Abstract
Rationale
Vascular calcification is highly correlated with morbidity and mortality, and is often associated with inflammation. Vitamin D may regulate vascular calcification, and has been associated with cardiovascular survival benefits.
Methods and Results
We developed a macrophage/SMC coculture system and examined the effects of vitamin D receptor activators (VDRAs), calcitriol and paricalcitol, on SMC matrix calcification. We found that treatment of SMC alone with VDRAs had little effect on phosphate-induced SMC calcification in vitro. On the other hand, coculture with macrophages promoted SMC calcification, and this was strikingly inhibited by VDRA treatment. Several VDRA-induced genes, including BMP2, TNFα, and osteopontin (OPN) were identified as candidate paracrine factors for VDRA’s protective effect. Of these, OPN was further investigated and found to contribute significantly to the inhibitory actions of VDRAs on calcification in macrophage/SMC cocultures.
Conclusion
The ability of VDRAs to direct a switch in the paracrine phenotype of macrophages from procalcific to anticalcific may contribute to their observed cardiovascular survival benefits.
Keywords: macrophage, vascular calcification, smooth muscle, coculture, osteopontin
Introduction
Vascular calcification is a common finding in patients with cardiovascular disease (CVD), diabetes and end-stage renal disease (ESRD). Vascular calcification has been positively correlated with coronary atherosclerotic plaque burden 1, 2, increased risk of myocardial infarction 3, 4 and plaque instability 5, 6. Furthermore, coronary calcium score is a strong predictor of incident coronary heart disease 7 and stroke 8. In diabetes mellitus, vascular calcification is strongly correlated with coronary artery disease and future cardiovascular events including lower extremity amputation 9, 10. In ESRD patients, vascular calcification is a strong prognostic marker of CVD mortality 11, 12 and is likely to be a major contributor to the 10–100 fold increase in cardiovascular mortality risk observed in these patients 13. Underscoring this problem, the American Heart Association has indicated that ESRD patients should be considered at the highest risk for CVD 14.
Vitamin D is a steroid hormone that plays an essential role in mineral metabolism, skeletal health and immunity. The active metabolite of Vitamin D, calcitriol (1, 25 (OH)2 Vitamin D), exerts the majority of its actions via the nuclear Vitamin D receptor (VDR). The complex of calcitriol and the VDR binds to Vitamin D response elements in the promoters of target genes and regulates gene expression. A broad spectrum of Vitamin D-regulated genes have been identified including those involved in bone and mineral metabolism, cell proliferation and differentiation, and immunomodulation (reviewed in 15, 16).
The role of Vitamin D in regulating vascular calcification appears to be complex. Hypervitaminosis D leads to ectopic calcification in people 17. Likewise, in experimental models, high levels of Vitamin D, either in non-uremic or uremic animals, induce vascular calcification 18, 19. These effects are in large part due to the stimulatory effect of Vitamin D on intestinal absorption of calcium and phosphate, thereby leading to elevated serum mineral levels that predispose to ectopic calcium deposition. On the other hand, clinical studies have shown that serum levels of calcitriol are inversely correlated with coronary artery calcification score in the general population, suggesting an inhibitory role of Vitamin D in the development of vascular calcification 20–22 In addition, Vitamin D deficiency is prevalent in ESRD patients 23–25 and they are routinely treated with VDRAs in order to prevent secondary hyperparathyroidism. In these patients, VDRA treatment has been shown to have cardiovascular survival benefits in several large, cross-sectional studies 26–28. Together, these findings suggest that the effects of Vitamin D on vascular health are complex and highly dose-dependent. Indeed, a recent clinical study showed a bimodal dose relationship between serum calcitriol levels and both carotid intimal/medial thickness and calcification score in children on dialysis 29.
While Vitamin D overload-induced cardiovascular disease is usually secondary to hypercalcemia, hyperphosphatemia, and vascular calcification, the mechanisms mediating the cardiovascular survival benefits of Vitamin D remain obscure. The possibility that survival benefits of VDRAs relate to regulation of vascular calcification was suggested by a recent study in uremic low density lipoprotein deficient (LDLR−/−) mice. Lower doses of VDRAs inhibited, while higher doses induced, atherosclerotic plaque calcification 30. In this regard, accumulating evidence indicates that vascular calcification is an actively regulated process involving several cell types, including vascular SMCs and macrophages, two key components of atherosclerotic lesions. Numerous molecules that either promote or inhibit vascular calcification have been identified, including those with the ability to regulate the osteochondrogenic transdifferentiation of SMCs 31. Accumulation of macrophages is associated with vascular calcification in human carotid 22 and coronary arteries 32, and several in vitro studies have shown that macrophages regulate vascular calcification by promoting an osteogenic phenotypic transition of SMC 33, 34. Thus, it is possible that the beneficial effects of VDRAs on vascular calcification may be mediated via direct effects either on SMC, macrophages or both. Thus, in the present study, we developed a macrophage/SMC coculture system to examine the effects of VDRA on SMC calcification in vitro.
