Abstract
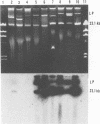
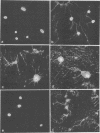
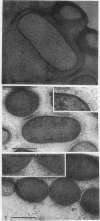
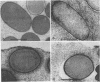
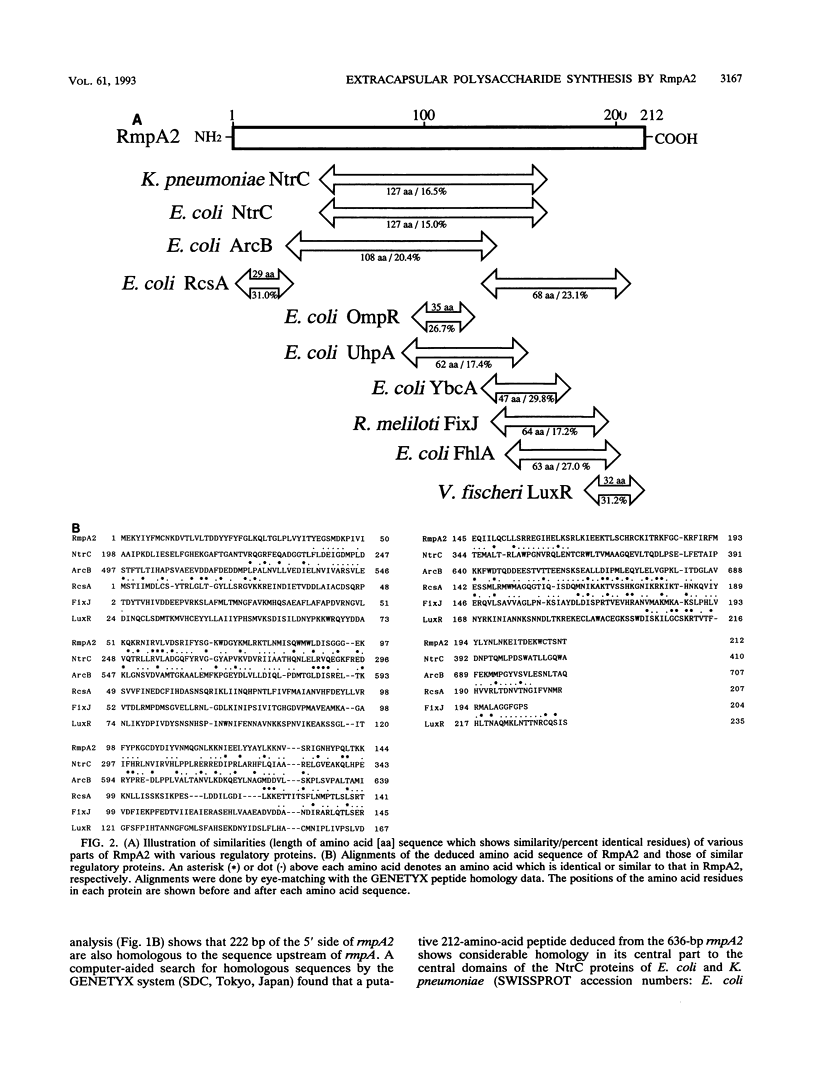
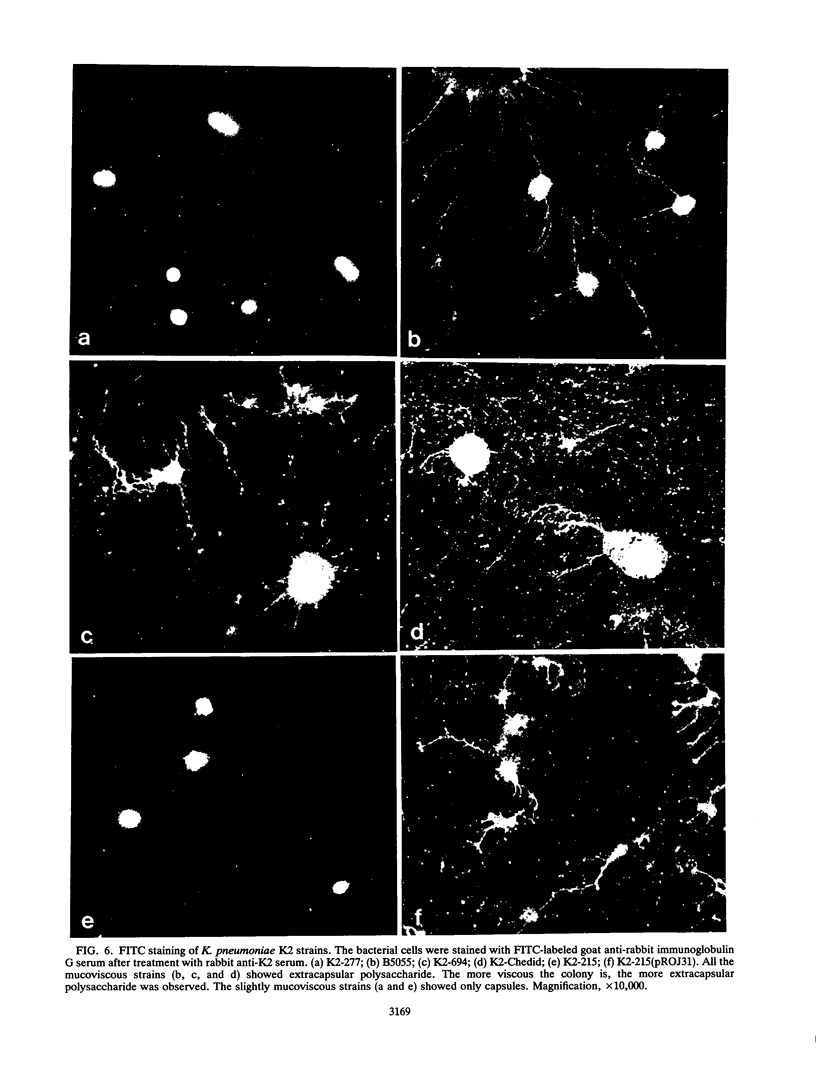
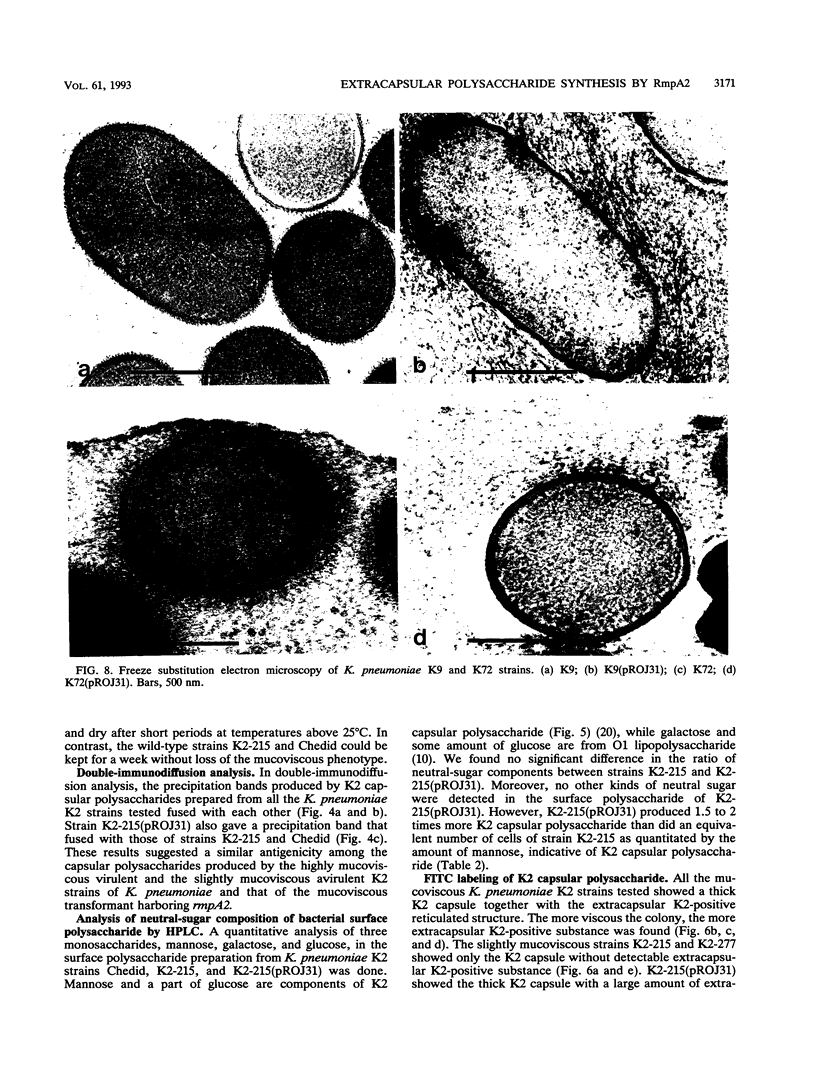
We determined the complete nucleotide sequence of a 2.1-kb HindIII-EcoRI fragment that was cloned from a resident large plasmid of Klebsiella pneumoniae Chedid, a highly virulent and mucoviscous strain of the O1:K2 serotype. This fragment encoded an ability to enhance K2 capsular polysaccharide synthesis in K. pneumoniae, and a 636-bp open reading frame (rmpA2) was found. The 411-bp rmpA reported to be involved in the virulence and mucoid phenotypes of K. pneumoniae by Nassif et al. (Mol. Microbiol. 3:1349-1359, 1989) was a part of rmpA2. Eighty percent homology in nucleotide sequence was found between rmpA2 and rmpA in the corresponding regions. The central domain of the deduced amino acid sequence of RmpA2 showed considerable homology to the central domains of NtrC of K. pneumoniae and Escherichia coli, to which the sigma factor of RNA polymerase binds. The C-terminal domain of RmpA2 also demonstrated considerable homology with the putative helix-turn-helix motifs of LuxR of Vibrio fischeri and FixJ of Rhizobium meliloti. Moreover, RmpA2 also showed some homology in its N- and C-terminal regions to those of RcsA, a transcriptional activator for colanic acid synthesis in E. coli. On the other hand, a sequence upstream of rmpA2 was found to be highly homologous to insertion sequence 3 of members of the family Enterobacteriaceae. Southern hybridization analysis suggested that rmpA2 exists on the large plasmids of all mucoviscous virulent K2 