Abstract
TCF-1 and LEF-1, the effector transcription factors of the canonical Wnt pathway, are known to be critical for normal thymocyte development. However, it is largely unknown if it has a role in regulating mature T cell activation and T cell-mediated immune responses. Here we demonstrate that like IL-7Rα and CD62L, TCF-1 and LEF-1 exhibit dynamic expression changes during T cell responses, being highly expressed in naïve T cells, downregulated in effector T cells, and upregulated again in memory T cells. Enforced expression of a p45 TCF-1 isoform limited the expansion of antigen-specific CD8 T cells in response to Listeria monocytogenes infection. However, when the p45 transgene was coupled with ectopic expression of stabilized β-catenin, more antigen-specific memory CD8 T cells were generated, with enhanced ability to produce IL-2. Moreover, these memory CD8 T cells expanded to a larger number of secondary effectors and cleared bacteria faster when the immunized mice were rechallenged with virulent L. monocytogenes. Furthermore, in response to vaccinia virus or lymphocytic choriomeningitis virus infection, more antigen-specific memory CD8 T cells were generated in the presence of p45 and stabilized β-catenin transgenes. Although activated Wnt signaling also resulted in larger numbers of antigen-specific memory CD4 T cells, their functional attributes and expansion after the secondary infection were not improved. Thus, constitutive activation of the canonical Wnt pathway favors memory CD8 T cell formation during initial immunization, resulting in enhanced immunity upon second encounter with the same pathogen.
Protective cellular immunity relies on antigen-specific CD4 and CD8 T cells, which exist at very low frequencies in naïve hosts (1-3). After infection or immunization, naïve T cells undergo massive clonal expansion, generating large numbers of antigen-specific effector T cells equipped with cytokines and/or cytolytic molecules to combat pathogens. The proliferative expansion phase is followed by a rapid decline of antigen-specific T cells, and such contraction of the effector T cells does not seem to be correlated with clearance of the pathogens (4). A fraction of antigen-specific T cells (approximately 5-10%) survive the contraction phase and form a pool of memory T cells, which will respond with accelerated expansion rate upon re-encounter with the same antigen. This immunological memory is the basis for vaccination, and much effort has been devoted to improve vaccine design through manipulating T cell responses to maximize memory T cell formation (1, 5-7). Three major classes of signals are known to be critical for T cell activation and transition to memory T cells, i.e., signal 1 from antigen stimulation of TCR, signal 2 derived from co-stimulatory molecules such as CD28, and signal 3 from pro-inflammatory cytokines including IL-12 and type I interferons (1, 2). Recent studies demonstrated that the effect of inflammatory cytokines such as IL-12 on effector and memory CD8+ T cells may be at least partly mediated by differential expression of the T-box transcription factor, T-bet (8, 9). It is not known whether additional signals such as morphogenic Wnt signaling and its downstream transcriptional programs have a role in modulating T cell responses.
Wnt proteins are secreted, lipid-modified glycoproteins that activate multiple signal transduction pathways to regulate a variety of cellular activities, including cell fate determination, proliferation, and gene expression (10, 11). The canonical Wnt pathway transduces signals via the intracellular mediator β-catenin. In the absence of interaction between Wnt and its Frizzled receptors, β-catenin is kept at a low level by a multi-molecular “destruction complex” containing casein kinase I and glycogen synthase kinase 3. These two kinases sequentially phosphorylate a set of conserved serine and threonine residues in the N-terminus of β-catenin, and the resulting phosphorylated footprint marks β-catenin for constant degradation by the proteosome. Under this condition, the Wnt effector transcription factors T cell factor-1 (TCF-1) and lymphoid enhancer-binding factor 1 (LEF-1) are associated with Groucho/transducin-like enhancer of split (TLE) corepressor proteins and act as transcriptional repressors. When Wnt ligand binds to Frizzled receptors and co-receptors, glycogen synthase kinase 3 activity is inhibited and the “destruction complex” is inactivated, resulting in accumulation of β-catenin in the cytoplasm. Upon entering the nucleus, β-catenin replaces Groucho/TLE, forms complexes with TCF-1/LEF-1, and activates the transcription of Wnt target genes. Although LEF-1-null mice did not display abnormalities in T cell development, inactivation of TCF-1 resulted in incomplete blocks at multiple early T cell developmental stages (12). TCF-1 and LEF-1 double deficiency completely arrested T cell development at the immature single positive thymocyte stage (13), indicating functional redundancy between TCF-1 and LEF-1 and a relatively dominant role of TCF-1 during T cell development. Ablation of β-catenin was initially found to perturb β-selection during thymocyte development (14), however, recent studies indicate that both β-catenin and γ-catenin are dispensable for thymopoiesis (15-17). Nevertheless, deletion of phosphorylation sites in the N-terminus of β-catenin led to accumulation and constitutive activation of the canonical Wnt pathway, and when driven by a T cell specific promoter, the stabilized β-catenin transgene enhances thymocyte production by extending thymocyte survival (18, 19).
In spite of its critical roles during T cell development, TCF-1 appeared to be dispensable for T cell proliferation and cytotoxic function upon activation in vitro (20), albeit the canonical Wnt signaling has been shown to be operative in naïve or activated T cells (17). A recent study demonstrated that overexpression of stabilized β-catenin can increase the survival of CD4+CD25+ regulatory T cells and induce an anergic phenotype in CD4+CD25- effector T cells (21). In the current study, we investigated the effect of constitutive activation of Wnt signaling on CD4 and CD8 T cell responses to infection.
Materials and Methods
Mice and infectious agents
βCat-Tg and p45-Tg were as described (19, 22). p45-Tg mice were backcrossed more than 10 times to C57BL/6 background, and βCat-Tg mice were backcrossed to C57BL/6 for 4 generations before crossed to p45-Tg. βCat and p45 single transgenic mice were used as breeders and littermates of all 4 genotypes (i.e., single transgenic mice, dTg, and no transgene controls) were used in this study for parallel comparisons. Listeria monocytogenes (LM), lymphocytic choriomeningitis virus (LCMV), and vaccinia virus (VacV)-infected mice were housed in accordance with institutional biosafety regulations. All animal experiments followed protocols approved by the Institutional Animal Care and Use Committee at the University of Iowa.
LM expressing ovalbumin (LM-Ova) was a gift from Dr. Hao Shen (University of Pennsylvania) and Dr. Leo Lefrancois (University of Connecticut) (23). Attenuated LM-Ova was created by introducing an in-frame deletion in the actA gene as described (referred to as actA-LM-Ova) (24). Naïve transgenic mice and littermate controls were first i.v. infected with 5 × 106 CFU (colony forming unit) actA-LM-Ova. Some of the mice were challenged with 7 × 105 CFU virulent LM-Ova via i.v. injection on day 45 after the primary infection. Bacteria were grown and quantified as previously described (25). The numbers of LM-Ova (CFUs) present in the livers and spleens were determined on day 3 after the secondary infection as previously described (25). In experiments evaluating CD8 T cell responses to viral infection, mice were infected via intraperitoneal injection with 3 × 106 PFU (plaque forming unit) of VacV expressing ovalbumin (VacV-Ova) (26) or 2 × 105 PFU of LCMV-Armstrong (27).