Materials and Methods
An expanded Materials and Methods section is available in the online Data Supplement (available at http://atvb.ahahournals.org).
Human SMC were from Clonetics Corporation (Palo Alto, CA). Human THP-1 and mouse P388D1 macrophages were from ATCC (CCL-46). Macrophage/SMC cocultures were performed in transwells. P388D1 cells deficient in OPN or vitamin D receptor were generated using the pSUPER RNA interference system (Oligoengine, Seattle, WA).
Results
Expression of VDR in THP-1, human SMCs and mouse macrophages
VDR expression profiles were examined in the various cell types used in our study, including differentiated and undifferentiated human THP-1 cells, human SMC and mouse P388D1 macrophages. As shown in supplementary figure IA, bands at 264 or 208 bp were amplified from human or mouse cells respectively. These results indicate that VDR is expressed in both human and mouse macrophages, as well as human SMC.
VDRAs have minimal direct effects on SMC calcification in vitro
Previous studies have provided contradictory results regarding Vitamin D action on SMC calcification in vitro, 35–37, 16, 38. In order to determine the effect of VDRAs in our system, human SMCs were incubated with 2.6 mM phosphate containing media (CM) in the presence of either calcitriol or paricalcitol at concentrations of 0.5, 5, 50 nM for 10 days, and calcium content of the extracellular matrix was determined. As shown in supplementary figure IB, there was a small (~20%) reduction in calcium content compared to vehicle-treated control in VDRA-treated SMCs, but these effects were not dose-dependent, suggesting that they were not VDR-mediated. Incubation of SMC with media containing 1.24 mM phosphate did not induce matrix calcification (data not shown), consistent with our previous studies 39–41.
Macrophages promote SMC calcification in cocultures
In order to determine the regulatory roles of macrophages in vascular calcification, macrophage/SMC cocultures were performed in 6-well transwells. SMCs and macrophages were cocultured in either GM or CM for 10 days and calcium content in the extracellular matrix of SMC layer was determined. As shown in Figure 1, macrophage coculture substantially increased SMC calcification (SMC vs. macrophage/SMC: 18.65 vs. 66.97 µg Ca/mg protein, p<0.05). However, no mineralization was observed in either SMC culture alone or macrophage/SMC coculture under normal phosphate conditions. The enhanced calcification observed in macrophage/SMC cocultures suggested that macrophages might release soluble factors that modulate the calcification capacity of SMCs.
Figure 1.
Macrophage coculture promotes SMC calcification in vitro. THP-1 macrophage/SMC cocultures were treated with GM or CM for 10 days. Calcium content of the SMC cultures was measured and presented as mean ± S.D. (n = 3). * Significant increase compared with SMC cultured alone (P <0.05).