strains but not on those of the slightly mucoviscous avirulent strains. Freeze substitution electron microscopy and fluorescent-antibody staining with anti-K2 serum revealed that K. pneumoniae Chedid has a dense and thick capsule (180 nm) with dense extracapsular substance, whereas K. pneumoniae K2-215, one of the slightly mucoviscous and avirulent strains, has a capsule which is looser and thinner (120 nm) than that of strain Chedid and no extracapsular substance. Introduction of rmpA2 into K2-215 as well as reference strains K. pneumoniae K9 and K72 resulted in a change of the colony phenotype to highly mucoviscous through abundant production of extracapsular substance which reacted with anti-K2, -K9, or -K72, respectively, as did their parental strains. From these results, it is suggested that RmpA2 belongs to the family of transcriptional regulators and confers a highly mucoviscous phenotype on cells of various serotypes of K. pneumoniae by enhancing extracapsular polysaccharide synthesis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa Y., Ohta M., Kido N., Mori M., Ito H., Komatsu T., Fujii Y., Kato N. Chromosomal beta-lactamase of Klebsiella oxytoca, a new class A enzyme that hydrolyzes broad-spectrum beta-lactam antibiotics. Antimicrob Agents Chemother. 1989 Jan;33(1):63–70. doi: 10.1128/aac.33.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa Y., Ohta M., Wacharotayankun R., Mori M., Kido N., Ito H., Komatsu T., Sugiyama T., Kato N. Biosynthesis of Klebsiella K2 capsular polysaccharide in Escherichia coli HB101 requires the functions of rmpA and the chromosomal cps gene cluster of the virulent strain Klebsiella pneumoniae Chedid (O1:K2). Infect Immun. 1991 Jun;59(6):2043–2050. doi: 10.1128/iai.59.6.2043-2050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATSHON B. A., BAER H., SHAFFER M. F. Immunologic paralysis produced in mice by Klebsiella pneumoniae type 2 polysaccharide. J Immunol. 1963 Jan;90:121–126. [PubMed] [Google Scholar]