Detection of antigen-specific T cells
Splenocytes were harvested on indicated days after primary or secondary infection, and stimulated with following peptides for 5-6 hrs in the presence of brefeldin A (BD Biosciences): 5 μM LLO190-201 peptide (NEKYAQAYPNVS) for CD4 responses after LM-Ova infection, 200 nM of Ova257-264 peptide (SIINFEKL) for CD8 responses after LM-Ova or VacV-Ova infection, 200 nM each of GP33-41 (KAVYNFATC), NP396-404 (FQPQNGQLI), NP205-212 (YTVKYPNL), or GP276-286 (SGVENPGGYCL) peptide for CD8 responses after LCMV infection. The stimulated cells were then surfaced stained, fixed and permeabilized, and intracellularly stained for IFN-γ, and in some cases IL-2 following standard protocols (28). For detection of CD62L, cells were pre-treated with 0.1 mM TAPI-2 (TNF-α Protease Inhibitor 2, Peptides International) for 30 min before peptide stimulation to prevent cleavage of surface CD62L (29). All fluorochrome-conjugated antibodies were from either eBioscience or BD Biosciences. The stained cells were analyzed on a FACSCalibur flow cytometer followed by data analysis using FlowJo software (Tree Star, Inc). For calculation of the absolute numbers of antigen-specific CD4 or CD8 T cells per spleen, total numbers of splenocytes were multiplied with the frequency of CD4+ or CD8+Thy1.2+IFN-γ+ cells after stimulation with the specific peptide. The number of cells producing IFN-γ in the absence of peptide was subtracted. It should be noted that β–Catenin transgene was coupled with a GFP expression indicator via an internal ribosome entry site (19). In the βCat-Tg mice, GFP was highly expressed in developing thymocytes but substantially attenuated in mature CD4 or CD8 T cells (19). We have confirmed this finding and that the GFP fluorescence channel can be used to detect highly expressed Thy1 marker or T cell receptor (TCR) Vβ subtypes on total or antigen-specific CD8 T cells (Data not shown and Supplemental Fig. 1).
Detection of BrdU uptake and activated Caspase-3/7 in antigen-specific CD8 T cells
To determine the proliferation rate of antigen-specific T cells at early contraction phase, the mice were i.p. injected with 1 mg of 5-bromo-2-deoxyuridine (BrdU) on day 7 post-infection with actA-LM-OVA and given 0.8 mg/ml BrdU in drinking water for additional 2 days. Splenocytes were isolated, incubated with Ova257-264 peptide, and surface-stained as above, followed by fixation and permeabilization procedures as recommended in the BrdU Flow Kit (BD Biosciences). Anti-BrdU and anti-IFN-γ antibodies were used simultaneously for intracellular staining to detect BrdU uptake in IFN-γ+ CD8 T cells. For detection of activated caspase-3 and -7 in antigen-specific T cells, splenocytes were peptide-stimulated and surface-stained as above, and then incubated with the fluorescent inhibitor of caspases reagent at 37°C for 60 min as recommended in the Vybrant FAM Caspase-3 and -7 assay kit (Invitrogen). The cells were then washed and used in intracellular staining for IFN-γ.
Isolation of antigen-specific T cells and quantitative RT-PCR
Naïve CD8 T cells were purified from uninfected OT-I transgenic mice via negative selection to >95% purity (StemCell Technologies). To obtain antigen-specific effector and memory T cells, naïve C57BL/6 (Thy1.2+) mice were adoptively transferred with 500 CD8 T cells from OT-I TCR transgenic mice (Thy1.1+), and one day later infected with actA-LM-Ova. Antigen-specific T cells were purified on day 5 (as effector T cells) and day 135 (as memory T cells) post-infection by labeling splenocytes with PE-conjugated Thy1.1 antibody followed by anti-PE coated magnetic beads (Miltenyi Biotec) according to manufacturer's directions. Labeled Thy1.1+ OT-1 Tg T cells were then positively enriched to >90% purity using the autoMACS (Miltenyi Biotec). Total RNA was then extracted and reverse-transcribed as previously described (30). Gene-specific probes and primer sets were from the Taqman Gene Expression Assay system (Applied Biosystems), and quantitative PCR was performed on an ABI 7300 Real Time PCR System (Applied Biosystems). β-actin was used to normalize the expression of other genes of interest. For each individual gene, its relative expression in naïve T cells was arbitrarily set to 1, and its expression changes in effector and memory T cells were calculated as fold repression or induction.
Results
Dynamic gene expression changes of TCF-1 and LEF-1 during T cell responses
To explore if the Wnt signaling pathway has a role in modulating mature T cell responses, we first measured relevant gene expression levels in naïve, effector and memory T cells. Effector and memory T cells were obtained 5 and 135 days post-infection from mice that had initially received low numbers of (∼500) OT-I CD8 T cells (31) and were then infected with an attenuated strain of L. monocytogenes (actA-LM) expressing ovalbumin (23). IL-7 receptor α chain (IL-7Rα) is known for its dynamic gene expression changes during T cell responses, being expressed in naïve T cells, downregulated in effectors, and restored to an even higher levels in memory T cells (32, 33). Consistent with previous observations, IL-7Rα transcripts were considerably lower in day 5 effector T cells than those in naïve T cells, whereas day 135 memory T cells showed higher IL-7Rα expression than naïve T cells (Fig. 1). We previously demonstrated that GA binding protein is required for transcriptional activation of the IL-7Rα gene (34), however, the expression of GABPα (the DNA binding subunit) and GABPβ1 (the transactivation subunit) were relatively stable during T cell responses (Fig. 1). In contrast, both TCF-1 and LEF-1 were dramatically downregulated in day 5 effector T cells compared to naïve T cells, but were partially restored in day 135 memory T cells. The expression levels of both transcription factors in memory T cells were still lower than naïve T cells, consistent with previous findings that antigen-experienced human CD8 T cells expressed less TCF-1 and LEF-1 than naïve cells (35). The corepressor Groucho/TLE family consists of 4 proteins of similar molecular weight and structure in mammals (36), and another corepressor C-terminal binding protein 1 (CtBP1) has been shown to interact with TCF family transcription factors (37). Interestingly, all 4 TLE and CtBP1 transcripts were decreased in effector T cells and partially upregulated in memory phase again, similar to the expression changes in TCF-1 and LEF-1 (Fig. 1). These findings collectively suggest that TCF-1/LEF-1-mediated activation and repression of Wnt downstream genes are both attenuated in effectors and restored in memory T cells, albeit to a lesser extent compared with naïve T cells.
Figure 1.
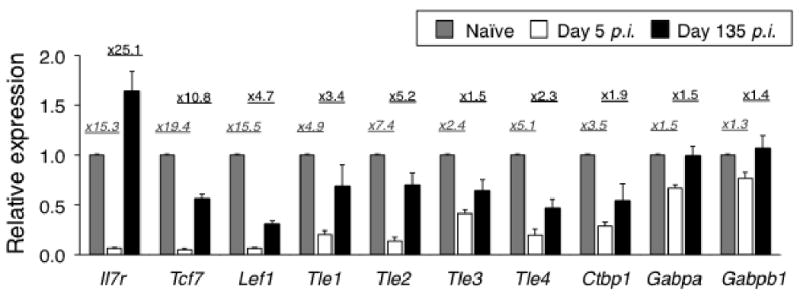
Dynamic regulation of Wnt pathway-related genes during CD8 T cell responses. Naïve, effector (day 5 p.i.), and memory (day 135 p.i.) OT-I CD8 T cells were separated as detailed in “Materials and Methods”. Total RNA was extracted and analyzed for expression of indicated transcripts using quantitative PCR. The expression level of each transcript in naïve T cells was arbitrarily set to 1, and its relatively expression in effector and memory T cells was shown. Fold repression in effector T cells compared with naïve cells (italicized) and fold induction in memory T cells compared with effector cells were shown on top of the bar graphs. The data are means ± s.e.m. of triplicate measurements of a total of 3 samples from 2 independent experiments.
