca1 fluorescence of kit-CLPs, CLPs, MPPs -derived cells and that of LSKF—derived cells of the same phenotype in each recipient. (n = 3; * significantly different from LSKF- cells).
In non-competitively transplanted C57BL/6 hosts, we calculated the ratio between the geometric mean of the Sca1 fluorescence between cells derived from kit-CLPs, CLPs, MPPs or LSKF- cells and the geometric mean of the Sca1 fluorescence of endogenous cells of the same phenotype in each recipient. The expression of Sca1 on pre- and pro-B cells was similar in all donor-derived populations (Fig 3D). However, iB derived from kit-CLPs and T1 cells derived from both CLPs and kit-CLPs expressed approximately twofold more Sca1 than those derived from LSKF- cells (Fig. 3D). Similar observations were made in donor-derived mature B cells. Likely because of the higher baseline expression of Sca1 on mature B cells (Fig. 3B) these differences were less pronounced, though they were still statistically significant (Fig. 3D). Sca1 expression on MPP-derived cells was variable, and in between that of cells generated from CLPs and kit-CLPs, and from LSKF- cells, respectively (Fig. 3D). No progenitor-dependent differences were observed in the expression of CD21, CD23, IgM or IgD in any of the donor-derived populations in the spleen (not shown).
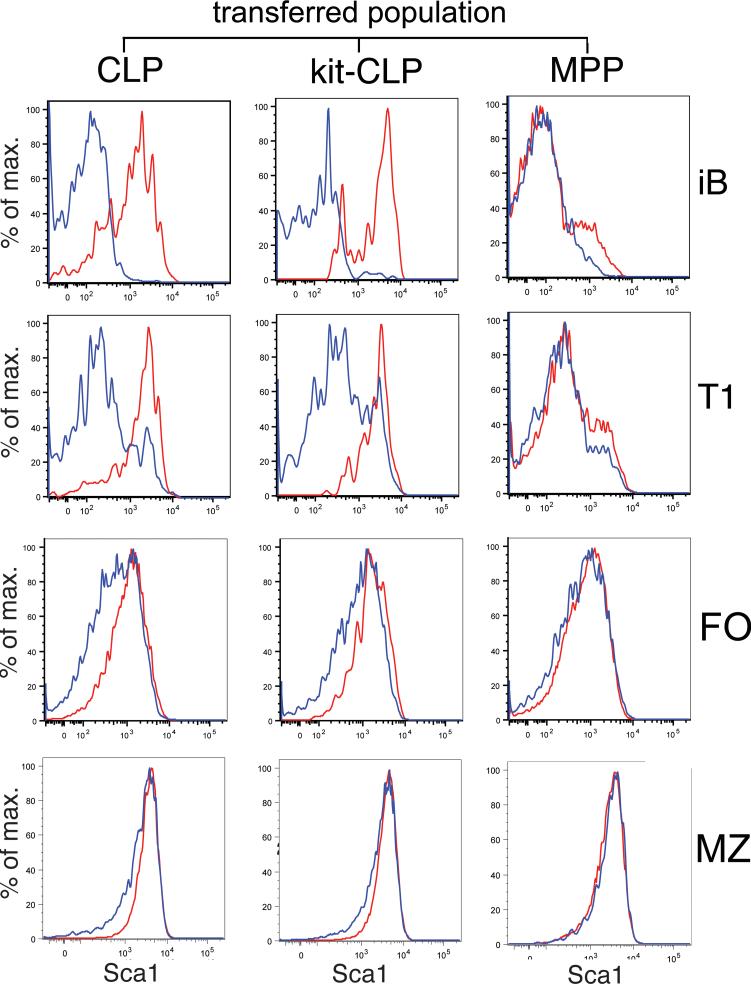
In competitively repopulated Rag1-/- hosts, we compared Sca1 expression by calculating the ratio between the geometric mean of Sca1 fluorescence between cells derived from either kit-CLPs, CLPs or MPPs and the geometric mean of the Sca1 fluorescence of mature B cells originating from the competing LSKF- cells, which served as an internal control (Fig. 3E, Fig. 4 for a representative example). As in C57BL/6 recipients, in Rag1-/- hosts expression of Sca1 on donor pro- and pre-B cells was similar irrespective of their progenitor cell origin (Fig. 3E). However, both CLPs and kit-CLPs produced iB and T1 cells that expressed tenfold more Sca1 than LSKF- cells. FO and MZ B cells originating from CLPs and kit-CLPs expressed threefold and 50 % more Sca1 than those derived from LSKF- cells, respectively. MPP-derived cells expressed Sca1 at levels slightly, though not statistically significantly higher than that of LSKF- cells (Fig. 3E, Fig. 4). There were no progenitor-dependent differences in the expression of CD21, CD23, IgM or IgD in any of the donor-derived populations in the spleen (not shown). It is