Abstract
Objectives
Clinical practice guidelines recommend ongoing testing (surveillance) for colorectal cancer survivors because they remain at risk for both local recurrences and second primary tumors. However, survivors often do not receive colorectal cancer surveillance. We used the Health Belief Model (HBM) to identify health beliefs that predict intentions to obtain routine colonoscopies among colorectal cancer survivors.
Methods
We completed telephone interviews with 277 colorectal cancer survivors who were diagnosed four years earlier, between 2003 and 2005, in North Carolina. The interview measured health beliefs, past preventive behaviors, and intentions to have a routine colonoscopy in the next five years.
Results
In bivariate analyses, most HBM constructs were associated with intentions. In multivariable analyses, greater perceived likelihood of colorectal cancer (OR=2.00, 95% CI=1.16–3.44) was associated with greater intention to have a colonoscopy. Survivors who already had a colonoscopy since diagnosis also had greater intentions of having a colonoscopy in the future (OR=9.47, 95% CI=2.08–43.16).
Conclusions
Perceived likelihood of colorectal cancer is an important target for further study and intervention to increase colorectal cancer surveillance among survivors. Other health beliefs were unrelated to intentions, suggesting that the health beliefs of colorectal cancer survivors and asymptomatic adults may differ due to the experience of cancer.
Keywords: Colorectal neoplasms, survivors, colonoscopy, health behavior
Introduction
With an estimated 150,000 new cases in 2008, colorectal cancer is the third most common cancer in US men and women.1 Because colorectal cancer survivors are at risk for both local recurrences and second primary tumors,2 clinical practice guidelines recommend ongoing testing (surveillance) for colorectal cancer. Although recommendations have varied regarding the specific test (e.g., colonoscopy, sigmoidoscopy) and frequency of surveillance, all guidelines published over the last decade recommend colorectal cancer surveillance to reduce the risk of mortality.3–11 In 2006, several organizations reached consensus that colorectal cancer survivors should get routine colonoscopies 1 and 3 years after surgery, and then every 5 years.4
Regardless of the guideline used as a standard for adherence, colorectal cancer survivors do not get adequate surveillance. Twenty-four to 40% do not receive colorectal cancer surveillance three years after diagnosis;12–14 this rate is estimated at 20% to 35% within 5 years.13–15 Prior studies have identified clinical and demographic characteristics of survivors who are less likely to receive colorectal cancer surveillance, including those who have rectal cancer (as opposed to colon cancer),12, 14–16 are younger,12–17 and are white,12, 14, 18 although the evidence for race is inconsistent.13, 15, 16 However, these risk factors are not mutable. To develop effective strategies to increase surveillance, it is important to identify potentially modifiable factors that can direct the content of an intervention.
The Health Belief Model (HBM) posits that individuals are more likely to engage in preventive health behavior if they have higher perceived likelihood of getting the disease, higher perceived severity of the disease, fewer perceived barriers to prevention, more perceived benefits of prevention, higher self-efficacy, and have more cues to taking action.19 The HBM has been used extensively to both predict screening behavior (including colorectal cancer) and design interventions to increase screening.20–28 However, the HBM has not been applied to colorectal cancer surveillance. This is critical because CRC survivors may differ from asymptomatic patients on elements of the HBM.
Thus, in a cohort of CRC survivors, we hypothesized that higher perceived likelihood of CRC, more perceived benefits to colonoscopy, fewer perceived barriers to colonoscopy, greater self-efficacy, and more cues to action would be associated with intention to have colonoscopy in the next 5 years. We had 3 secondary hypotheses. First, poor continuity of care would be associated with underuse of preventive care among survivors.29–31 Second, survivors who had undergone colorectal cancer screening before their colorectal cancer diagnosis would have greater intentions to have a surveillance colonoscopy than those who did not. Finally, having had surveillance colonoscopy would be associated with greater intentions to have colonoscopy in the future.
Methods
Sample
Data came from the Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium, a national, population-based cohort study of lung and colorectal cancer. Detailed descriptions of CanCORS have been published elsewhere.32, 33 Briefly, patients diagnosed with colorectal cancer or lung cancer from May 2003 through October 2005 were identified from cancer registries at 8 study sites across the country. The present study was restricted to CanCORS patients in North Carolina, using survey questions developed solely for this study. Eligible participants for the present ancillary study included CanCORS participants from 33 counties in North Carolina who completed baseline and 1-year interviews, were diagnosed with stage I-III colorectal cancer, were alive without metastasis, and were not undergoing treatment for their original cancer. Participants who were unable to have colonoscopies (i.e., did not have enough remaining colon) were not eligible to participate.