Constitutively active Wnt signaling limits CD8 T cell expansion and contraction but favors formation of memory CD8 T cells
The dynamic expression changes of TCF-1 and LEF-1 along with IL-7Rα are suggestive of their potential functional importance during T cell responses. It has been shown that enforced expression of IL-7Rα alone was not sufficient to protect effector T cells from contraction (28, 38). Since stabilized β-catenin and expression of TCF-1 extended thymocyte survival (19, 22), we hypothesized that constitutive activation of Wnt signaling may provide survival signals to effector T cells and thus result in a larger memory T cell pool. To test this, we used two lines of transgenic mice, expressing a stabilized β-catenin and a p45 isoform of TCF-1, respectively (19, 22). The stabilized β-catenin has an internal deletion of 20 amino acids containing four critical phosphorylation sites, preventing proteosome-mediated degradation. The stabilized β-catenin transgene (referred as βCat-Tg hereafter) is driven by the CD4 promoter, which allows transgene expression in both CD4 and CD8 T cells (19, 39). Among multiple TCF-1 isoforms, the p45 TCF-1 protein contains an N-terminal β-catenin interaction domain and can at least partially restore normal thymic cellularity and thymocyte development in TCF-1-deficient mice (22). The p45 transgene was driven by H-2Kb promoter coupled with IgH enhancer, resulting in persistent expression in lymphocytes including T lineage cells (hereafter referred to as p45-Tg) (22). We crossed βCat-Tg and p45-Tg to generate double transgenic mice (dTg), and the expression of both transgenes may help ensure constitutively active Wnt signaling in effector T cells in which the endogenous TCF-1 proteins are downregulated.
To make sure that the expression of βCat and p45 transgenes does not affect the TCR repertoire, we first used a panel of antibodies to detect TCR Vβ subtypes on splenic CD8 and CD4 T cells and observed no significant changes (Supplemental Fig. 1A and 1B for CD8 T cells, data not shown for CD4 T cells). It was shown previously that the expression of constitutive active β-catenin extended thymocyte survival by upregulating Bcl-XL (19, 22) and that transgenic expression of Bcl-XL resulted in a bias toward 3′ Jα usage in the TCRα subtypes (40). To determine if βCat-Tg functionally altered the repertoire of antigen-specific T cells, we measured their functional avidity, which would likely be affected by skewed TCRα subtypes (41, 42). We infected βCat-Tg, p45-Tg, dTg, and wild-type (WT) littermate controls with the attenuated actA-LM-Ova (28). On day 7 post-infection, we isolated splenocytes, incubated them with 10-fold serial dilutions of Ova257-264 peptide, and measured the fraction of cells producing IFN-γ. As shown in Supplemental Fig. 1C, no apparent differences in T cell functional avidity were observed among the various transgenic strains, suggesting that the TCR repertoire was not detectably skewed by the βCat and/or p45 transgenes.
To measure T cell responses in vivo, we infected WT, βCat-Tg, p45-Tg, and dTg mice with actA-LM-Ova (28), and then measured the Ova257-264-specific CD8 T cell responses using intracellular cytokine staining for peptide-stimulated IFN-γ on days 5, 7, 9, 14 and 42 post-infection. During the clonal expansion phase (5-9 days post-infection), all groups of mice showed expansion of Ova-specific CD8 T cells, as manifested by IFN-γ production upon Ova peptide stimulation, with the peak response appearing on day 7 in all mouse strains (Fig. 2A and 2G). Effector CD8 T cells in each group showed similarly diminished expression of CD62L and IL-7Rα, similar CD27high subsets and IL-2 production, except that the frequency of IL-2+IFN-γ+ CD8 T cells in βCat-Tg mice appeared smaller (Fig. 2A). The T cell expansion, in terms of frequency and absolute numbers of antigen-specific CD8 T cells, was slightly increased by ectopic expression of stabilized β-catenin alone but was limited by the forced expression of p45 TCF-1 isoform, especially in the presence of both transgenes (Fig. 2B). The limited T cell expansion was also reflected in decreased total CD8 T cell frequency in splenocytes (Fig. 2C). These results suggest that down-regulation of TCF-1 might be necessary for optimal T cell expansion.
Figure 2.
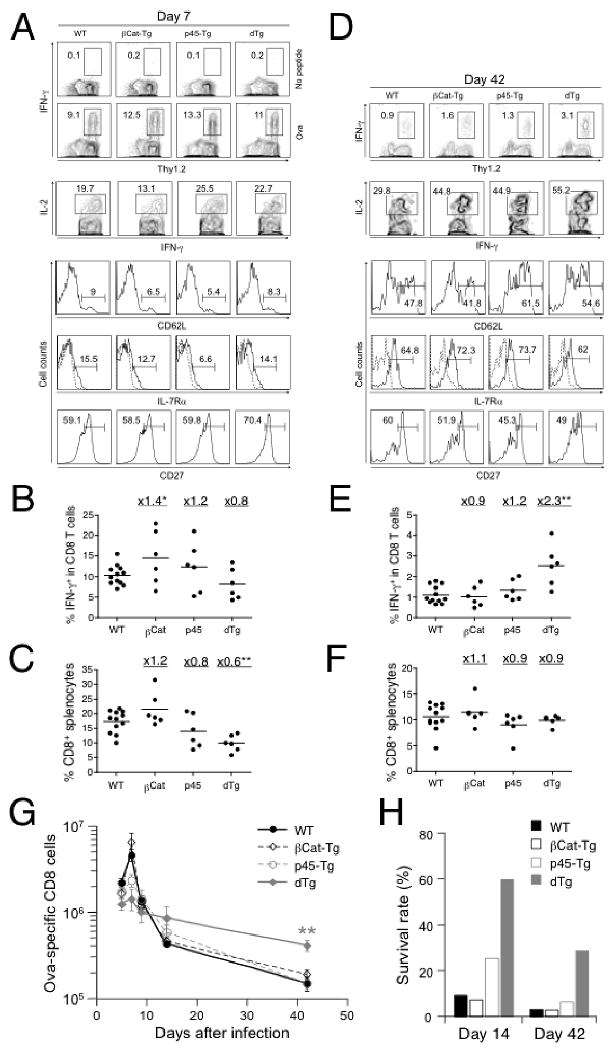
Constitutive activation of Wnt signaling favors formation of memory CD8 T cells. (A) and (D) WT, βCat-Tg, p45-Tg, and dTg mice were infected with actA-LM-Ova and the CD8 T cell response to Ova257-264 was monitored in the spleen at day 7 (A) and day 42 (D) post-infection using intracellular cytokine staining for IFN-γ. The percentages of IFN-γ+ CD8 cells are shown in the contour plots in the absence (shown for A) or presence of Ova peptide stimulation. IFN-γ+ cells were further analyzed for IL-2+, CD62Lhigh, IL-7Rα+, or CD27high subsets, and their percentages were shown. Staining with isotype control for CD62L and IL-7Rα was displayed as dotted line in histograms showing IL-7Rα staining. Data are representative of 2 independent experiments with at least 3 mice examined in each experiment. (B) Frequency of IFN-γ+ cells in CD8 and (C) frequency of CD8 cells in splenocytes on day 7 post-infection. (E) Frequency of IFN-γ+ cells in CD8 and (F) frequency of CD8 cells in splenocytes on day 42 post-infection. Fold changes of mean values for each transgenic strain versus WT mice are shown for (B-C) and (E-F). (G) Kinetics of Ova-specific CD8 T cell responses shown as total numbers at indicated time points. Data are means ± s.e.m. in one of the two independent experiments with similar results (n = 3). (H) Survival rate of Ova-specific CD8 T cells at contraction and memory phases. The mean value of Ova-specific CD8 T cell numbers at days 14 and 42 (as in G) was normalized to respective peak response on day 7. The percentage of survived cells is shown. *, p<0.05; **, p<0.01 by t-test for each group of Tg mice versus WT controls.