interesting to note that lymphopenia differentially affected Sca1 expression depending on the progenitor origin of the cells, as the difference in Sca1 expression on iB, T1, MZ and FO cells derived from kit-CLPs and CLPs on one hand and LSKF- cells on the other hand was more pronounced in Rag1-/- than C57BL/6 hosts (Fig. 3D,E). Furthermore, while kit-CLPs generated iB cells with elevated Sca1 expression in both C57BL/6 and Rag1-/- host, CLPs only generated such iB cells in lymphopenic Rag1-/- hosts. These data indicate that, not only in terms of MZ/FO ratio, but also in terms of Sca1 expression, the B cell development program that emanates from CLPs and kit-CLPs and is more responsive to lymphopenia than that originating from LSKF- cells.
Fig. 4. Representative example of Sca1 expression.
Histograms of Sca1 expression on donor-derived iB, T1, MZ and FO B cells in Rag1-/- mice three weeks after competitive co-transplantation of 1,500 LSKF- cells with 1,500 CLPs, kit-CLPs or MPPs (representative of 3 independent experiments).
As Sca1 is an activation marker on lymphoid cells (48), we hypothesized that Sca1hi B cells derived from CLPs and from kit-CLPs would be more activated that Sca1lo B cells generated from LSKF- cells. We transferred these populations into Rag1-/- mice, isolated donor-derived MZ and FO B cells, and cultured these in the presence of LPS. As expected, MZ B cells proliferated more vigorously than FO B cells (49), but no progenitor cell-dependent differences were observed (Fig. 5A). Similarly, no progenitor-origin-dependent difference in the induction of the activation markers, CD86 and MHC II, were found (not shown). Finally, we transferred kit-CLPs (not shown), CLPs (Fig. 5B, upper panel), and LSKF- cells (Fig. 5B, lower panel) into Rag1-/- mice, and analyzed anti-IgM-induced Ca2+-flux of donor-derived FO and MZ B cells three weeks later, using spleen cells from wt C57BL/6 mice added before addition of anti-IgM as an internal control. While Ca2+-flux was consistently higher in MZ than in FO B cells, there were no differences in Ca2+-flux dependent on progenitor origin of the cells (Fig. 5B). These data suggest that differences in Sca1 expression between cells derived from kit-CLPs or CLPs on one hand, and from LSKF- cells on the other hand are unlikely to be explained by a difference in activation status.
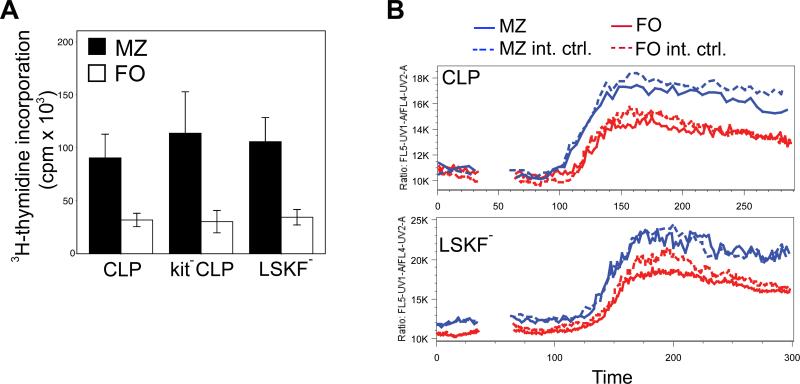
Fig. 5. Functional analysis of FO and MZ B cells according to progenitor origin.