Data sources
Data for this study came from three sources. First, for eligible participants providing informed consent, medical record audits were conducted by abstractors trained by a central CanCORS team to ascertain cancer-related medical visits and procedures. Specifically, data on clinical characteristics, disease status, vital status, demographics, treatments, diagnostic procedures, and participation in clinical trials were collected. Second, baseline interviews with participants were conducted approximately 4 months after diagnosis. These interviews assessed cancer care decision making, symptoms, satisfaction with care, demographics, and other topics for which survivors are an important source of information. Finally, follow-up interviews were conducted approximately four years after diagnosis only for North Carolina CanCORS participants in this ancillary study. These follow-up interviews assessed colorectal cancer surveillance, health beliefs, and intentions to have colonoscopy in the future. This study was approved by the University of North Carolina at Chapel Hill Institutional Review Board.
Dependent variables
Our outcome was reported intention to receive a colonoscopy in the next 5 years; this period was chosen because all guidelines recommended that this cohort should have at least 1 colonoscopy in the next 5 years. Intention was chosen as an outcome because we were unable to conduct a later assessment of actual colonoscopy use, and intention has been shown to be strongly associated with behavior.34
To assess intentions, the interviewer described colonoscopies and then asked about the participant’s expectation of having a colonoscopy, which was phrased as: “How likely are you to have a colonoscopy in the next 5 years?” Response options ranged from 1 (extremely unlikely) to 5 (extremely likely).
Independent variables: health beliefs
Perceived likelihood of getting colorectal cancer
We asked subjects “Assuming you do NOT get colonoscopies at regular intervals, what do you think is your chance of getting cancer in your colon or rectum again in your lifetime?” The wording was designed to include both local recurrences and second primary colorectal cancers. Response options ranged from 1 = “no chance” to 5 = “certain to get it”.
Perceived benefits and barriers
Perceived benefits of, and barriers to, colonoscopy were assessed by adapting validated scales of these attributes with respect to colorectal cancer screening.26 The 9 benefit items asked about the effectiveness of colonoscopy and the benefits of early detection. The barriers measures included 5 items that addressed concerns about undergoing colonoscopy (including negative results) and structural barriers (e.g., cost, transportation). The adaptations from the original measures reflect differences between screening colonoscopy for asymptomatic adults and surveillance colonoscopy for colorectal cancer survivors. The 5-point response scale for each item ranged from 1 (strongly disagree) to 5 (strongly agree). Perceived benefits and perceived barriers each formed a reliable scale (Cronbach’s alpha = 0.75 and 0.77, respectively), and each set of items was combined into an averaged summary measure.
Self-efficacy
Participants were asked how confident they were that they could get a colonoscopy when they are due for one (1 = “not at all confident” to 5 = “extremely confident”).
Cue to action
One item assessed whether, since having surgery, any physician recommended that the participant receive routine colonoscopies; this item was scored as 1 (yes) or 0 (no).
Independent variables: preventive behaviors
Screening history
Participants were asked whether they had received colorectal cancer screening before being diagnosed with colorectal cancer using measures created and tested by Vernon et al., with yes (1) and no (0) as response options.35
Visit to primary care provider
Participants were asked whether or not they had seen a primary care provider since being diagnosed with colorectal cancer (1=yes, 0=no).
Surveillance colonoscopy use
The interviewer asked participants if they had undergone any colonoscopies since having surgery for colorectal cancer, using a measure created and validated by Vernon et al. (1=yes, 0=no).35 Current guidelines suggest that participants should have received at least one colonoscopy since having surgery for their cancer.
Covariates
Other predictors of colonoscopy intentions included age, sex, education (up to and including high school graduate or more than high school graduate), income (continuous in $10,000 increments), insurance status (yes/no), site of the tumor (colon or rectum), stage at diagnosis, and severity of comorbidities. Using data abstracted from medical records, severity of comorbidities was assigned as none, mild, moderate, or severe using the Adult Comorbidity Evaluation (ACE-27) index.36–38 Stage at diagnosis was determined using collaborative staging if available. If unavailable, stage was determined from the registry or medical records.
Analysis
For variables with missing data (shown in Table 1), we imputed missing data using an iterative multivariable regression technique in Stata.39, 40 For all analyses, data were analyzed using two-tailed tests, with p values less than 0.05 considered statistically significant, in Stata.41 Statistical significance of predictors for all logit and ordered logit tests was determined using Wald Χ2 tests.42
Table 1.