During the contraction phase (day 14 post-infection), approximately 90% of the antigen-specific CD8 T cells was eliminated in WT mice as expected. When normalized to their respective peak responses, a similar contraction was seen in βCat-Tg, whereas a higher percentage of cells survived in p45-Tg (25%) and dTg (60%) mice (Fig. 2G and 2H). When the mice were examined at the memory phase (day 42 post-infection), dTg mice had higher frequencies and increased numbers of antigen-specific CD8 cells compared with WT or single transgenic mice (Fig. 2D, 2E, and 2G), despite a similar CD8 frequency in splenocytes among all transgenic and control strains (Fig. 2F). When normalized to the peak response, more antigen-specific CD8 T cells survived and became memory cells in dTg mice (29% in dTg versus 3-6% in WT and single Tg mice, Fig. 2H). The memory CD8 T cells in all the mouse strains examined exhibited similar CD27high subsets and similar upregulation of CD62L and IL-7Rα (Fig. 2D). These observations collectively indicate that constitutive activation of Wnt signaling in T cells favors the generation of memory CD8 T cells.
Heightened secondary expansion and bacteria clearance in double transgenic mice
Effective immunological memory is characterized by an accelerated expansion of memory cells upon re-encounter of the same antigen. Interestingly, the antigen-specific memory CD8 T cells showed increased capacity to produce IL-2 upon in vitro restimulation with Ova peptide in βCat-Tg or p45-Tg mice (Fig. 2D and 3A), and IL-2+IFN-γ+ CD8 T cells in dTg mice were significantly elevated in absolute numbers (Fig. 3B). To further determine if the increased number and IL-2 production by dTg memory CD8 T cells confers functional advantages, we challenged the immune mice (45 days after the primary infection) with virulent LM-Ova. After the secondary infection, Ova-specific CD8 T cells in all mouse strains expanded rapidly, and those in dTg mice were detected at higher frequencies and absolute numbers (Fig. 3C and 3D) as compared to WT controls (1.5- and 2.4-fold increase in cell number in dTg mice compared to controls at days 3 and 5, respectively), albeit the increase did not reach a statistical significance. It is noteworthy that the secondary expansion of antigen-specific CD8 T cells was not limited by the enforced expression of the p45 TCF-1 protein, which is different from the primary CD8 response. The contraction following the secondary CD8 response was more moderate as compared to that after the primary response (1), and dTg mice continued to exhibit a 3.2-fold increase of Ova-specific CD8 T cells over WT mice (Fig. 3D). Significantly more “secondary memory” CD8 T cells were detected on day 42 after secondary infection in dTg (3.6-fold increase over WT littermates, p<0.05) (Fig. 3D). Thus, in the presence of constitutively active Wnt signaling, the increased primary memory CD8 T cells, when rechallenged, gave rise to larger numbers of secondary effector and memory T cells.
Figure 3.
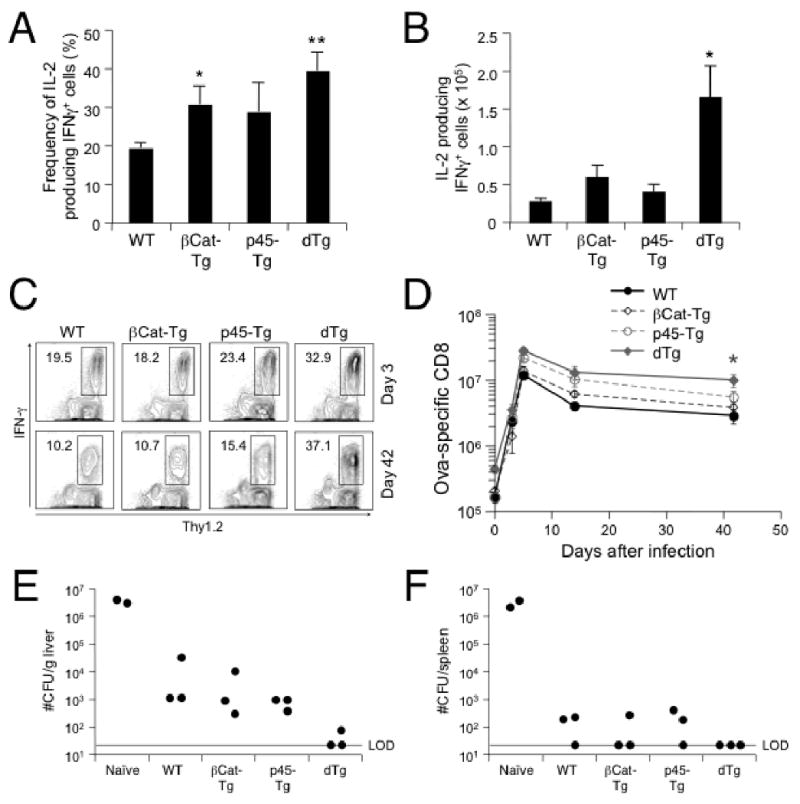
Memory CD8 T cells generated in the presence of constitutively active Wnt signaling manifested enhanced functionality. (A) Frequency and (B) numbers of IL-2 producing Ova-specific memory CD8 T cells. The numbers were calculated from the frequency of IL-2+IFN-γ+ cells (A) and the absolute number of Ova-specific CD8 T cells on day 42 post-infection as in Fig. 2G. (C) and (D) Secondary antigen-specific CD8 T cell responses. Mice of indicated genotypes were first immunized with act-LM-Ova as in Fig. 2, and detection of Ova-specific CD8 T cells on day 42 post-immunization was confirmed in periphery blood leukocytes by intracellular staining for IFN-γ (data not shown). The immunized mice were then infected with virulent LM-Ova, and CD8 responses to Ova were determined. Ova257-264-specific CD8 T cells at day 3 and day 42 after secondary infection were detected as Thy1.2+IFN-γ+ CD8 cells, with the percentages shown in (C). Total numbers of antigen-specific CD8 T cells per spleen in each group are shown in (D) as means ± s.e.m. (E) and (F) Clearance of secondary bacterial infection by primary CD8 memory T cells. Naïve or immunized mice were infected with virulent LM-Ova and 3 days later, livers and spleens were harvested and CFUs were determined. Data are reported as CFU numbers per gram of liver (E) or per spleen (F). LOD, limit of detection. Each symbol represents one mouse. All data are from one of 2 independent experiments with similar results. *, p<0.05; **, p<0.01 by t-test for each group of Tg mice versus WT controls.
An important feature of memory T cells is to provide protection upon encounter with the same pathogen. We next determined protection from virulent LM-Ova by measuring bacterial numbers (CFUs) in livers and spleens on day 3 after rechallenging naïve or immunized mice. Whereas the bacteria expanded drastically in both liver and spleen of naïve mice, the immunized mice effectively controlled the infection as many fewer CFUs were seen in WT, single Tg, and dTg mice, especially in the spleen (Fig. 3E and 3F). Interestingly, virulent LM-Ova was almost completely cleared in the liver of dTg mice, which is likely accounted for by a larger expansion of antigen-specific CD8 T cells observed in these mice.