Inhibition of SMC calcification in macrophage/SMC cocultures by VDRAs
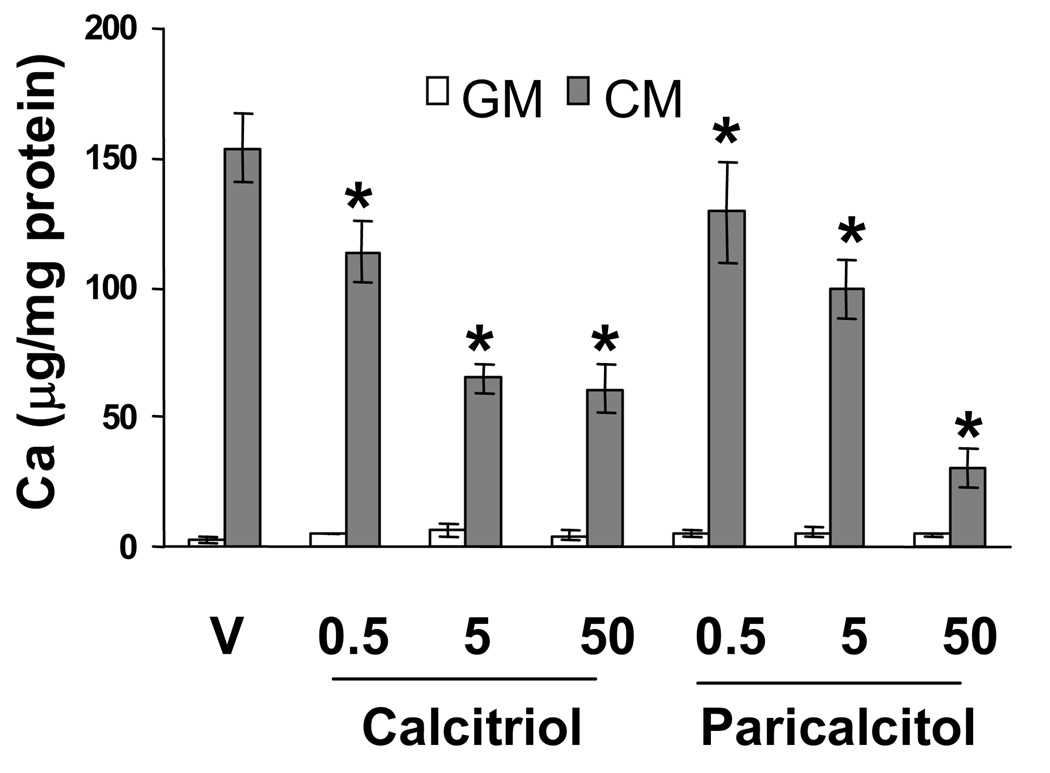
To determine the effect of VDRAs on SMC calcification in macrophage/SMC coculture, the two cell types were incubated with GM or CM in the presence or absence of various concentrations of calcitriol or paricalcitol for 10 days. As shown in Figure 2, both calcitriol and paricalcitol dose-dependently inhibited SMC calcification induced by CM with maximal ~7.5 fold inhibition observed with 50 nM paricalcitol (vehicle vs. 50 nM paricalcitol: 153.74 vs. 30 µg Ca/mg protein). On the other hand, VDRAs had no effect on SMC calcification in GM. Von Kossa staining confirmed the results of the biochemical calcium assay in the cocultures, and indicated that the effect of VDRAs was to decrease cell matrix-associated mineralization (data not shown).
Figure 2.
VDRAs inhibit SMC calcification in macrophage/SMC coculture. THP-1 macrophage/SMC cocultures were treated with various concentrations of calcitriol or paricalcitol in GM or CM for 10 days. Calcium content of the SMC cultures was measured and presented as mean ± S.D. (n = 3). * Significant decrease compared with vehicle (P <0.05).
To confirm that the actions of the VDRAs were indeed mediated by the VDR, we performed knockdown of the VDR in P388D1 macrophages prior to coculture. P388D1 macrophages treated with VDR specific siRNA had extremely low levels of VDR mRNA compared to cells treated with a control siRNA indicating robust knockdown efficiency (supplementary figure IIA). Using these cells, the effect of VDRAs on calcification in macrophage-SMC cocultures was again examined. As shown in supplementary Figure IIb, P388D1 macrophages deficient in VDR (VDR siRNA) did not inhibit calcium deposition in response to either calcitriol or paricalcitol treatment, whereas macrophages that expressed normal levels of VDR (CT siRNA) showed significant inhibition of calcification in response to VDRA treatment.
The findings of VDR-dependent inhibition of SMC calcification by macrophages in response to VDRA treatment were in striking contrast to the minimal effects on calcification observed with VDRA treatment of SMC alone and the robust induction of SMC calcification observed following coculture with untreated macrophage. Together, these data suggest that VDRAs, acting through the VDR, induce a procalcific to anticalcific paracrine switch in macrophages.