Comparison of sample characteristics between 1-year and 4-year interviews
| Characteristic | 1-year interviewa (N=601) % | 4-year interview (N=277) % |
|---|---|---|
| Age | ||
| Less than 65 | 47 | 52 |
| 65 or older | 53 | 48 |
| Income | ||
| Less than $50,000 | 40 | 40 |
| $50,000 or more | 45 | 45 |
| Missing | 15 | 15 |
| Insurance status | ||
| Uninsured | 3 | 3 |
| Insured | 76 | 84 |
| Missing | 20 | 13 |
| Education | ||
| Less than high school | 35 | 42 |
| High school or more | 62 | 49 |
| Missing | 3 | 9 |
| Stage | ||
| I | 24 | 32 |
| II | 24 | 29 |
| III | 28 | 34 |
| Local (I or II) | 24 | 5 |
| Gender | ||
| Female | 52 | 53 |
| Male | 48 | 47 |
| Race | ||
| White | 77 | 79 |
| Non-white | 23 | 21 |
| Missing | 0 | 0 |
| Comorbidities | ||
| None | 24 | 27 |
| Mild | 30 | 40 |
| Moderate | 17 | 17 |
| Severe | 9 | 9 |
| Missing | 20 | 8 |
| Site of disease | ||
| Colon | 58 | 72 |
| Rectum | 17 | 17 |
| Missing | 25 | 10 |
| Saw a primary care provider in first year b | - | 96 |
| Screening for CRC before diagnosis | - | 48 |
| Had colonoscopy since diagnosis | - | 86 |
Note: CRC = colorectal cancer. % = percentage of observations.
Percentages may not add to 100 due to rounding.
Both samples exclude participants with missing stage, stage 0 or stage IV disease.
Primary care visits, CRC screening, and colonoscopy after diagnosis were only assessed during 4-year interview.
Descriptive analyses
We measured the associations between intention and each health belief, use of primary care, history of screening, and use of surveillance using bivariate models. We used bivariate ordered logit models for the 5-category intention variable.
Main analysis
We assessed predictors of intention to undergo a colonoscopy using multivariable ordered logit regression models for the 5-category intention variable. Covariates included age, level of education, sex, race/ethnicity, severity of comorbidities, site of tumor, stage at diagnosis, income, and whether the participant had any insurance. We report odds ratios, 95% confidence intervals, and p values.
Results
Description of the sample
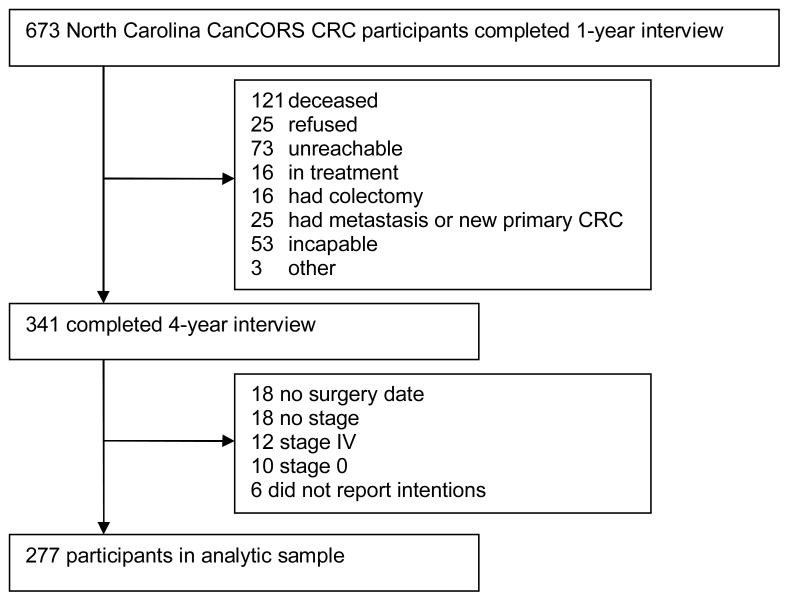
Three hundred forty-one participants completed the follow-up interview at 4 years post-diagnosis, between April 2007 and September 2008 (Figure 1). Of these, 64 were excluded from the analysis due to not having stage reported, being stage IV, or being stage 0 (N=40); not having had surgery (N=18); or not having responded to the intention question (N=6), yielding a final sample of 277 participants. Compared with participants who completed the one-year interview (and were not diagnosed with stage 0 or stage IV disease or did not have staging information), participants who completed the follow-up interview were more likely to have colon cancer but were otherwise comparable. (Table 1)
Figure 1.