(A) LPS-induced proliferation, measured by 3H-thymidine incorporation, of MZ and FO B cells generated from 1,500 CLPs, kit-CLPs and LSKF- cells three weeks after transfer into Rag1-/- mice (n = 3 independent experiments, all differences between MZ and FO B cells P < 0.05). (B) Ca2+-flux, as measured by ratiometric analysis of Indo-1 fluorescence gated on FO and MZ B cells generated 3 weeks after adoptive transfer of 1,500 CD45.1+ CLPs (upper panel) and LSKF- cells (lower panel) into Rag1-/- mice. Wt CD45.2+ C57BL/6 spleen cells were added to the samples as an internal control, and analyzed according to the same MZ/FO gates.
We conclude that B cell development from either CLPs or kit-CLPs results in B cells that express more Sca1 than those derived directly from LSKF- cells.
Different potential of Sca1+ and Sca1- iB cells
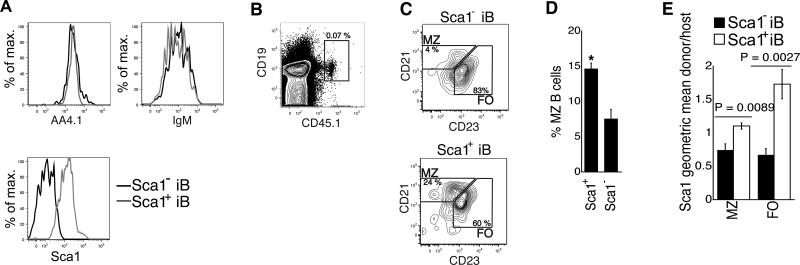
As expression of Sca1 on donor-derived iB cells was associated with the increased MZ bias of donor-derived mature B cells, and as a fraction of iB cells (AA4.1hiB220+IgM+) in wt mice express Sca1 (Fig. 3A), we tested the hypothesis that high expression of Sca1 on iB cells confers increased propensity to generate MZ B cells. We injected 5×105 Sca1+ and Sca1- iB cells from CD45.1+ C57BL/6 donors into unconditioned CD45.2+ C57BL/6 recipients (see Fig. 6A for representative sort purities). At least a fraction of iB cells are poised to leave the bone marrow and home to the spleen and could therefore be expected to show some engraftment in the absence of lymphopenia. Up to 5 % of injected donor cells were found in the spleen (0.005 to 0.07 % of total spleen population, Fig. 6B). After four to six days, the MZ fraction of the donor-derived mature B cells was twofold higher after transfer of Sca1hi iB cells than of Sca1lo iB cells (Fig. 6C,D). Furthermore, Sca1+ iB cells generated FO and MZ B cells that expressed more Sca1 than endogenous FO and MZ B cells, while FO and MZ B cells generated from Sca1- iB cells expressed less Sca1 than their host counterparts (Fig. 6E). We conclude that higher Sca1 expression in iB cells is associated with the generation of B cells with a higher expression of Sca1 and with increased tendency to generate MZ B cells, even in unconditioned hosts. These results are consistent with the fact the CLPs and kit-CLPs generate iB cells that express more Sca1 than iB cells generated from LSKF- cells, and that mature B cells derived from these progenitors express more Sca1 and display a higher MZ/FO differentiation ratio than those derived from LSKF- cells.
Fig. 6. Potential of Sca1+ and Sca1- iB cells.