- Beveridge T. J., Graham L. L. Surface layers of bacteria. Microbiol Rev. 1991 Dec;55(4):684–705. doi: 10.1128/mr.55.4.684-705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Mortimer P. M., Mansfield V., Germanier R. Seroepidemiology of Klebsiella bacteremic isolates and implications for vaccine development. J Clin Microbiol. 1986 Apr;23(4):687–690. doi: 10.1128/jcm.23.4.687-690.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenico P., Diedrich D. L., Straus D. C. Extracellular polysaccharide production by Klebsiella pneumoniae and its relationship to virulence. Can J Microbiol. 1985 May;31(5):472–478. doi: 10.1139/m85-088. [DOI] [PubMed] [Google Scholar]
- Edmondson A. S., Cooke E. M. The production of antisera to the Klebsiella capsular antigens. J Appl Bacteriol. 1979 Jun;46(3):579–584. doi: 10.1111/j.1365-2672.1979.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiseleva B. S., Krasnogolovets V. N. Rol' Klebsiella pneumoniae v étiologii bakterial'nogo sepsisa. Zh Mikrobiol Epidemiol Immunobiol. 1983 Feb;(2):20–25. [PubMed] [Google Scholar]
- Lawrence J. G., Ochman H., Hartl D. L. The evolution of insertion sequences within enteric bacteria. Genetics. 1992 May;131(1):9–20. doi: 10.1093/genetics/131.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum K. L., Whitfield C. The rcsA gene of Klebsiella pneumoniae O1:K20 is involved in expression of the serotype-specific K (capsular) antigen. Infect Immun. 1991 Feb;59(2):494–502. doi: 10.1128/iai.59.2.494-502.