Regulation of macrophage gene expression by VDRAs
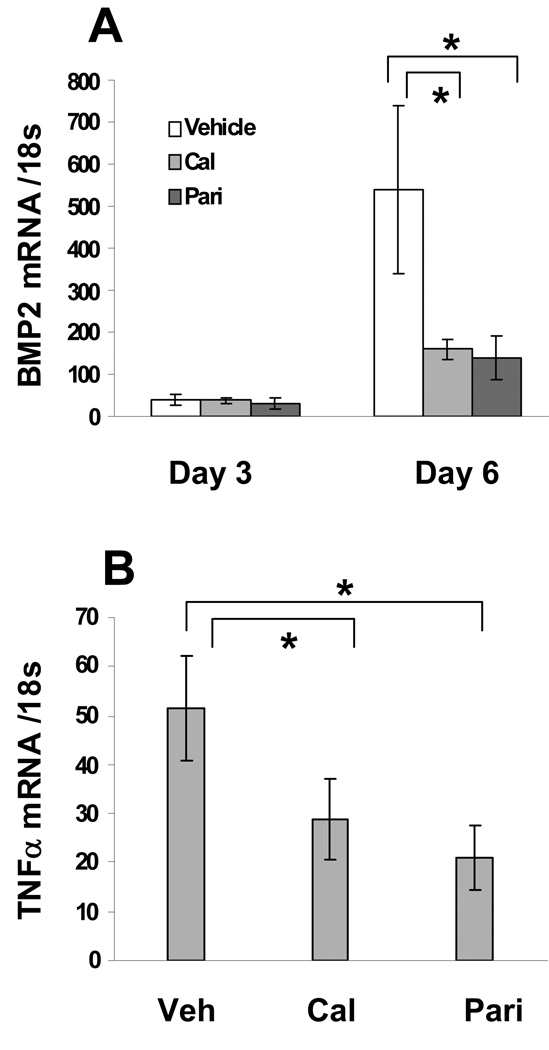
To characterize the VDRA-induced macrophage phenotype switch, we tested several candidate Vitamin D responsive genes that were known to generate secreted proteins and that have been implicated in biomineralization. Thus, macrophages were treated with 50 nM calcitriol or paricalcitol for 3 or 6 days and the expression levels of BMP2, TNFα, OPN and TGFβ were determined by QPCR. Figure 3A shows that calcitriol and paricalcitol equivalently inhibited BMP2 expression, with more than 70% inhibition observed following 6 days of treatment. TNFα mRNA levels were also reduced by VDRA treatment at day 3 (Figure 3B), though this effect disappeared by day 6 (data not shown). As shown in Figure 4A, both calcitriol and paricalcitol increased OPN mRNA levels with a maximal 2 fold increase observed with calcitriol. Consistent with mRNA results, both calcitriol and paricalcitol increased protein levels of OPN comparably (Figure 4B). Furthermore, OPN induction by VDRAs was blocked by VDR siRNA (supplementary figure III), indicating that the effect was mediated by VDR. VDRA treatment did not alter levels of TGFβ mRNA in macrophages (data not shown).
Figure 3.
VDRAs inhibit BMP2 and TNFα mRNA levels. Total RNA was obtained from THP-1 macrophages treated with 50 nM calcitriol or paricalcitol for 3 and 6 days for BMP2 (A) and 3 days for TNFα (B) and analyzed by QPCR. Results were normalized to 18s rRNA and are presented as mean ± S.D. (n = 3). * Significant decrease compared with vehicle (P <0.05).
Figure 4.
VDRAs induce OPN expression in macrophages. (A) Total RNA was obtained from THP-1 macrophages treated with 50 nM calcitriol or paricalcitol for 3 and 6 days respectively. The levels of OPN mRNA were determined by QPCR and normalized to 18s rRNA. (B) Conditioned media were collected from THP-1 macrophages treated with 50 nM of calcitriol or paricalcitol for 6 days. OPN protein levels were determined by ELISA. Data are presented as mean ± S.D. (n = 3).
Downregulation of OPN production in macrophages prevents VDRA-mediated inhibition of SMC calcification in coculture
To determine whether synthesis of OPN by VDRA-treated macrophages was required for their inhibitory effect on SMC calcification, we generated OPN deficient macrophages via siRNA transduction of P388D1 mouse macrophages. An ~80% reduction of OPN mRNA was achieved in OPN siRNA cells compared to CT siRNA cells as determined by QPCR (supplementary figure IV, A). To determine knockdown efficiency of siRNA on activated macrophages, CT siRNA and OPN siRNA macrophages were cultured in the presence of PMA, a known inducer of OPN 43. Consistent with mRNA data (supplementary figure IV, A), secreted OPN levels were 88% lower in OPN siRNA compared to CT siRNA cells in the absence of PMA (supplementary figure IV, B). PMA significantly increased OPN secretion in CT siRNA cells, and this effect was almost completely blocked in OPN siRNA cells (95% reduction in OPN siRNA vs. CT siRNA) (supplementary figure IV, B). Thus, OPN siRNA cells were deficient in both constitutive and inducible OPN expression.
In order to determine the specificity and identify potential off-target effects of OPN siRNA, mRNA levels of BMP2 and TNFα were examined in both OPN siRNA and CT siRNA cells. BMP2 mRNA was not detected in either cell type in the presence or absence of VDRA (data not shown). TNFα expression was similar in OPN siRNA and CT siRNA cells under basal conditions, and VDRA treatment inhibited TNFα equivalently in the two cell types (supplementary figure IV, C ).