(A) Representative example of Sca1, IgM and AA4.1 expression of sorted Sca1+ and Sca1- iB cells from the BM of C57BL/6 mice. >95 % of the cells fell in the sort windows in each experiment. (B) Representative example of the detection of donor cells 4 days after transfer of 5.105 CD45.1+ iB cells into a unconditioned CD45.2+ C57BL/6 host. (C) Expression of CD21 and CD23 on donor-derived cells 4 days after adoptive transfer of and Sca1+ and Sca1- iB cells from congenic CD45.1+ mice into unconditioned CD45.2+ C57BL/6 mice. (D) Fraction of MZ B cells among donor-derived cells 4 days (in one of the 4 experiments, 6 days) after adoptive transfer of Sca1+ and Sca1- iB cells from congenic CD45.1+ into unconditioned CD45.2+ C57BL/6 mice (n = 4, P = 0.006). (E) Average Sca1 expression on FO and MZ B cells generated from adoptively transferred Sca1+ and Sca1- iB cells, calculated as the ratio between geometric mean of Sca1 fluorescence of donor-derived cells and that of host cells of the same phenotype (MZ or FO) in each recipient (n = 4).
CLPs, kit-CLPs and LSKF- cells differ in B1 potential
CLPs and kit-CLPs behaved very similarly with respect to their potential to generate MZ and FO B cells. Both populations differ, however, as kit-CLPs have lower myeloid potential, TdT and Rag expression than CLPs (21,22). In the peritoneal cavity (PC), both B2 (B220+Mac1-) and B1 (B220loMac1+) cells occur (Fig. 7A) (25,26). B1 cells are additionally distinguished from B2 cells by their higher IgM expression (Fig. 7A) (25,26). To further examine functional differences between CLPs and kit-CLPs, we assessed their potential to generate B1 B cells by measuring relative B1 potential as the ratio between B1 and B2 cells in the PC (see Fig. 7A for representative analysis gates). Both in sublethally irradiated C57BL/6 and Rag1-/- hosts, the B1/B2 ratio of transferred kit-CLPs was significantly stronger than that of CLPs and MPPs. The B1/B2 ratio of LSKF- cells appeared somewhat higher than that of CLPs and MPPs in both types of recipients, but this difference failed to reach statistical significance both using parametric and non-parametric tests (Fig. 7B,C). Similar to splenic B2 cells, Sca1 expression was higher on B1 cells derived from the kit-CLPs than from LSKF- cells (shown in Fig. 7D for Rag1-/- competitively repopulated with LSKF- cells and kit-CLPs). We also note that the B1/B2 ratio was similar in Rag1-/- and C57BL/6 hosts, suggesting that, in contrast to MZ B cell development, B1 development is not affected by lymphopenia (Fig. 7B,C). Typical Ig heavy chains rearrangement of B1 cells, VH11 and VHQ52 (50), were present in all B1 populations, irrespective of progenitor origin (Fig. 7E). Furthermore, consistent with the observation that CD5 is typically expressed on B1 cells derived from fetal and not from adult progenitors (45), CD5 expression on B1 cells derived from all progenitor populations under study here was low to absent (not shown). We conclude that CLPs and kit-CLP differ in their B1 potential, and that B1 B cells originating from kit-CLPs express more Sca1 than those originating from LSKF- cells.
Fig. 7. B1 potential of progenitor populations.
(A) Representative analysis windows for the detection of B1 and B2 cells in the PC of transplanted mice (the example shown is a sublethally irradiated C57BL/6 host transplanted with kit-CLPs). (B) B1/B2 ratio in donor-derived cells in the PC 3 weeks after transfer of 3,000 CLPs, kit-CLPs, MPPs, or LSKF- cells into sublethally irradiated C57BL/6 mice (n = 6, * significantly different from CLPs and MPPs cells). (C) B1/B2 ratio in donor-derived cells in the PC 3 weeks after transfer of 1,500 CD45.1+ CLPs, kit-CLPs or MPPs together with 1,500 CD45.1+CD45.2+ LSKF- cells into sublethally irradiated Rag1-/- mice (n = 4; * significantly different from CLPs and MPPs). (D) Representative example of Sca1 expression on B 1 cells derived from LSKF- cells and from kit-CLPs in a Rag1-/- recipient competitively transplanted with both populations. (E) Nested DNA PCR for the rearrangement of VH11 and VHQ52 in B1 cells isolated by cell sorting from Rag1-/- mice after adoptive transfer of CLPs, kit-CLPs or LSKF- cells.