Constitutively active Wnt signaling suppresses Caspase3/7 activation
Our observations above indicate that forced expression of the p45 TCF-1 isoform and a constitutively active β-catenin limited the contraction of antigen-specific CD8 effectors and resulted in increased numbers of memory T cells. These antigen-specific memory CD8 T cells generated more secondary effector T cells upon rechallenge, leading to a more effective reduction in bacterial burden. The reduced contraction of effector T cells in dTg mice may be a result of increased proliferation and/or survival of antigen-specific T cells. To distinguish these possibilities, we i.p. injected BrdU into dTg and WT littermate controls on day 7 post-infection with actA-LM-Ova, and measured BrdU incorporation in Ova-specific CD8 T cells on day 9, the early stage of contraction phase. As shown in Fig. 4A, the proliferation rates of Ova-specific CD8 T cells were similar in both WT and dTg mice. We next determined the expression levels of pro-survival Bcl-2 family members in Ova-specific T cells. Consistent with previous findings that Bcl-2 expression is downregulated in antigen-specific effector T cells in response to lymphocytic choriomeningitis virus (LCMV) infection (43), the Ova-specific T cells in both WT and dTg mice expressed similarly low levels of Bcl-2 (Supplemental Fig. 2A). In thymocytes, activation of β-catenin upregualtes Bcl-XL and increases their survival (19, 22). We purified Ova-specific CD8 T cells by cell sorting of MHC-I/SIINFEKL tetramer-positive cells from both WT and dTg mice and by quantitative RT-PCR measured transcript levels of Bcl-XL and Mcl-1, other pro-survival Bcl-2 family members. Both genes showed a minimal increase in expression (<1.5 fold on average) in dTg Ova-specific T cells compared with those in WT littermates (Supplemental Fig. 2B), consistent with previous observations that β-catenin transgene did not increase Bcl-XL expression in mature T cells (19).
Figure 4.
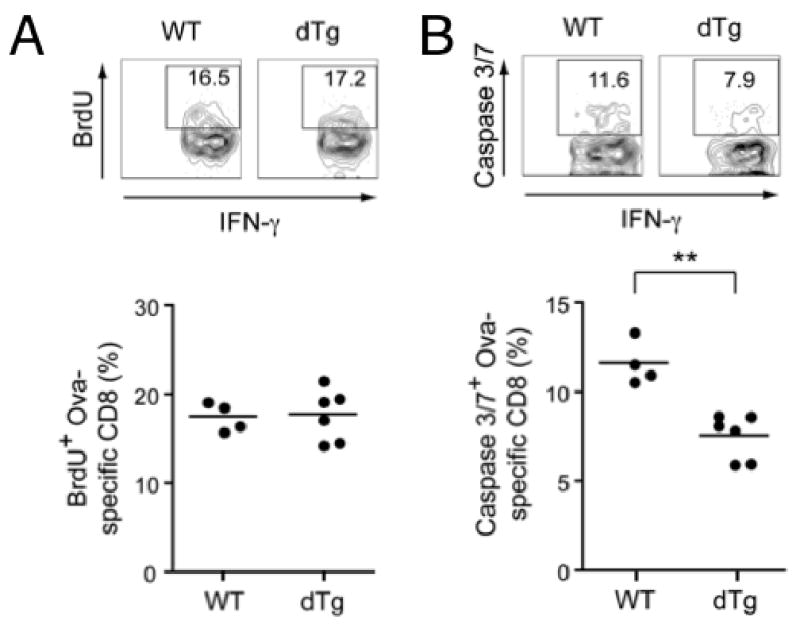
Detection of BrdU uptake and caspase activation in antigen-specific effector CD8 T cells. (A) Proliferation rate of effector CD8 T cells at early contraction phase. WT and dTg mice were infected with actA-LM-Ova, and given BrdU on day 7 via intraperitoneal injection and in drinking water for 2 days. Percentages of BrdU+ cells in IFN-γ+ CD8 effectors are shown. (B) Activation of caspase-3/7 in effector CD8 T cells at early contraction phase. Splenocytes were isolated from WT and dTg mice on day 9 post-infection, and activated Caspase-3/7 were detected using the FLICA methodology. Percentages of cells having activated Caspase-3/7 are shown. Representative flow cytometric profiles were shown on upper panels and accumulative data from 2 independent experiments are shown in lower panels. **, p<0.01 by t-test.
Apoptotic signals converge on activation of effector caspases, via either intrinsic (such as withdrawal of growth factors) or extrinsic pathways (such as Fas engagement by Fas ligand) (44, 45). To further discern the apoptotic status of antigen-specific CD8 T cells during the contraction phase, we detected activated Caspase-3 and -7 using a fluorescent inhibitor of caspases (FLICA) methodology, in which the active center of activated caspases is bound by the flouromethyl ketone moiety of fluorochrome-labeled FLICA (46). As shown in Fig. 4B, a smaller portion of Ova-specific CD8 effector cells from dTg mice had active Caspase 3/7 compared with those from WT controls. Collectively, the reduced contraction of effector T cells in the presence of active Wnt signaling is at least partly explained by reduced caspase activation and hence alleviated apoptosis, which is probably independent of Bcl-2 and other pro-survival Bcl-2 family members.
Constitutively active Wnt signaling favors memory CD8 T cell formation in response to viral infection
We next investigated if constitutively active Wnt signaling can promote memory CD8 T cell formation in response to other forms of infectious agents in addition to bacterial infection. Vaccinia virus and LCMV are known to elicit cellular immune responses mainly mediated by CD8 T cells. We first infected WT and dTg mice with vaccinia virus expressing ovalbumin (VacV-Ova) (26, 47). On day 42 post-infection, we measured Ova-specific memory CD8 T cells in the spleen by intracellular staining for IFN-γ after the Ova peptide stimulation. The Ova-specific memory CD8 T cells in dTg mice exhibited on average an approximately 2.5 fold increase compared with WT littermate controls (Fig. 5A). We next infected the mice with LCMV-Armstrong and determined memory phase CD8 responses in the spleen to multiple epitopes including the immunodominant epitopes located in glycoprotein GP33-41, GP276-286 and nucleoprotein NP396-404, and subdominant epitopes NP205-212 (48). By measuring peptide-stimulated IFN-γ production, dTg mice were found to have more memory CD8 T cells to GP33-41, NP396-404, and NP205-212 epitopes than the WT control mice (Fig. 5B), and all these increases are statistically significant. On the other hand, GP276-286-specific memory CD8 T cells in dTg mice showed only a small increase (Fig. 5B). Taken together, these results suggested that activation of Wnt signaling positively impacted the generation of memory CD8 T cells in response to both bacterial and viral infections.
Figure 5.