Flowchart of study participants
Note: CRC = colorectal cancer
Participants were interviewed a mean of 45 months since diagnosis (standard deviation [s.d.] = 2.3 months) and a mean of 44 months since their primary cancer surgery (s.d. = 2.5 months). Most (72%) survivors had colon (non-rectal) cancer; 53% were female; and 79% were white. Virtually all (96%) had visited a primary care physician since their diagnosis, and 86% reported having had a colonoscopy since having surgery. Forty-eight percent reported that they had had screening for colorectal cancer before being diagnosed (Table 1).
Levels of intentions and health beliefs
Intentions to have a colonoscopy were very high; 88% reported that they were extremely likely to have a colonoscopy in the next 5 years. Participants strongly endorsed (mean = 4.72) benefits of colonoscopy. In general, participants reported few barriers to getting colonoscopy (mean = 1.66). (Table 2) The cost of colonoscopy was the greatest barrier (mean = 2.13).
Table 2.
Mean values of health beliefs
| Health Belief | N | Mean (s.d.) |
|---|---|---|
| Perceived likelihood of getting CRC again if you do not get regular colonoscopies1 | 248 | 2.84 (0.98) |
| Perceived benefits summary score 2 | 275 | 4.72 (0.44) |
| Perceived barriers summary score2 | 277 | 1.66 (0.58) |
| Self-efficacy3 | ||
| How confident are you that you can get a colonoscopy when you are due? | 275 | 4.80 (0.63) |
| N | % | |
| Cues to action | ||
| Have any of your doctors recommended that you have a colonoscopy? | 272 | 92% |
Note: s.d. = standard deviation, CRC = colorectal cancer.
Response options were 1=no chance to 5=certain to get it
Response options were 1=strongly disagree to 5=strongly agree
Response options were 1= not at all confident to 5= extremely confident
Survivors felt their chance of getting colorectal cancer was, on average, between slight and moderate. However, they varied widely about their likelihood of getting colorectal cancer (s.d. = 0.98); 39% felt there was a slight chance or no chance of getting colorectal cancer again, whereas 28% felt there was a high chance or that they were certain they would get colorectal cancer again.
Participants reported feeling quite confident that they could obtain a colonoscopy when they were due (mean self-efficacy = 4.81). Ninety-two percent of participants reported that at least one doctor recommended routine colonoscopies.
Primary Hypotheses: Health beliefs and intentions
In bivariate analyses, most health beliefs were associated with intention to have a colonoscopy. Survivors who had higher perceived likelihood (OR=2.02, 95% CI = 1.29–3.18), greater perceived benefits (OR = 2.54. 95% CI = 1.26–5.14), lower perceived barriers (OR=0.28, 95% CI = 0.16–0.50), and a recommendation for a colonoscopy from a physician (OR=14.85, 95% CI = 5.91–37.31) were more likely to have greater intentions to undergo colonoscopy. Self-efficacy was not associated with intentions to have a colonoscopy in the future.
In multivariable analyses (Table 3), greater perceived likelihood of getting colorectal cancer again was associated with higher expectations of receiving a colonoscopy (OR = 1.83, p <0.05). Perception of barriers, perception of benefits, physician recommendation, and self-efficacy were not associated with intentions.
Table 3.
Multivariable health belief predictors of intention to have colonoscopy in 5 years. a (N=273)
| Predictor | Intention |
|---|---|
| OR (95% CI) | |
| Perceived likelihood | 2.00 (1.16–3.44) |
| Perceived benefits | 1.08 (0.33–3.53) |
| Perceived barriers | 0.51 (0.23–1.13) |
| Self-efficacy (confident that could get colonoscopy) | 1.76 (0.86–3.61) |
| Physician recommended colonoscopy b | 2.70 (0.53 – 13.73) |
| Seen a primary care provider since diagnosis b | 0.12 (0.00 – 3.68) |
| History of screening before diagnosis b | 1.90 (0.64 – 5.62) |
| Had colonoscopy after diagnosis b | 9.47 (2.08 – 43.16) |
Adjusted for age, education, sex, race/ethnicity, severity of comorbidities, site of tumor, stage at diagnosis, income, whether insured
Reference groups for dichotomous variables are: did not receive recommendation, did not see a primary care provider, did not have screening before diagnosis, and did not have colonoscopy after diagnosis.