Discussion
We have shown here that progenitors in three distinct populations, CLPs, kit-CLPs and LSKF- cells display quantitatively different potentials to give rise to various types of mature B cells. The LSKF- fraction contains a B cell precursor that is functionally distinct from CLPs and kit-CLPs and gives rise in the short term to a FO-biased B cell development program. Alternatively, it is possible that the HSCs within this fraction give rise to such a B cell precursor in the short term. CLPs and kit-CLPs are more MZ-biased than LSKF- cells, in particular in conditions of lymphopenia, while kit-CLPs have the strongest B1 potential. Distinct progenitor populations give rise to a maximal output of mature B cells, such as B1 cells, at different time points, dependent on the overall maturation state of the respective progenitor populations (51). We observed, however, that all populations investigated here are capable of generating mature B cells within three weeks of transfer, and that the ratios between the types of mature B cell generated at this early time point differ remarkably. These qualitative differences in B cell potential are surprising. Harman et al. (22), for example, reported that each donor population (CLPs and kit-CLPs) generated B cell subsets at comparable frequencies 3 months after transfer into non-lymphopenic B6 hosts. However, a problem in the interpretation of later time points with respect to qualitative differences in B cell potential is that local homeostatic forces have had an opportunity to alter the fate of mature cells. It has been shown previously that small resting B cells can assume a MZ phenotype in conditions of lymphopenia. Furthermore, B1 cells can undergo homeostatic proliferation (25), and, very likely, MZ B cells can do the same, as in conditions where B cell production has ceased due to conditional deletion of Rag1, MZ and B1 compartment are maintained (36). Therefore, any differences in the intrinsic potential of distinct progenitor populations may become more difficult to detect at later time points because of the effect of local homeostatic forces. Thus assessment of B cell potential at an early time point more likely reveals intrinsic differences in B cell potential. Another caveat in the interpretation of our data, and of any experiments probing the potential of progenitor populations in vivo, is that adoptive transfer into lymphopenic or conditioned hosts may not reflect the function and potential of these progenitors in steady-state. However, we also observed differences of the MZ/FO ratio after adoptive transfer of Sca1+ and Sca1- iB cells into lymphocyte-replete, unconditioned hosts, which is as close to steady-state as is currently possible.
The progeny of CLPs and kit-CLPs does not only differ from that of LSKF- cells in terms of MZ/FO ratio, but also in Sca1 expression, as B cells derived from CLPs and kit-CLPs express more Sca1 than those derived from the LSKF- fraction. MPPs share B cell differentiation characteristics with both LSKF- cells and CLPs, and are therefore likely a heterogeneous transitional population with respect to early B cell development. Before the iB stage, however, Sca1 expression is similar in cells derived from CLPs, kit-CLPs, MPPs or LSKF- cells. Since Sca1 is induced by inflammatory cytokines (52), it is possible that Sca1 expression is a reflection of differential response of the cells to stress, either caused by irradiation or by lymphopenia. However, the adoptive transfer experiments of iB cells were performed in unconditioned, lymphocyte-replete host, arguing against this notion. As Sca1 is an activation marker (48), it is also possible that CLPs and kit-CLPs generate intrinsically more ‘activated’ B cells and are therefore more readily recruited into the MZ population, which have a more activated phenotype than FO B cells (27,28). However, the absence of progenitor origin-dependent differences in LPS-induced proliferation, CD86 and MHC II expression, as well as in anti-IgM-induced Ca2+-flux suggest that the activation status of Sca1hi and Sca1lo B cells is similar. It is therefore possible that from the iB stage on, Sca1 expression may be a reflection of lineal origin. The difference in Sca1 expression is too subtle to use this marker to determine the progenitor origin of FO or MZ B cells in unmanipulated wt mice, however.