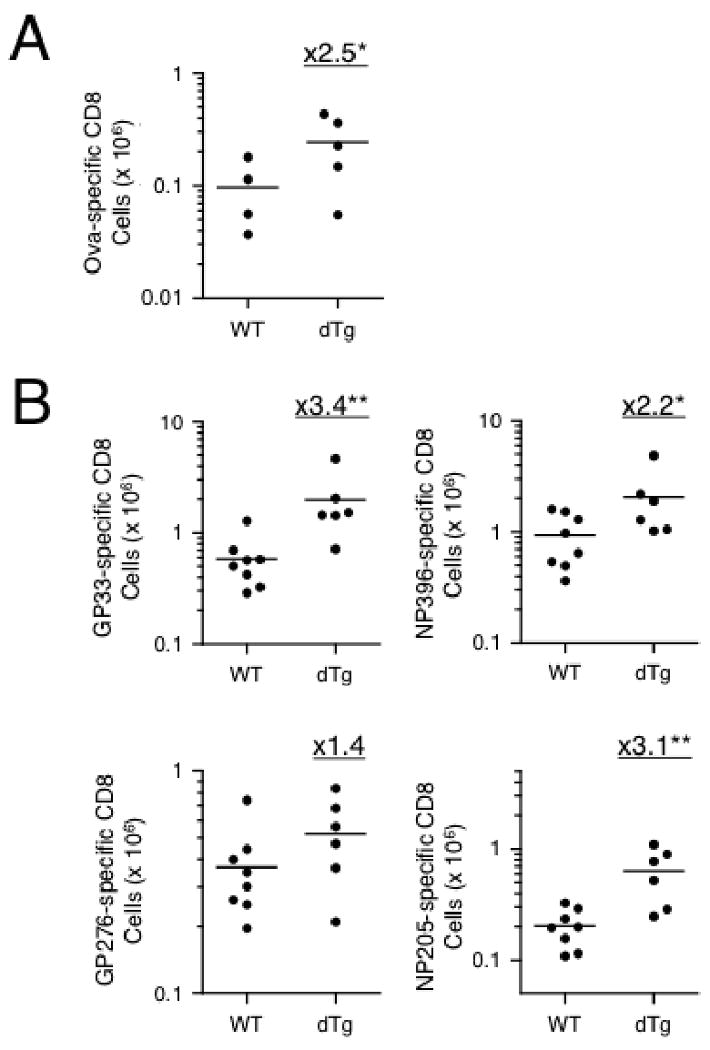
Constitutive activation of Wnt signaling enhances memory CD8 T cell formation in response to viral infection. (A) Ova-specific memory CD8 T cells after VacV-Ova infection. WT and dTg mice were i.p. infected with vaccinia virus expressing Ovalbumin, and the Ova-specific CD8 T cells on day 42 post-infection were determined by intracellular staining for IFN-γ. (B) Antigen-specific memory CD8 T cells after LCMV infection. WT and dTg mice were i.p. infected with LCMV-Armstrong, and 42 days later memory CD8 T cells specific for dominant epitopes GP33-41, NP396-404, GP276-286, and subdominant epitopes NP205-212 were determined by ICS for IFN-γ. Fold changes of mean values were shown. *, p<0.05; **, p<0.01 by t-test.
Constitutively active Wnt signaling favors memory CD4 T cell formation but does not improve secondary expansion
Finally we determined whether the enforced Wnt signaling similarly affected CD4 T cell responses by detecting listeriolysin O (LLO)190-201 peptide-specific CD4 cells at various stages after actA-LM-Ova infection. The peak expansion of LLO190-201-specific effector CD4 T cells in WT and βCat-Tg mice occurred at day 7 after LM infection (Fig. 6A and 6G), with βCat-Tg mice exhibiting moderately increased frequency of the antigen-specific CD4 cells (Fig. 6B). On the other hand, the frequency of antigen-specific CD4 T cells in p45-Tg and dTg mice was similar to the WT controls (Fig. 6B). The frequency of CD4 T cells in the spleens was also similar among WT, βCat-Tg, and p45-Tg mice, but slightly decreased in dTg mice (Fig. 6C). The absolute numbers of antigen-specific CD4 T cells were similar among all transgenic strains and WT littermate controls on day 5 post-infection, however, those in p45-Tg and dTg mice had already started to decline on day 7 post-infection (Fig. 6G). Despite the change in kinetics, antigen-specific CD4 T cells in all mouse strains showed similar capacity of IL-2 production, similar downregulation of CD62L and IL-7Rα, and comparable portions of CD27high subsets (Fig. 6A).
Figure 6.
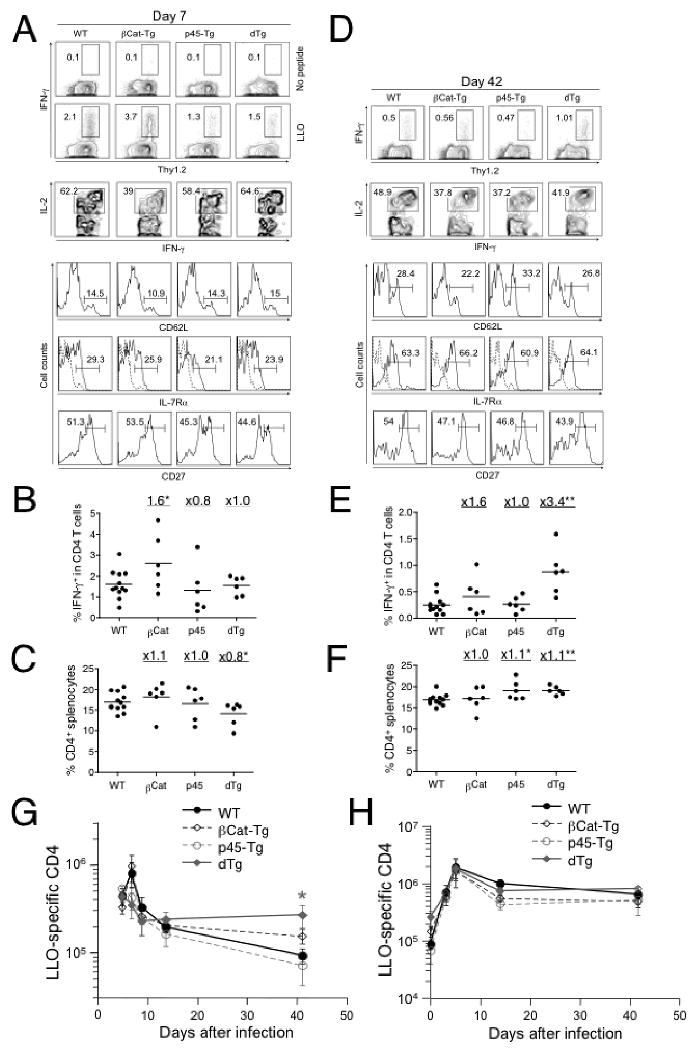
Constitutively active Wnt signaling increases primary but not secondary memory CD4 T cells. Mice were infected as in Fig. 2, and the CD4 responses to LLO190-201 were determined on various days post-infection. (A) and (D) Detection and characterization of LLO190-201-specific CD4 T cells at day 7 (A) and day 42 (D) post-infection. The percentages of Thy1.2+IFN-γ+ CD4 cells are shown in the contour plots in the absence (shown for A) or presence of LLO peptide stimulation. IFN-γ+ CD4 cells were further analyzed for IL-2+, CD62Lhigh, IL-7Rα+, or CD27high subsets in memory CD4 cells, and their percentages were shown. Staining with isotype control for CD62L and IL-7Rα was displayed as dotted line in histograms showing IL-7Rα staining. Data are representative of 2 independent experiments with at least 3 mice examined in each experiment. (B) Frequency of IFN-γ+ cells in CD4 and (C) frequency of CD4 cells in splenocytes on day 7 post-infection. (E) Frequency of IFN-γ+ cells in CD4 and (F) frequency of CD4 cells in splenocytes on day 42 post-infection. Fold changes of mean values for each transgenic strain versus WT mice are shown for (B-C) and (E-F). (G) Kinetics of LLO-specific CD4 T cell responses shown as total numbers at indicated time points. (H) Secondary antigen-specific CD4 T cell responses. Mice were first infected with act-LM-Ova and 45 days later re-challenged with virulent LM-Ova as in Fig. 3C. CD4 responses to LLO190-201 were determined on days 3, 5, 14 and 42 after the secondary infection. Total numbers of antigen-specific CD4 T cells per spleen in each group are shown as means ± s.e.m. Data are representative of 2 independent experiments with at least 3 mice examined in each experiment. *, p<0.05; **, p<0.01 by t-test for each group of Tg mice versus WT controls.
