Abstract
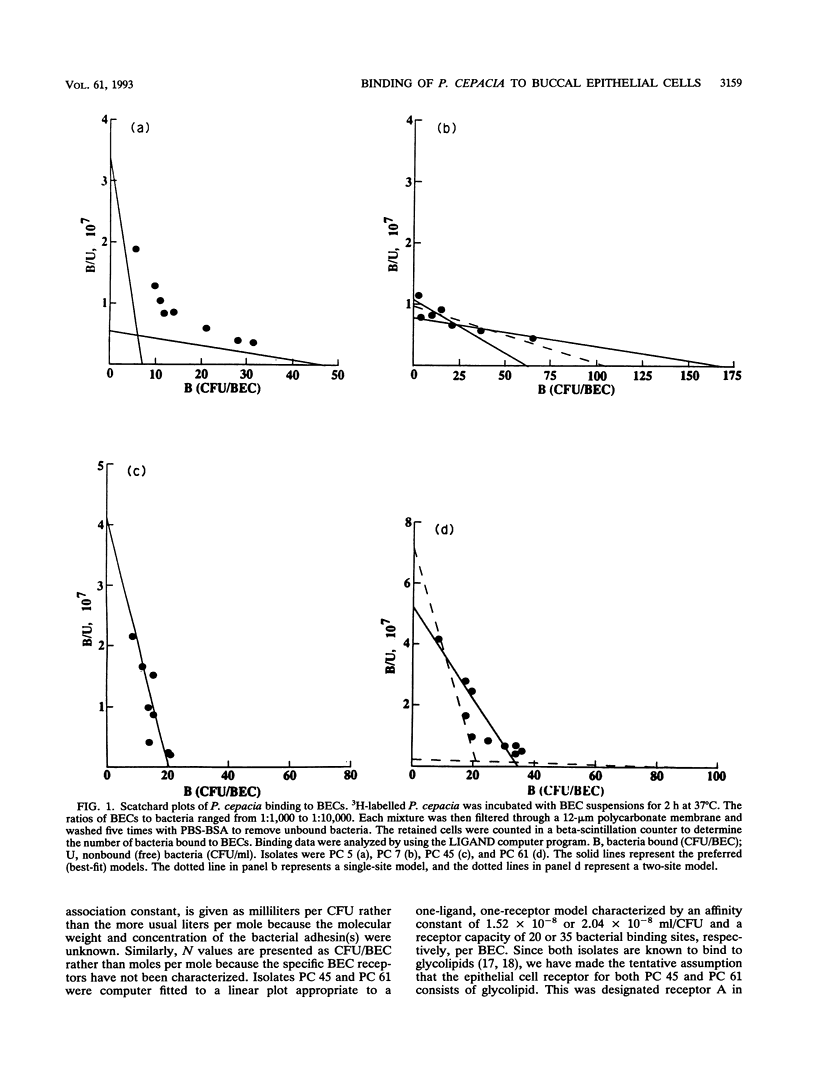
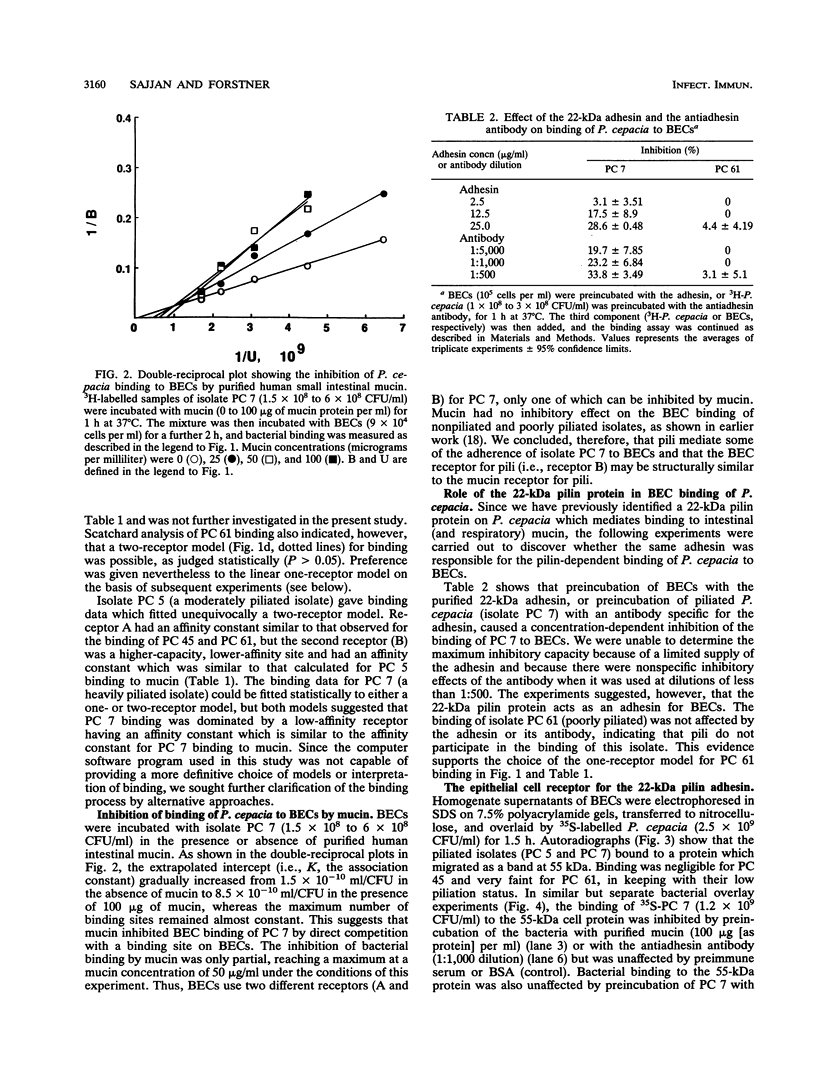
Previously we have shown that many isolates of Pseudomonas cepacia obtained from the sputum of patients with cystic fibrosis exhibit specific binding to purified mucins. The binding was mediated by a 22-kDa protein located on peritrichous pili of the bacteria. Nonpiliated bacteria did not bind to mucin. In the present study we found that both piliated and nonpiliated P. cepacia bind to buccal epithelial cells (BECs) obtained from health human volunteers. Scatchard plot analyses of binding data with the LIGAND computer program suggest the presence of at least two classes of cell receptors (A and B) for piliated P. cepacia (isolates PC 5 and PC 7) and a single class of receptors (A) for nonpiliated P. cepacia (isolates PC 45 and PC 61). The affinity constants for receptor A varied from 1.7 x 10(-9) to 4.7 x 10(-8) ml/CFU. Receptor B had a lower affinity constant (2.5 x 10(-10) to 1.2 x 10(-9) ml/CFU) but a greater saturation capacity. Receptor B was similar in affinity to the mucin receptor for piliated P. cepacia (3.3 x 10(-10) to 1.3 x 10(-9) ml/CFU). Purified mucin partially inhibited the binding of piliated bacteria to BECs by competing with BEC receptor site B. The purified 22-kDa pilin adhesin and an antiadhesin antibody also caused partial inhibition. One BEC receptor for piliated isolates of P. cepacia was identified as a 55-kDa protein as shown by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of BEC homogenate supernatants, Western blotting (immunoblotting), and bacterial overlay assays. Preincubation of piliated bacteria with either mucin or the antiadhesin antibody abolished binding to the 55-kDa BEC receptor. In summary, our results indicate that piliated P. cepacia interacts with BECs by using at least two different adhesin-receptor systems. One adhesin (not examined) is common to piliated and nonpiliated P. cepacia, but the other system is the pilus-localized 22-kDa mucin-binding adhesin and its 55-kDa BEC receptor protein. Because it mediates adherence to both mucin and epithelial cells, the 22-kDa adhesin may be an important virulence determinant in cystic fibrosis lung infections in which mucins are abnormally adhesive on mucosal surfaces.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doig P., Todd T., Sastry P. A., Lee K. K., Hodges R. S., Paranchych W., Irvin R. T. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect Immun. 1988 Jun;56(6):1641–1646. doi: 10.1128/iai.56.6.1641-1646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G. Colonization factor antigens of human pathogens. Curr Top Microbiol Immunol. 1990;151:129–145. doi: 10.1007/978-3-642-74703-8_7. [DOI] [PubMed] [Google Scholar]