Although lymphopenia can induce homeostatic proliferation of MZ and B1 B cells (36,37), and although B cell development emanating from CLPs and kit-CLPs is, both in terms of Sca1 expression and MZ bias, more responsive to lymphopenia than the program originating directly from the LSKF- compartment, the observed variation in differentiation ratios cannot be explained by varying degrees of host lymphopenia. In C57BL/6 hosts, the injected populations competed with endogenous B cell development. Therefore, the progeny of all transferred populations encountered a similar degree of lymphopenia during differentiation and upon arrival in the spleen. Furthermore, total B cell number was similar in recipients of CLPs, kit-CLPs and LSKF- cells, while the MZ/FO ratio and the expression of Sca1 in B cells generated from these progenitors was different. Similarly, in Rag1-/- recipients the MZ/FO ratio of LSKF--derived cells was equally low despite varying degrees of B cell reconstitution by competing MPPs, CLPs or kit-CLPs. Finally, the difference in MZ/FO ratio between both programs is also observed in unconditioned hosts, as demonstrated by the transplants of iB cells (see Fig. 6). The observed variation in differentiation ratios among CLPs, kit-CLPs and LSKF- cells must therefore be caused by cell intrinsic mechanisms.
If all B cells were derived from CLPs, then no matter which progenitor population upstream from CLPs (MPPs or LSKF- cells) is transferred in vivo, the various types of peripheral B cells should be recovered in similar ratios. The only expected difference would be a developmental delay of the earliest compared to the most differentiated population. Our observations do not fit this model, as we find that CLPs, kit-CLPs and LSKF- cells have intrinsically distinct potentials to generate mature B cells. We suggest a model where LSKF- B cell progenitors generate a FO-biased B cell population with a Sca1 expression at the lower end of the Sca1 distribution on mature B cells, while CLPs and kit-CLPs belong to a program that generates B cell populations with a stronger tendency to generate MZ B cells, and with a Sca1 expression at the upper end of the Sca1 distribution on mature B cells. Importantly, CLP and kit-CLP-derived B cell development is more responsive to lymphopenia than LSKF--derived B cell development, further underscoring the functional distinction between both programs. As such, our findings have two major implications. First, this model differs from models of early hematopoietic differentiation where at each successive stage of differentiation, dichotomous cell fate choices lead to progressive specification of progenitors to a given lineage (1). This model was already challenged by the observations that erythroid and megakaryocytic potential is lost before HSCs reach the MPP stage (2), and that T cell development independent of CLPs is possible (14-18). These controversies are not yet resolved however (3,10,11). A second major implication of our observations is that progenitor cell origin adds another layer of control to B cell fate determination. Although Ig gene rearrangements are not yet completed at the CLP, kit-CLP, MPP or LSKF- stage, these observations do not rule out a role for BCR signaling (27-35) however, as the various differentiation programs may differ in the expression of proteins that regulate BCR signaling.
The lineage relation between kit-CLPs and CLPs is unclear. Both populations generate B cells with elevated MZ/FO ratio and Sca1 expression, and therefore likely belong to the same B cell differentiation program. However, in many respects, kit-CLPs appear more ‘committed’ to this program, as their MZ/FO ratio was higher than that of CLPs, at least in Rag1-/- mice, and as in C57BL/6 hosts, kit-CLPs but not CLPs also generated Sca1hi iB cells. These findings, in addition to their lower proliferative capacity (21) would place kit-CLPs downstream of CLPs. However, kit-CLPs have a broader differentiation potential, as they can generate B1 cells more efficiently than do CLPs. Furthermore, kit-CLPs are also characterized by lower expression of Rag genes (21,22) compared to CLPs. As Rag genes are progressively upregulated during lymphoid commitment (7,19,20), these expression data would place kit-CLPs upstream of CLPs. It is possible that CLPs and kit-CLPs both originate from a progenitor within the MPP population, and represent distinct, parallel progenitors within the same differentiation program.
In conclusion, the findings presented here indicate that progenitor origin contributes to cell fate choices of mature B cells and suggest that CLP-independent B cell development programs may exist.