Note: OR = odds ratio, CI = confidence interval
Secondary hypotheses: Health care use and intentions
In bivariate analyses, having seen a primary care physician in the year since diagnosis was not associated with expectations to have colonoscopies in the future. Those who had a colonoscopy after diagnosis were more likely to have greater intentions to have a colonoscopy in the future (OR = 10.33, 95% CI = 4.62–23.1). Having had screening before diagnosis was not associated with intentions.
Similarly, in multivariable analyses, having seen a primary care physician since diagnosis and having been screened before diagnosis were unrelated to participants’ surveillance intentions. Those who had colonoscopy since surgery for their disease had greater intentions of having colonoscopy in the future (OR=9.47, 95% CI = 2.08–43.16).
Discussion
Intentions and behavior
Although colorectal cancer survivors are at increased risk for a second primary cancer or a local recurrence,2, 4 they often receive less surveillance than recommended.12–18, 43 Because of their history of colorectal cancer, one might expect colorectal cancer survivors to hold beliefs that would support surveillance. Using a population-based study of patients with recently diagnosed colorectal cancer, we used the HBM to identify potentially modifiable predictors of long-term (4-year) colorectal cancer surveillance among colorectal cancer survivors.
Overall, participants expressed high intentions to receive colonoscopy in the next 5 years. Given that these participants have already been diagnosed with, and successfully treated for, colorectal cancer, it is not surprising that they would intend to engage in preventive behavior in the future. However, this high level of intention and the high level of reported colonoscopy use contrasts with the low rates of surveillance found in other studies.12–18 This may be because our sample consists of survivors who have not had a second primary cancer and are well enough and motivated to participate in three interviews during a 4-year period. In addition, most participants were insured and did not perceive major barriers to colonoscopy.
Association of health beliefs and intentions
Perceived likelihood of recurrent or new colorectal cancer was an important predictor of expectation to undergo colonoscopy. This association had not been investigated before among cancer survivors. The few relevant studies suggest that survivors are concerned about recurrence. A multi-state survey study found that 68% of colorectal cancer 1-year survivors feel fearful that their illness will return,44 the top-ranked concern among a list of 29 concerns. In another survey study of 96 colorectal cancer survivors who responded at variable times after completion of their treatment, 92% of colorectal cancer survivors reported believing that they were at risk for recurrence.45 Our finding suggests that this perceived risk of recurrence may be related to later surveillance, although this association needs to be confirmed in a prospective study.
Although the perceived likelihood of getting colorectal cancer was high in this study, we observed substantial variation. Some survivors clearly underestimated their likelihood of recurrent or new colorectal cancer; 8% reported having no chance of a recurrent colorectal cancer, even if they were not to get regular colonoscopies. If perceived likelihood drives not just intention but actual surveillance behavior, addressing this erroneous perception presents a useful target for intervention. To take appropriate steps to protect themselves, survivors should understand their risk and perceive that it is modifiable through their own actions.46, 47 A Cochrane review of interventions that use risk communication to help people make informed decisions about screening found that tailored risk communications had a modest effect on screening behavior.48
The cue to action assessed in this study – recommendation for a colonoscopy from a physician – was unrelated to intentions in multivariable analyses. Physician recommendations have been shown to be related to colonoscopy screening in previous studies.49–51 Our finding indicates that a physician recommendation may not be necessary to influence intentions to have a surveillance colonoscopy. The operationalization of cues to action has not been systematically studied in the screening literature,19 nor in the surveillance literature, suggesting that physician recommendations may not be the most relevant cue to action for survivors.
Other health beliefs that have been shown to predict colorectal cancer screening did not predict surveillance intentions in multivariable analyses among our sample of survivors. The health beliefs that have been shown to be associated with colorectal cancer screening include perceived benefits of undergoing colorectal cancer screening23, 26, 52 (although effects are mixed22, 25), perceived barriers to undergoing colorectal cancer screening,22, 25, 26, 52 and self-efficacy.49, 52 Our null results may reflect a difference in the role of health beliefs between screening in the general population and surveillance among colorectal cancer survivors. After experiencing the diagnosis and treatment of cancer, barriers may seem minimal, benefits to colonoscopy may seem obvious, and the task of getting a colonoscopy (after more invasive treatments) may not seem insurmountable, causing a floor effect in perceived barriers and a ceiling effect in perceived benefits and self-efficacy. More sensitive measures may need to be created to assess these variables among survivors.