Although p45-Tg and dTg mice exhibited an early decline in the frequency of antigen-specific CD4 T cells, their numbers were similar in all mouse strains at day 14 post-infection (Fig. 6G). Whereas LLO190-201-specific CD4 T cells in WT and single Tg mice further declined by day 42 post-infection, those in dTg mice persisted at a higher frequency and total number (Fig. 6D, 6E, and 6G). The splenic CD4 frequency in all mouse strains at the memory phase was similar (Fig. 6F). In contrast to their numbers, memory CD4 T cells in all transgenic strains and WT controls exhibited similar re-expression of CD62L and IL-7Rα on the cell surface. Unlike memory CD8 T cells, dTg memory CD4 T cells from dTg mice did not show an improved ability to produce IL-2 upon peptide stimulation (Fig. 6D). These data suggest that enforced expression of p45 and a stabilized β-catenin enhances the generation of memory CD4 T cells, which does not seem to be accompanied by obvious functional changes.
We also assessed the secondary responses of memory CD4 T cells after re-challenge with virulent LM-Ova. The antigen-specific CD4 T cells in all mouse strains expanded rapidly (Fig. 6H and supplemental Fig. 3). Although there were more primary memory CD4 T cells to start with in dTg mice (Fig. 6G), they expanded to a similar “ceiling” level as seen in WT and single Tg mice (Fig. 6H). The LLO-specific CD4 cells contracted similarly and stabilized on a similar level as “secondary memory” CD4 cells in WT and dTg mice. On the other hand, if any, fewer LLO-specific CD4 cells were detected on days 14 and 42 after secondary infection in βCat-Tg and p45-Tg mice (Fig. 6H and supplemental Fig. 3). Collectively, these data suggests that activation of the Wnt pathway does not improve the secondary response of CD4 cells, even though more memory CD4 cells were generated during the initial immunization.
Discussion
The canonical Wnt pathway, with TCF-1 and LEF-1 as the effector transcription factors, is known to play critical roles in T cell development, but its function in peripheral T cells remains relatively unknown. Here we report that constitutive activation of the Wnt pathway, by the enforced expression of a stabilized β-catenin together with a β-catenin receptive p45 TCF-1 isoform, resulted in an increased frequency and number of primary memory CD8 T cells accompanied by an increased ability to produce IL-2. Moreover, upon re-challenge the enlarged memory CD8 T cell pool expanded to larger numbers of secondary effector T cells and the pathogen was cleared faster. Future studies should determine if primary memory dTg CD8 cells have enhanced per cell protective capacity. Nevertheless, our results indicated that constitutive activation of the canonical Wnt pathway favors formation of memory CD8 T cells, leading to a “win by numbers” after re-encountering the same pathogen. On the other hand, although CD4 memory T cells were also increased, enforced Wnt signaling did not improve the secondary expansion of antigen-specific CD4 cells and their IL-2 production. The distinct effects of Wnt signaling on CD4 and CD8 cells are not surprising, since several intrinsic differences between CD4 and CD8 responses have been noted. For examples, CD4 expansion upon activation is less pronounced than that of CD8 cells, and formation of memory CD4 T cells requires prolonged antigen contact (3, 49, 50). Our observations thus suggest a specific role of the canonical Wnt signaling pathway in promoting memory CD8 T cell formation and enhancing their functionality.
One implication of our study is the potential use of Wnt ligands as adjuvants in vaccination to enhance memory CD8 T responses. Current strategies to improve vaccination efficiency include the use of epitopes with enhanced affinity of binding to the TCR, the use of co-stimulatory molecules and of cytokines as adjuvants (5, 51). The addition of non-cytokine factors, such as Wnt ligands, may further improve the T cell responses. We found that p45 overexpression limited initial CD8 T cell expansion, however, when coupled with stabilized β-catenin expression, the contraction of antigen-specific CD8 T cells was also reduced, resulting in increased numbers of memory CD8 cells. Our observations are in line with a recent finding that in vitro treatment of CD8 T cells with pharmacological inhibitors of glycogen synthase kinase 3, hence stabilizing β-catenin and activating Wnt pathway, blocked their differentiation to effector T cells but promoted them to adopt a CD8 memory stem cell phenotype (52). There are at least 19 known Wnt proteins in mammals, and 10 known Fzds receptors for Wnt ligands (10, 11). Using reporter assays, it has been shown that Wnt signaling is active in both naïve and activated T cells (17). Future studies need to be directed towards identification of Wnt ligand and Fzd receptor pairs that are critical in mature T cell function for potential adjuvant applications. It should be noted that the expression of stabilized β-catenin alone was not sufficient to improve CD8 T cell memory. This may be explained by the downregulation of TCF-1 and LEF-1 in effector T cells, which may render them unresponsive to activation of Wnt signaling. We previously demonstrated that downregulation of IL-7Rα in effector T cells was mediated by the phosphoinositide 3-kinase (PI3-K) and Akt pathway, and that inhibition of the PI3-K/Akt pathway in activated T cells allowed retention of higher IL-7Rα expression (53). Elucidation of the signaling pathway(s) leading to TCF-1/LEF-1 downregulation and intervention of this pathway may ultimately help maximize the beneficial effects of active Wnt signaling.
Activation of the canonical Wnt signaling has diverse effects on hematopoietic cells, and the effects are dependent on the cell context and the way how Wnt signals are delivered. For example, purified Wnt ligands can expand hematopoietic stem cells (HSCs) in vitro and enhance their self-renewal and reconstitution activities (54, 55), however, constitutive or induced expression of stabilized β-catenin in mouse HSCs had adverse effects on HSC maintenance and blocked multi-lineage differentiation (56, 57). The stabilized β-catenin transgene used in our study was previously shown to extend thymocyte survival (19). In agreement with this observation, we found that coupled with p45 expression, stabilized β-catenin partly protected effector CD8 cells from massive apoptosis during the contraction phase. The reduced apoptosis/contraction in dTg mice can be at least in part explained by suppression of caspase activation. In contrast to upregulation of Bcl-XL by stabilized β-catenin in thymocytes, we did not find substantial upregulation of pro-survival Bcl-2 family members including Bcl-2, Bcl-XL, and Mcl-1, which is suggestive of differential regulatory roles of Wnt pathway in developing and mature T cells. A recent study described the induction of anergy in non-regulatory T cells (CD4+CD25-) following retroviral transduction with a stable β-catenin (21). We observed a reduced expansion of antigen-specific CD4 and CD8 cells in the presence of the p45 transgene alone or in combination with the stabilized βCat transgene during the primary responses. We do not believe that these cells are anergic because memory cells derived from these cells expanded robustly and cleared bacterial infection upon secondary challenge. The discrepancy may lie in difference in the experimental system and how Wnt activation is achieved. Nevertheless, it should be kept in mind that when and how Wnt signaling is activated may determine the outcomes of prophylactic or therapeutic interventions.
Supplementary Material
Acknowledgments
We thank Dr. Zuoming Sun (University of Illinois) for providing transgenic mice expressing a stabilized β-catenin, Thomas Wirth and Nhat-Long Pham for useful discussion, and Lecia Epping for technical assistance.
Abbreviations used in this paper
- LM
Listeria monocytogenes
- LCMV
lymphocytic choriomeningitis virus
- VacV
vaccinia virus
- LLO
listeriolysin O
- TCF-1
T cell factor 1
- LEF-1
lymphoid enhancer-binding factor 1
- TLE
transducin-like enhancer of split
- IL-7Rα
IL-7 receptor α chain
- BrdU
5-bromo-2-deoxyuridine
Footnotes
This work was supported in part by internal funds to HHX from Department of Microbiology, University of Iowa and in part by NIH grants to HHX (AI042767 and HL095540), to VPB (AI183286), and to JTH (AI46653, AI42767, AI70073, and AI59752).
Disclosure
The authors have no financial conflicts of interest.