- Girod S., Zahm J. M., Plotkowski C., Beck G., Puchelle E. Role of the physiochemical properties of mucus in the protection of the respiratory epithelium. Eur Respir J. 1992 Apr;5(4):477–487. [PubMed] [Google Scholar]
- Hasty D. L., Ofek I., Courtney H. S., Doyle R. J. Multiple adhesins of streptococci. Infect Immun. 1992 Jun;60(6):2147–2152. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Roberts D. D., Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogfelt K. A. Bacterial adhesion: genetics, biogenesis, and role in pathogenesis of fimbrial adhesins of Escherichia coli. Rev Infect Dis. 1991 Jul-Aug;13(4):721–735. doi: 10.1093/clinids/13.4.721. [DOI] [PubMed] [Google Scholar]
- Kuehn M., Lent K., Haas J., Hagenzieker J., Cervin M., Smith A. L. Fimbriation of Pseudomonas cepacia. Infect Immun. 1992 May;60(5):2002–2007. doi: 10.1128/iai.60.5.2002-2007.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler H., Svanborg-Eden C. Host epithelial glycoconjugates and pathogenic bacteria. Am J Respir Cell Mol Biol. 1990 May;2(5):409–411. doi: 10.1165/ajrcmb/2.5.409. [DOI] [PubMed] [Google Scholar]
- Lewin L. O., Byard P. J., Davis P. B. Effect of Pseudomonas cepacia colonization on survival and pulmonary function of cystic fibrosis patients. J Clin Epidemiol. 1990;43(2):125–131. doi: 10.1016/0895-4356(90)90175-o. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992 Jan;174(1):1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Mantle M., Fahim R. E., Specian R. D., Bennick A., Kawagishi S., Sherman P., Forstner J. F. The putative 'link' glycopeptide associated with mucus glycoproteins. Composition and properties of preparations from the gastrointestinal tracts of several mammals. Biochem J. 1989 Jul 15;261(2):637–647. doi: 10.1042/bj2610637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiman L., Cacalano G., Prince A. Pseudomonas cepacia adherence to respiratory epithelial cells is enhanced by Pseudomonas aeruginosa. Infect Immun. 1990 Aug;58(8):2578–2584. doi: 10.1128/iai.58.8.2578-2584.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan S. U., Forstner J. F. Identification of the mucin-binding adhesin of Pseudomonas cepacia isolated from patients with cystic fibrosis. Infect Immun. 1992 Apr;60(4):1434–1440. doi: 10.1128/iai.60.4.1434-1440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan U. S., Corey M., Karmali M. A., Forstner J. F. Binding of Pseudomonas cepacia to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Invest. 1992 Feb;89(2):648–656. doi: 10.1172/JCI115631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick M. J., Madara J. L., Fields B. N., Normark S. J. Molecular cross talk between epithelial cells and pathogenic microorganisms. Cell. 1991 Nov 15;67(4):651–659. doi: 10.1016/0092-8674(91)90061-3. [DOI] [PubMed] [Google Scholar]