Past behaviors and intentions
The other predictor of greater intentions to get a routine colonoscopy was having already had at least one routine colonoscopy since diagnosis. Because this study excluded survivors who were diagnosed with a second primary cancer or recurrence, any prior colonoscopy either had normal results or indicated precancerous polyps that were presumably removed. It is therefore not surprising that survivors who had both interest in and access to an earlier colonoscopy, and which had yielded normal results or a reduction in future cancer risk, would report an expectation to continue with preventive care.
Limitations and conclusions
There are several limitations of these findings. First, because the data are cross-sectional, we cannot make causal statements about the association between health beliefs and intentions. Second, intentions may not reflect actual behavior, although a meta-analysis of 10 meta-analyses found that intentions strongly predict behaviors across studies.34 Additionally, a meta-analysis by Webb and Sheeran of 47 experiments manipulating intentions showed that changes in intention led to corresponding changes in behavior.53 Finally, this study was conducted in a single state, in a highly insured population with high use of primary care, which may limit its generalizability.
The National Cancer Institute and other organizations have prioritized research on cancer survivorship to better assess long-term health services use and health outcomes.29 We extended research on cancer behaviors from screening to surveillance among a diverse sample of registry-ascertained colorectal cancer survivors. Because of their history of cancer, survivors may evaluate benefits, barriers, likelihood of future disease, self-efficacy, and cues to action differently from those encouraged to have cancer screening. Moreover, they are at greater risk than the rest of the population for contracting a future colorectal cancer.
We found that among colorectal cancer survivors four years after diagnosis, perceived likelihood of getting colorectal cancer again is associated with interest in ongoing routine colonoscopies. To facilitate surveillance and improve poor adherence to colonoscopy guidelines, it is important to identify drivers of colonoscopy utilization and pragmatic interventions that capitalize on these findings.
Implications for Cancer Survivors
To maximize interest in surveillance, it may be useful to create interventions for survivors that inform survivors of their continued risk of colorectal cancer. One such intervention is a survivorship care plan, which was designed by the Institute of Medicine to communicate important information to survivors as they navigate the transition between cancer care and post-treatment care.29 Survivorship care plans contain information for survivors including a summary of their cancer diagnosis and treatment, recommendations for future care, and resources for cancer survivors. Survivorship care plans, which already are being developed and disseminated, may be ideal platforms to communicate important information about risks of second malignancies to cancer survivors in order to improve adherence to surveillance guidelines.
Acknowledgments
This research was supported, in part, by grants from the National Institutes of Health (P30 DK034987, U01 CA093326) and by a George Bennett Dissertation fellowship from the Foundation for Informed Medical Decision Making.
References
- 1.Cancer Facts & Figures – 2008. American Cancer Society (ACS); Atlanta, Georgia: 2008. [Google Scholar]
- 2.Green RJ, et al. Surveillance for second primary colorectal cancer after adjuvant chemotherapy: an analysis of Intergroup 0089. Ann Intern Med. 2002;136(4):261–9. doi: 10.7326/0003-4819-136-4-200202190-00005. [DOI] [PubMed] [Google Scholar]
- 3.Desch CE, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23(33):8512–9. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 4.Rex DK, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2006;130(6):1865–71. doi: 10.1053/j.gastro.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer, 2003. CA Cancer J Clin. 2003;53(1):27–43. doi: 10.3322/canjclin.53.1.27. [DOI] [PubMed] [Google Scholar]
- 6.Winawer S, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale - update based on new evidence. Gastroenterology. 2003;124(2):544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 7.The NCCN Colorectal Screening Clinical Practice Guidelines in Oncology (Version 1.2006) National Comprehensive Cancer Network; 2006. [DOI] [PubMed] [Google Scholar]