Acknowledgments
This work was supported by grant NIH R01 AG16327 to HWS.
Abbreviations used in this paper
- HSC
hematopoietic stem cells
- LSKF-
lin-Sca1+kit+flt3-
- MPP
multipotential progenitors
- CLP
common lymphoid progenitors
- MZ
marginal zone
- FO
follicular
- T
transitional
- iB
immature B cells
- BM
bone marrow
- PC
peritoneal cavity
- wt
wild type
References
- 1.Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 2.Adolfsson J, Mansson R, Buza-Vidas N, Hultquis A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Forsberg EC, Serwold T, Kogan S, Weissman IL, Passegue E. New evidence supporting megakaryocyte-erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell. 2006;126:415–426. doi: 10.1016/j.cell.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 4.Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(-)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–69. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 5.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc. Natl. Acad. Sci USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 7.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 8.Kouro T, Kumar V, Kincade PW. Relationships between early B- and NK- lineage lymphocyte precursors in bone marrow. Blood. 2002;100:3672–3680. doi: 10.1182/blood-2002-02-0653. [DOI] [PubMed] [Google Scholar]
- 9.Izon D, Rudd K, DeMuth W, Pear WS, Clendenin C, Lindsley RC, Allman D. A common pathway for dendritic cell and early B cell development. J. Immunol. 2001;167:1387–1392. doi: 10.4049/jimmunol.167.3.1387. [DOI] [PubMed] [Google Scholar]
- 10.Karsunky H, Inlay MA, Serwold T, Bhattacharya D, Weissman IL. Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages. Blood. 2008;111:5562–5570. doi: 10.1182/blood-2007-11-126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serwold T, Ehrlich LI, Weissman IL. Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood. 2009;113:807–815. doi: 10.1182/blood-2008-08-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J. Exp. Med. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manz MG, Traver D, Miyamoto T, Weissman IL, Akashi K. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood. 2001;97:3333–3341. doi: 10.1182/blood.v97.11.3333. [DOI] [PubMed] [Google Scholar]
- 14.Allman D, Sambandam A, Kim S, Miller JP, Pagan A, Well D, Meraz A, Bhandoola A. Thymopoiesis independent of common lymphoid progenitors. Nat. Immunol. 2003;4:168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nat. Immunol. 2004;5:953–960. doi: 10.1038/ni1101. [DOI] [PubMed] [Google Scholar]
- 16.Bhandoola A, von Boehmer H, Petrie HT, Zúñiga-Pflücker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26:678–689. doi: 10.1016/j.immuni.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452:764–767. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- 18.Wada H, Masuda K, Satoh R, Kakugawa K, Ikawa T, Katsura Y, Kawamoto H. Adult T-cell progenitors retain myeloid potential. Nature. 2008;452:768–772. doi: 10.1038/nature06839. [DOI] [PubMed] [Google Scholar]
- 19.Welner RS, Pelago R, Kincade PW. Evolving views on the genealogy of B cells. Nat. Rev. Immunol. 2008;82:95–106. doi: 10.1038/nri2234. [DOI] [PubMed] [Google Scholar]
- 20.Hardy RR, Kincade PW, Dorshkind K. The protean nature of cells in the B lymphocyte lineage. Immunity. 2007;26:703–714. doi: 10.1016/j.immuni.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Kumar R, Fossati V, Israel M, Snoeck HW. Lin-Sca1+Kit- bone marrow cells contain primitive lymphoid-committed precursors that are distinct from common lymphoid progenitors. J. Immunol. 2008;181:7507–7513. doi: 10.4049/jimmunol.181.11.7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harman BC, Northrup DL, Allman D. Resolution of unique Sca-1highc-Kit- lymphoid-biased progenitors in adult bone marrow. J. Immunol. 2008;181:7514–7524. doi: 10.4049/jimmunol.181.11.7514. [DOI] [PubMed] [Google Scholar]
- 23.Hardy RR, Hayakawa K. B cell development pathways. Annu. Rev. Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 24.Schlissel MS. Regulating antigen-receptor gene assembly. Nat. Rev. Immunol. 2003;3:890–899. doi: 10.1038/nri1225. [DOI] [PubMed] [Google Scholar]