OPN siRNA and CT siRNA cells were then cocultured with SMC in CM in the presence of 50 nM calcitriol and paricalcitol for 10 days. Calcium content of the extracellular matrix of SMC layer was measured. As shown in Figure 5, OPN deficiency in macrophages almost completely abrogated the ability of VDRAs to inhibit SMC calcification in coculture. These results indicate that OPN upregulation and secretion from cocultured macrophages is a critical factor required for the observed VDRA-mediated inhibition of SMC calcification.
Figure 5.
Downregulation of OPN production in P388D1 macrophages prevents VDRA-mediated inhibition of SMC calcification in coculture. Cocultures of SMC with either CT siRNA or OPN siRNA macrophages were treated with 50 nM calcitriol or paricalcitol in CM for 10 days. Calcium content of the SMC culture was measured and presented as mean ± S.D. (n = 3). * Significant decrease compared with vehicle (P <0.05).
Discussion
Vascular calcification occurs in two different patterns in the arterial wall depending on disease state. In atherosclerosis, calcification occurs predominantly in the arterial intima associated with inflamed and necrotic regions of the plaque. In arteriosclerosis, calcification occurs predominantly in the arterial media in the absence of inflammation, Both types of calcification are observed in ESRD patients and are thought to contribute to the increased cardiovascular disease risk in these patients 44, 45.
The majority of ESRD patients are Vitamin D deficient and are treated with VDRAs to prevent secondary hyperparathyroidism. VDRA treatment has cardiovascular survival benefits in these patients. The mechanisms mediating the cardiovascular survival benefits of VDRAs remain to be identified. SMCs contain VDRs and have been previously reported to respond to VDRA treatment with alterations in proliferation and changes in gene expression 17, 18. However, past studies have provided contradictory results regarding VDRA action on SMC calcification in vitro. Several studies reported that VDRA treatment induced calcification in bovine and rat SMC 35–37. In contrast, Wolisi and Wu-Wong failed to observe VDRA effect on mineralization in either bovine or human vascular SMC 16, 38. Our studies are in agreement with the latter studies, since we found very little specific effect of VDRA treatment on calcification in human SMC culture. These disparate results are most likely due to different experimental conditions and/or cell sources, which are known to affect SMC susceptibility to calcification 39.
Macrophages play a crucial role in the pathogenesis of various diseases and conditions, such as atherosclerosis, autoimmune diseases and chronic inflammation. Macrophages accumulate in atherosclerotic lesions and are associated with various stages of calcification in human carotid arteries 22. In plaques, macrophages colocalize with SMC and may regulate their function via paracrine factors. Thus, we used macrophage/SMC cocultures to model cell interactions that exist in atherosclerotic plaques. We found that coculture with macrophages substantially increased SMC calcification compared to SMC cultured in the absence of macrophages. The procalcific action of macrophages was most likely mediated by the production of soluble paracrine factors, since macrophage/SMC cocultures were performed in transwells without cell-cell contact. Our studies are in agreement with previous studies demonstrating that monocyte/macrophage coculture enhanced SMC calcification in vitro 33, 34.
Interestingly, we found that treatment of macrophage/SMC cocultures with VDRAs converted the stimulatory effect of macrophages on SMC calcification to a striking inhibitory effect. VDRA mediated effects were abolished when VDR deficient macrophages were used in the macrophage/SMC cocultures. These findings indicate a crucial role of macrophage in regulation of SMC calcification by VDRA acting through the macrophage VDR. Our studies also provide a potential mechanism for the recent findings of Mathew et al who showed that physiological doses of VDRAs inhibited aortic calcification as well as osteogenic gene expression in uremic, high fat fed LDLR−/− mice 30.
Most of VDRA’s biological actions are mediated by transcriptional regulation of VDRA-responsive genes. In the present study, we found that BMP2 and TNFα were highly expressed by macrophages, but that VDRA treatment strikingly inhibited their expression. BMP2 is a bone morphogenetic protein that promotes bone formation and mineralization in vivo, and SMC calcification in vitro 46. TNFα is an important inflammatory mediator that is expressed at high levels in classically activated macrophages that has been implicated in SMC calcification in vitro and in vivo 34, 47, 48. Thus, VDRA treatment reduces production of procalcifying molecules by macrophages and this likely contributes to their ability to attenuate SMC calcification in coculture. In contrast, Wu-Wong et al used DNA microarray covering 22,000 different human genes to characterize the VDR-mediated gene expression profile in human SMCs treated with calcitriol and paricalcitol 49. A total of 181 VDRA target genes were identified. However, BMP2, TNFα and OPN were not among these target genes. Whether VDRA treatment alters circulating levels of these factors is not known, but would be predicted from our studies.