- 8.The NCCN Colorectal Screening Clinical Practice Guidelines in Oncology (Version 1.2005) National Comprehensive Cancer Network, Inc; 2005. [DOI] [PubMed] [Google Scholar]
- 9.Anthony T, et al. Practice parameters for the surveillance and follow-up of patients with colon and rectal cancer. Dis Colon Rectum. 2004;47(6):807–17. doi: 10.1007/s10350-004-0519-x. [DOI] [PubMed] [Google Scholar]
- 10.Benson AB, 3rd, et al. 2000 update of American Society of Clinical Oncology colorectal cancer surveillance guidelines. J Clin Oncol. 2000;18(20):3586–8. doi: 10.1200/JCO.2000.18.20.3586. [DOI] [PubMed] [Google Scholar]
- 11.Simmang CL, et al. Practice parameters for detection of colorectal neoplasms. The Standards Committee, The American Society of Colon and Rectal Surgeons. Dis Colon Rectum. 1999;42(9):1123–9. doi: 10.1007/BF02238562. [DOI] [PubMed] [Google Scholar]
- 12.Cooper GS, Payes JD. Temporal trends in colorectal procedure use after colorectal cancer resection. Gastrointest Endosc. 2006;64(6):933–40. doi: 10.1016/j.gie.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Elston Lafata J, et al. Sociodemographic differences in the receipt of colorectal cancer surveillance care following treatment with curative intent. Med Care. 2001;39(4):361–72. doi: 10.1097/00005650-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Rolnick S, et al. Racial and age differences in colon examination surveillance following a diagnosis of colorectal cancer. J Natl Cancer Inst Monogr. 2005;(35):96–101. doi: 10.1093/jncimonographs/lgi045. [DOI] [PubMed] [Google Scholar]
- 15.Rulyak SJ, et al. Clinical and sociodemographic factors associated with colon surveillance among patients with a history of colorectal cancer. Gastrointest Endosc. 2004;59(2):239–47. doi: 10.1016/s0016-5107(03)02531-8. [DOI] [PubMed] [Google Scholar]
- 16.Cooper GS, et al. Geographic and patient variation among Medicare beneficiaries in the use of follow-up testing after surgery for nonmetastatic colorectal carcinoma. Cancer. 1999;85(10):2124–31. [PubMed] [Google Scholar]
- 17.Knopf KB, et al. Bowel surveillance patterns after a diagnosis of colorectal cancer in Medicare beneficiaries. Gastrointest Endosc. 2001;54(5):563–71. doi: 10.1067/mge.2001.118949. [DOI] [PubMed] [Google Scholar]
- 18.Ellison GL, et al. Racial differences in the receipt of bowel surveillance following potentially curative colorectal cancer surgery. Health Serv Res. 2003;38(6 Pt 2):1885–903. doi: 10.1111/j.1475-6773.2003.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janz NK, Champion V, Strecher VJ. The Health Belief Model. In: Glantz K, Rimer BK, Marcus Lewis F, editors. Health Behavior and Health Education. Jossey-Bass; San Francisco: 2002. pp. 45–66. [Google Scholar]
- 20.Palmer RC, et al. Familial risk and colorectal cancer screening health beliefs and attitudes in an insured population. Prev Med. 2007;45(5):336–41. doi: 10.1016/j.ypmed.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Emmons KM, et al. Tailored computer-based cancer risk communication: correcting colorectal cancer risk perception. J Health Commun. 2004;9(2):127–41. doi: 10.1080/10810730490425295. [DOI] [PubMed] [Google Scholar]
- 22.Macrae FA, et al. Predicting colon cancer screening behavior from health beliefs. Prev Med. 1984;13(1):115–26. doi: 10.1016/0091-7435(84)90044-6. [DOI] [PubMed] [Google Scholar]
- 23.Codori AM, et al. Health beliefs and endoscopic screening for colorectal cancer: potential for cancer prevention. Prev Med. 2001;33(2 Pt 1):128–36. doi: 10.1006/pmed.2001.0862. [DOI] [PubMed] [Google Scholar]
- 24.Gipsh K, Sullivan JM, Dietz EO. Health belief assessment regarding screening colonoscopy. Gastroenterol Nurs. 2004;27(6):262–7. doi: 10.1097/00001610-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Janz NK, et al. Colorectal cancer screening attitudes and behavior: a population-based study. Prev Med. 2003;37(6 Pt 1):627–34. doi: 10.1016/j.ypmed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Rawl S, et al. Validation of scales to measure benefits and barriers of colorectal cancer screening. Journal of Psychosocial Oncology. 2001;19(34):47–63. [Google Scholar]
- 27.James AS, Campbell MK, Hudson MA. Perceived barriers and benefits to colon cancer screening among African Americans in North Carolina: how does perception relate to screening behavior? Cancer Epidemiol Biomarkers Prev. 2002;11(6):529–34. [PubMed] [Google Scholar]