- 25.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 2001;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 26.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat. Rev. Immunol. 2007;7:213–219. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 27.Pillai S, Cariappa A, Moran S. Marginal zone B cells. Annu. Rev. Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 28.Martin F, Kearney JF. Marginal-zone B cells. Nat. Rev. Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 29.Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat. Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 30.Lam KP, Rajewsky K. B cell antigen receptor specificity and surface density together determine B-1 versus B-2 cell development. J. Exp. Med. 1999;190:471–477. doi: 10.1084/jem.190.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin F, Kearney JF. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19 and btk. Immunity. 2000;12:39–49. doi: 10.1016/s1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
- 32.Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K, Pillai S. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14:603–615. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 33.Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, Silver J, Hardy RR. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 34.Hayakawa K, Asano M, Shinton SA, Gui M, Wen LJ, Dashoff J, Hardy RR. Positive selection of anti-thy-1 autoreactive B-1 cells and natural serum autoantibody production independent from bone marrow B cell development. J. Exp. Med. 2003;197:87–99. doi: 10.1084/jem.20021459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen L, Brill-Dashoff J, Shinton SA, Asano M, Hardy RR, Hayakawa K. Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 2005;23:297–308. doi: 10.1016/j.immuni.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Hao Z, Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J. Exp. Med. 2001;194:1151–1150. doi: 10.1084/jem.194.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)- mice. J. Exp. Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agenes F, Freitas AA. Transfer of small resting B cell into immunodeficient hosts results in the selection of a self-renewing activated B cell population. J. Exp. Med. 1999;189:319–329. doi: 10.1084/jem.189.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srivastava B, Quinn WJ, 3rd., Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J. Exp. Med. 2005;202(9):1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–412. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 41.Saito T, Chiba S, Ichikawa M, Kunisato A, Asai T, Shimizu K, Yamaguchi T, Yamamoto G, Seo S, Kumano K, Nakagami-Yamaguchi E, Hamada Y, Aizawa S, Hirai H. Notch2 is p referentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 2003;18:675–85. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 42.Tan JB, Xu K, Cretegny K, Visan I, Yuan JS, Egan SE, Guidos CJ. Lunatic and manic fringe cooperatively enhance marginal zone B cell precursor competition for delta-like 1 in splenic endothelial niches. Immunity. 2009;30(2):254–263. doi: 10.1016/j.immuni.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Kantor AB, Stall AM, Adam S, Herzenberg LA, Herzenberg LA. Differential development of progenitor activity for three B cell lineages. Proc. Natl. Acad. Sci. USA. 1992;89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 46.Tung JW, Mrazek MD, Yang Y, Herzenberg LA, Herzenberg LA. Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse. Proc. Natl. Acad. Sci. U S A. 2006;103:6293–6298. doi: 10.1073/pnas.0511305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 48.Shevach EM, Korty PE. Ly-6: a multigene family in search of a function. Immunol. Today. 1989;10:195–200. doi: 10.1016/0167-5699(89)90324-1. [DOI] [PubMed] [Google Scholar]
- 49.Oliver AM, Martin F, Gartland GL, Carter R. H. RH, Kearney JF. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur. J. Immunol. 1997;27:2366–2374. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- 50.Seidl KJ, Wilshire JA, MacKenzie JD, Kantor AB, Herzenberg LA, Herzenberg LA. Predominant VH genes expressed in innate antibodies are associated with distinctive antigen-binding sites. Proc. Natl. Acad. Sci. USA. 1999;96:2262–2267. doi: 10.1073/pnas.96.5.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc. Natl. Acad. Sci. USA. 2009;106:5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sinclair A, Daly B, Dzierzak E. The Ly-6E.1 (Sca-1) gene requires a 3′ chromatin-dependent region for high-level gamma-interferon-induced hematopoietic cell expression. Blood. 1996;87:2750–2761. [PubMed] [Google Scholar]