In addition to inhibition of procalcifying gene expression, VDRA treatment induced OPN levels in macrophages. OPN is a secreted protein that has been shown to inhibit SMC calcification in vitro and in vivo 42, 50–52. Inhibition of calcification by OPN is highly dependent on its level of phosphorylation, with non-phosphorylated OPN showing little anticalcific activity 53–55. Vehicle-treated macrophages expressed some OPN constitutively, but both OPN mRNA and secreted protein levels were increased following VDRA treatment. Interestingly, we observed that 50 nM paricalcitol was more potent at inhibiting SMC calcification compare with calcitriol, though paricalcitol had less effect on OPN mRNA and protein than calcitriol. There may be several explanations for this observation. First, it is possible that paricalcitol preferentially induced a more highly phosphorylated form of osteopontin. Extent of phosphorylation is known to dramatically affect the potency of osteopontin as an inhibitor of calcification 54. Since the ELISA and Western blots do not distinguish between specific phosphorylated forms or extent of phosphorylation of OPN, we cannot rule out this possibility. Second, it is possible that paricalcitol inhibited other procalcifying molecules or induced other anticalcific proteins that contribute to the overall effect. BMP2 and TNFα do not appear to explain this effect, since both were inhibited equivalently by calcitriol and paricalcitol.
The functional importance of OPN induction was revealed by treatment of macrophages with OPN siRNA. OPN deficient macrophages no longer suppressed SMC calcification following VDRA treatment as compared to OPN sufficient macrophages. These results suggest a potentially important role of macrophage-derived OPN in the regulation of vascular calcification by VDRAs. While it is likely that a secreted form of OPN that could bind apatite and inhibit crystal growth was responsible for this inhibitory activity based on previous mechanistic studies 52–54, we cannot exclude the possibility that an intracellular form of OPN might also be important since the siRNA strategy employed in our studies would inhibit both extracellular and intracellular forms of OPN. An intracellular form of OPN arising from an internal translation initiation site has recently been described that controls dendritic cell function and interferon-alpha production 56, 57.
In conclusion, our studies are the first to demonstrate an anticalcific effect of VDRAs in vitro. Our studies suggest that classically activated macrophages promote vascular calcification by releasing procalcifying factors, such as BMP2 and TNFα and low levels of the anticalcific molecules OPN. VDRA treatment, on the other hand, induces a procalcific to anticalcific paracrine switch in macrophages, whereby levels of BMP2 and TNFα are reduced and levels of OPN are elevated. These findings suggest a novel mechanism for the survival benefit of VDRAs observed in the general population, ESRD patients, and experimental animal models.
Supplementary Material
Acknowledgements
Sources of Funding: Studies were funded by a sponsored research agreement from Abbott and NIH grants HL62329 and HL081785 to CMG.
References
- 1.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 2.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, Schwartz RS. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 3.Loecker TH, Schwartz RS, Cotta CW, Hickman JR., Jr Fluoroscopic coronary artery calcification and associated coronary disease in asymptomatic young men. J Am Coll Cardiol. 1992;19:1167–1172. doi: 10.1016/0735-1097(92)90319-i. [DOI] [PubMed] [Google Scholar]
- 4.Beadenkopf WG, Daoud AS, Love BM. Calcification in the coronary arteries and its relationship to arteriosclerosis and myocardial infarction. Am J Roentgenol. 1964;92:865–871. [PubMed] [Google Scholar]
- 5.Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, Nakamura Y, Yamashita H, Yamagishi H, Takeuchi K, Naruko T, Haze K, Becker AE, Yoshikawa J, Ueda M. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–3429. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty: an observational study using intravascular ultrasound. Circulation. 1992;86:64–70. doi: 10.1161/01.cir.86.1.64. [DOI] [PubMed] [Google Scholar]
- 7.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 8.Vliegenthart R, Hollander M, Breteler MM, van der Kuip DA, Hofman A, Oudkerk M, Witteman JC. Stroke is associated with coronary calcification as detected by electron-beam CT: the Rotterdam Coronary Calcification Study. Stroke. 2002;33:462–465. doi: 10.1161/hs0202.103071. [DOI] [PubMed] [Google Scholar]