- 28.Katz ML, et al. Improving colorectal cancer screening by using community volunteers: results of the Carolinas cancer education and screening (CARES) project. Cancer. 2007;110(7):1602–10. doi: 10.1002/cncr.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hewitt M, Greenfield S, Stovall E, editors. From Cancer Patient to Cancer Survivor: Lost in Transition. Institute of Medicine and National Research Council; 2006. [Google Scholar]
- 30.Earle CC. Failing to plan is planning to fail: improving the quality of care with survivorship care plans. J Clin Oncol. 2006;24(32):5112–6. doi: 10.1200/JCO.2006.06.5284. [DOI] [PubMed] [Google Scholar]
- 31.Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101(8):1712–9. doi: 10.1002/cncr.20560. [DOI] [PubMed] [Google Scholar]
- 32.Cancer Care Outcomes Research & Surveillance Consortium 2007. 2008 April 9; Available from: http://healthservices.cancer.gov/cancors/
- 33.Ayanian JZ, et al. Understanding Cancer Treatment and Outcomes: The Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22(15):2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Sheeran P. Intention-behavior relations: A conceptual and empirical review. In: Stroebe W, Hewstone M, editors. European Review of Social Psychology. John WIley and Sons; 2002. pp. 1–36. [Google Scholar]
- 35.Vernon SW, et al. Measures for ascertaining use of colorectal cancer screening in behavioral, health services, and epidemiologic research. Cancer Epidemiol Biomarkers Prev. 2004;13(6):898–905. [PubMed] [Google Scholar]
- 36.Johnston A, et al. Validation of a comorbidity education program. J of Registry Management. 2001;28(3):125–131. [Google Scholar]
- 37.Piccirillo J. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110(4):593–602. doi: 10.1097/00005537-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Piccirillo J, et al. The measurement of comorbidity by cancer registries. J of Registry Management. 2003;30(4):8–14. [Google Scholar]
- 39.Royston P. Multiple imputation of missing values: Update of ice. Stata Journal. 2005;5(4):527–536. [Google Scholar]
- 40.Royston P. Multiple imputation of missing values. Stata Journal. 2004;4(3):227–241. [Google Scholar]
- 41.Stata Statistical Software: Release 10. StataCorp LP: College Station, TX; 2007. [Google Scholar]
- 42.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. New York: Wiley-Interscience; 2000. [Google Scholar]
- 43.Elston Lafata J, et al. Routine surveillance care after cancer treatment with curative intent. Med Care. 2005;43(6):592–9. doi: 10.1097/01.mlr.0000163656.62562.c4. [DOI] [PubMed] [Google Scholar]
- 44.Baker F, et al. Adult cancer survivors: how are they faring? Cancer. 2005;104(11 Suppl):2565–76. doi: 10.1002/cncr.21488. [DOI] [PubMed] [Google Scholar]
- 45.Cardella J, et al. Compliance, attitudes and barriers to post-operative colorectal cancer follow-up. J Eval Clin Pract. 2008;14(3):407–15. doi: 10.1111/j.1365-2753.2007.00880.x. [DOI] [PubMed] [Google Scholar]
- 46.Lipkus IM, Green LG, Marcus A. Manipulating perceptions of colorectal cancer threat: implications for screening intentions and behaviors. J Health Commun. 2003;8(3):213–28. doi: 10.1080/10810730305684. [DOI] [PubMed] [Google Scholar]
- 47.Lipkus IM, et al. Increasing colorectal cancer screening among individuals in the carpentry trade: test of risk communication interventions. Prev Med. 2005;40(5):489–501. doi: 10.1016/j.ypmed.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Edwards AG, et al. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev. 2006;(4):CD001865. doi: 10.1002/14651858.CD001865.pub2. [DOI] [PubMed] [Google Scholar]
- 49.Friedman LC, Webb JA, Everett TE. Psychosocial and medical predictors of colorectal cancer screening among low-income medical outpatients. J Cancer Educ. 2004;19(3):180–6. doi: 10.1207/s15430154jce1903_14. [DOI] [PubMed] [Google Scholar]
- 50.Christie J, et al. Predictors of endoscopy in minority women. J Natl Med Assoc. 2005;97(10):1361–8. [PMC free article] [PubMed] [Google Scholar]
- 51.Levy BT, et al. Colorectal cancer testing among patients cared for by Iowa family physicians. Am J Prev Med. 2006;31(3):193–201. doi: 10.1016/j.amepre.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Menon U, et al. Beliefs associated with fecal occult blood test and colonoscopy use at a worksite colon cancer screening program. J Occup Environ Med. 2003;45(8):891–8. doi: 10.1097/01.jom.0000083038.56116.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Webb TL, Sheeran P. Does Changing Behavioral Intentions Engender Behavior Change? A Meta-Analysis of the Experimental Evidence. Psychological Bulletin. 2006;132(2):249–268. doi: 10.1037/0033-2909.132.2.249. [DOI] [PubMed] [Google Scholar]