References
- 1.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 4.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 5.Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nat Rev Immunol. 2001;1:209–219. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- 6.Esser MT, Marchese RD, Kierstead LS, Tussey LG, Wang F, Chirmule N, Washabaugh MW. Memory T cells and vaccines. Vaccine. 2003;21:419–430. doi: 10.1016/s0264-410x(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 7.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 8.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan NI, Bendall LJ. Role of WNT signaling in normal and malignant hematopoiesis. Histol Histopathol. 2006;21:761–774. doi: 10.14670/HH-21.761. [DOI] [PubMed] [Google Scholar]
- 11.Staal FJ, Clevers HC. WNT signalling and haematopoiesis: a WNT-WNT situation. Nat Rev Immunol. 2005;5:21–30. doi: 10.1038/nri1529. [DOI] [PubMed] [Google Scholar]
- 12.Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 13.Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R. Redundant regulation of T cell differentiation and TCRalpha gene expression by the transcription factors LEF-1 and TCF-1. Immunity. 1998;8:11–20. doi: 10.1016/s1074-7613(00)80454-9. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta-catenin impairs T cell development. Nat Immunol. 2003;4:1177–1182. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- 15.Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, Radtke F. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 17.Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, Kuttler F, Malanchi I, Birchmeier W, Leutz A, Huelsken J, Held W. Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood. 2008;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- 18.Mulroy T, Xu Y, Sen JM. beta-Catenin expression enhances generation of mature thymocytes. Int Immunol. 2003;15:1485–1494. doi: 10.1093/intimm/dxg146. [DOI] [PubMed] [Google Scholar]
- 19.Xie H, Huang Z, Sadim MS, Sun Z. Stabilized beta-catenin extends thymocyte survival by up-regulating Bcl-xL. J Immunol. 2005;175:7981–7988. doi: 10.4049/jimmunol.175.12.7981. [DOI] [PubMed] [Google Scholar]
- 20.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998;161:3984–3991. [PubMed] [Google Scholar]
- 21.Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med. 2008;14:162–169. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- 22.Ioannidis V, Beermann F, Clevers H, Held W. The beta-catenin--TCF-1 pathway ensures CD4(+)CD8(+) thymocyte survival. Nat Immunol. 2001;2:691–697. doi: 10.1038/90623. [DOI] [PubMed] [Google Scholar]
- 23.Pope C, Kim SK, Marzo A, Masopust D, Williams K, Jiang J, Shen H, Lefrancois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 24.Tvinnereim AR, Hamilton SE, Harty JT. CD8(+)-T-cell response to secreted and nonsecreted antigens delivered by recombinant Listeria monocytogenes during secondary infection. Infect Immun. 2002;70:153–162. doi: 10.1128/IAI.70.1.153-162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 26.Restifo NP, Bacik I, Irvine KR, Yewdell JW, McCabe BJ, Anderson RW, Eisenlohr LC, Rosenberg SA, Bennink JR. Antigen processing in vivo and the elicitation of primary CTL responses. J Immunol. 1995;154:4414–4422. [PMC free article] [PubMed] [Google Scholar]
- 27.Badovinac VP, Hamilton SE, Harty JT. Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity. 2003;18:463–474. doi: 10.1016/s1074-7613(03)00079-7. [DOI] [PubMed] [Google Scholar]
- 28.Haring JS, Jing X, Bollenbacher-Reilley J, Xue HH, Leonard WJ, Harty JT. Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J Immunol. 2008;180:2855–2862. doi: 10.4049/jimmunol.180.5.2855. [DOI] [PubMed] [Google Scholar]
- 29.Jabbari A, Harty JT. Simultaneous assessment of antigen-stimulated cytokine production and memory subset composition of memory CD8 T cells. J Immunol Methods. 2006;313:161–168. doi: 10.1016/j.jim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Xue HH, Bollenbacher-Reilley J, Wu Z, Spolski R, Jing X, Zhang YC, McCoy JP, Leonard WJ. The transcription factor GABP is a critical regulator of B lymphocyte development. Immunity. 2007;26:421–431. doi: 10.1016/j.immuni.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 32.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 33.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 34.Xue HH, Bollenbacher J, Rovella V, Tripuraneni R, Du YB, Liu CY, Williams A, McCoy JP, Leonard WJ. GA binding protein regulates interleukin 7 receptor alpha-chain gene expression in T cells. Nat Immunol. 2004;5:1036–1044. doi: 10.1038/ni1117. [DOI] [PubMed] [Google Scholar]
- 35.Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, Callan MF. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J Immunol. 2006;176:1439–1446. doi: 10.4049/jimmunol.176.3.1439. [DOI] [PubMed] [Google Scholar]
- 36.Gasperowicz M, Otto F. Mammalian Groucho homologs: redundancy or specificity? J Cell Biochem. 2005;95:670–687. doi: 10.1002/jcb.20476. [DOI] [PubMed] [Google Scholar]
- 37.Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25:7492–7504. doi: 10.1038/sj.onc.1210056. [DOI] [PubMed] [Google Scholar]
- 38.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawada S, Scarborough JD, Killeen N, Littman DR. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 1994;77:917–929. doi: 10.1016/0092-8674(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 40.Guo J, Hawwari A, Li H, Sun Z, Mahanta SK, Littman DR, Krangel MS, He YW. Regulation of the TCRalpha repertoire by the survival window of CD4(+)CD8(+) thymocytes. Nat Immunol. 2002;3:469–476. doi: 10.1038/ni791. [DOI] [PubMed] [Google Scholar]
- 41.Messingham KA, Badovinac VP, Jabbari A, Harty JT. A role for IFN-gamma from antigen-specific CD8+ T cells in protective immunity to Listeria monocytogenes. J Immunol. 2007;179:2457–2466. doi: 10.4049/jimmunol.179.4.2457. [DOI] [PubMed] [Google Scholar]
- 42.Slifka MK, Whitton JL. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 43.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 44.Siegel RM. Caspases at the crossroads of immune-cell life and death. Nat Rev Immunol. 2006;6:308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- 45.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 46.Smolewski P, Grabarek J, Halicka HD, Darzynkiewicz Z. Assay of caspase activation in situ combined with probing plasma membrane integrity to detect three distinct stages of apoptosis. J Immunol Methods. 2002;265:111–121. doi: 10.1016/s0022-1759(02)00074-1. [DOI] [PubMed] [Google Scholar]
- 47.Wirth TC, Badovinac VP, Zhao L, Dailey MO, Harty JT. Differentiation of central memory CD8 T cells is independent of CD62L-mediated trafficking to lymph nodes. J Immunol. 2009;182:6195–6206. doi: 10.4049/jimmunol.0803315. [DOI] [PubMed] [Google Scholar]
- 48.Gairin JE, Mazarguil H, Hudrisier D, Oldstone MB. Optimal lymphocytic choriomeningitis virus sequences restricted by H-2Db major histocompatibility complex class I molecules and presented to cytotoxic T lymphocytes. Journal of virology. 1995;69:2297–2305. doi: 10.1128/jvi.69.4.2297-2305.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stockinger B, Bourgeois C, Kassiotis G. CD4+ memory T cells: functional differentiation and homeostasis. Immunol Rev. 2006;211:39–48. doi: 10.1111/j.0105-2896.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 50.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 51.Salerno-Goncalves R, Sztein MB. Cell-mediated immunity and the challenges for vaccine development. Trends Microbiol. 2006;14:536–542. doi: 10.1016/j.tim.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xue HH, Kovanen PE, Pise-Masison CA, Berg M, Radovich MF, Brady JN, Leonard WJ. IL-2 negatively regulates IL-7 receptor alpha chain expression in activated T lymphocytes. Proc Natl Acad Sci U S A. 2002;99:13759–13764. doi: 10.1073/pnas.212214999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 55.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 56.Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 57.Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