- 9.Olson JC, Edmundowicz D, Becker DJ, Kuller LH, Orchard TJ. Coronary calcium in adults with type 1 diabetes: a stronger correlate of clinical coronary artery disease in men than in women. Diabetes. 2000;49:1571–1578. doi: 10.2337/diabetes.49.9.1571. [DOI] [PubMed] [Google Scholar]
- 10.Lehto S, Niskanen L, Suhonen L, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in noninsulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 11.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 12.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 13.Foley RN, Parfrey PS. Cardiovascular disease and mortality in ESRD. J Nephrol. 1998;11:239–245. [PubMed] [Google Scholar]
- 14.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 15.Mathieu C, Decallonne B, van Etten E. 1,25-dihydroxyvitamin D3: the endocrine system meets the immune system. Verh K Acad Geneeskd Belg. 2002;64:71–80;. [PubMed] [Google Scholar]
- 16.Wolisi GO, Moe SM. The role of vitamin D in vascular calcification in chronic kidney disease. Semin Dial. 2005;18:307–314. doi: 10.1111/j.1525-139X.2005.18407.x. [DOI] [PubMed] [Google Scholar]
- 17.Kerr DN. Hypercalcemia and metastatic calcification. Cardiovasc Res. 1997;36:293–297. doi: 10.1016/s0008-6363(97)00243-5. [DOI] [PubMed] [Google Scholar]
- 18.Mizobuchi M, Finch JL, Martin DR, Slatopolsky E. Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int. 2007;72:709–715. doi: 10.1038/sj.ki.5002406. [DOI] [PubMed] [Google Scholar]
- 19.Bas A, Lopez I, Perez J, Rodriguez M, Aguilera-Tejero E. Reversibility of calcitriol-induced medial artery calcification in rats with intact renal function. J Bone Miner Res. 2006;21:484–490. doi: 10.1359/JBMR.051211. [DOI] [PubMed] [Google Scholar]
- 20.Doherty TM, Tang W, Dascalos S, Watson KE, Demer LL, Shavelle RM, Detrano RC. Ethnic origin and serum levels of 1alpha,25-dihydroxyvitamin D3 are independent predictors of coronary calcium mass measured by electron-beam computed tomography. Circulation. 1997;96:1477–1481. doi: 10.1161/01.cir.96.5.1477. [DOI] [PubMed] [Google Scholar]
- 21.Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, Demer LL. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 22.Jeziorska M, McCollum C, Woolley DE. Calcification in atherosclerotic plaque of human carotid arteries: associations with mast cells and macrophages. J Pathol. 1998;185:10–17. doi: 10.1002/(SICI)1096-9896(199805)185:1<10::AID-PATH71>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez EA, Sachdeva A, Oliver DA, Martin KJ. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol. 2004;24:503–510. doi: 10.1159/000081023. [DOI] [PubMed] [Google Scholar]
- 24.LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, Graves KL, Moe SM. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45:1026–1033. doi: 10.1053/j.ajkd.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 25.Shah N, Bernardini J, Piraino B. Prevalence and correction of 25(OH) vitamin D deficiency in peritoneal dialysis patients. Perit Dial Int. 2005;25:362–366. [PubMed] [Google Scholar]
- 26.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 27.Shoji T, Shinohara K, Kimoto E, Emoto M, Tahara H, Koyama H, Inaba M, Fukumoto S, Ishimura E, Miki T, Tabata T, Nishizawa Y. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19:179–184. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 28.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA, Jr, Thadhani R. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 29.Shroff R, Egerton M, Bridel M, Shah V, Donald AE, Cole TJ, Hiorns MP, Deanfield JE, Rees L. A bimodal association of vitamin D levels and vascular disease in children on dialysis. J Am Soc Nephrol. 2008;19:1239–1246. doi: 10.1681/ASN.2007090993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathew S, Lund RJ, Chaudhary LR, Geurs T, Hruska KA. Vitamin D Receptor Activators Can Protect against Vascular Calcification. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2007080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Abbadi M, Giachelli CM. Mechanisms of vascular calcification. Adv Chronic Kidney Dis. 2007;14:54–66. doi: 10.1053/j.ackd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Hoshiga M, Alpers CE, Smith LL, Giachelli CM, Schwartz SM. Alpha-v beta-3 integrin expression in normal and atherosclerotic artery. Circ Res. 1995;77:1129–1135. doi: 10.1161/01.res.77.6.1129. [DOI] [PubMed] [Google Scholar]
- 33.Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation. 2002;105:650–655. doi: 10.1161/hc0502.102969. [DOI] [PubMed] [Google Scholar]
- 34.Shioi A, Katagi M, Okuno Y, Mori K, Jono S, Koyama H, Nishizawa Y. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circ Res. 2002;91:9–16. doi: 10.1161/01.res.0000026421.61398.f2. [DOI] [PubMed] [Google Scholar]
- 35.Cardus A, Parisi E, Gallego C, Aldea M, Fernandez E, Valdivielso JM. 1,25- Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF-mediated pathway. Kidney Int. 2006;69:1377–1384. doi: 10.1038/sj.ki.5000304. [DOI] [PubMed] [Google Scholar]
- 36.Jono S, Nishizawa Y, Shioi A, Morii H. 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation. 1998;98:1302–1306. doi: 10.1161/01.cir.98.13.1302. [DOI] [PubMed] [Google Scholar]
- 37.Shalhoub V, Shatzen E, Henley C, Boedigheimer M, McNinch J, Manoukian R, Damore M, Fitzpatrick D, Haas K, Twomey B, Kiaei P, Ward S, Lacey DL, Martin D. Calcification inhibitors and Wnt signaling proteins are implicated in bovine artery smooth muscle cell calcification in the presence of phosphate and vitamin D sterols. Calcif Tissue Int. 2006;79:431–442. doi: 10.1007/s00223-006-0126-z. [DOI] [PubMed] [Google Scholar]
- 38.Wu-Wong JR, Noonan W, Ma J, Dixon D, Nakane M, Bolin AL, Koch KA, Postl S, Morgan SJ, Reinhart GA. Role of phosphorus and vitamin D analogs in the pathogenesis of vascular calcification. J Pharmacol Exp Ther. 2006 doi: 10.1124/jpet.106.101261. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98:905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 40.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 41.Yang H, Curinga G, Giachelli CM. Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int. 2004;66:2293–2299. doi: 10.1111/j.1523-1755.2004.66015.x. [DOI] [PubMed] [Google Scholar]
- 42.Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161:2035–2046. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glesne D, Vogt S, Maser J, Legnini D, Huberman E. Regulatory properties and cellular redistribution of zinc during macrophage differentiation of human leukemia cells. J Struct Biol. 2006;155:2–11. doi: 10.1016/j.jsb.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz U, Buzello M, Ritz E, Stein G, Raabe G, Wiest G, Mall G, Amann K. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant. 2000;15:218–223. doi: 10.1093/ndt/15.2.218. [DOI] [PubMed] [Google Scholar]
- 45.Ibels LS, Alfrey AC, Huffer WE, Craswell PW, Anderson JT, Weil R., 3rd Arterial calcification and pathology in uremic patients undergoing dialysis. Am J Med. 1979;66:790–796. doi: 10.1016/0002-9343(79)91118-5. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Yang HY, Giachelli CM. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 48.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102:2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 49.Wu-Wong JR, Nakane M, Ma J, Ruan X, Kroeger PE. Effects of Vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis. 2006;186:20–28. doi: 10.1016/j.atherosclerosis.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 50.Speer MY, McKee MD, Guldberg RE, Liaw L, Yang HY, Tung E, Karsenty G, Giachelli CM. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J Exp Med. 2002;196:1047–1055. doi: 10.1084/jem.20020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wesson JA, Johnson RJ, Mazzali M, Beshensky AM, Stietz S, Giachelli C, Liaw L, Alpers CE, Couser WG, Kleinman JG, Hughes J. Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J Am Soc Nephrol. 2003;14:139–147. doi: 10.1097/01.asn.0000040593.93815.9d. [DOI] [PubMed] [Google Scholar]
- 52.Wada T, McKee MD, Steitz S, Giachelli CM. Calcification of vascular smooth muscle cell cultures inhibition by osteopontin. Circulation Research. 1999;84:166–178. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]
- 53.Pampena DA, Robertson KA, Litvinova O, Lajoie G, Goldberg HA, Hunter GK. Inhibition of hydroxyapatite formation by osteopontin phosphopeptides. Biochem J. 2004;378:1083–1087. doi: 10.1042/BJ20031150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jono S, Peinado C, Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275:20197–20203. doi: 10.1074/jbc.M909174199. 1087. [DOI] [PubMed] [Google Scholar]
- 55.Gericke A, Qin C, Spevak L, Fujimoto Y, Butler WT, Sorensen ES, Boskey AL. Importance of phosphorylation for osteopontin regulation of biomineralization. Calcif Tissue Int. 2005;77:45–54. doi: 10.1007/s00223-004-1288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci U S A. 2008;105:7235–7239. doi: 10.1073/pnas.0802301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, Cantor H. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.