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meno Y., Amako K. Morphological evidence for penetration of anti-O antibody through the capsule of Klebsiella pneumoniae. Infect Immun. 1990 May;58(5):1421–1428. doi: 10.1128/iai.58.5.1421-1428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta K., Ohta M., Mori M., Hasegawa T., Nakashima I., Kato N. Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect Immun. 1983 Apr;40(1):56–61. doi: 10.1128/iai.40.1.56-61.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Ohta M., Agata N., Kido N., Arakawa Y., Ito H., Komatsu T., Kato N. Identification of species and capsular types of Klebsiella clinical isolates, with special reference to Klebsiella planticola. Microbiol Immunol. 1989;33(11):887–895. doi: 10.1111/j.1348-0421.1989.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Nassif X., Honoré N., Vasselon T., Cole S. T., Sansonetti P. J. Positive control of colanic acid synthesis in Escherichia coli by rmpA and rmpB, two virulence-plasmid genes of Klebsiella pneumoniae. Mol Microbiol. 1989 Oct;3(10):1349–1359. doi: 10.1111/j.1365-2958.1989.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoons-Smit A. M., Verweij-van Vught A. M., MacLaren D. M. The role of K antigens as virulence factors in Klebsiella. J Med Microbiol. 1986 Mar;21(2):133–137. doi: 10.1099/00222615-21-2-133. [DOI] [PubMed] [Google Scholar]
- Springer E. L., Roth I. L. Ultrastructure of the capsule of Klebsiella pneumoniae and slime of Enterobacter aerogenes revealed by freeze etching. Arch Mikrobiol. 1973 Nov 19;93(4):277–286. doi: 10.1007/BF00427925. [DOI] [PubMed] [Google Scholar]
- Stout V., Torres-Cabassa A., Maurizi M. R., Gutnick D., Gottesman S. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J Bacteriol. 1991 Mar;173(5):1738–1747. doi: 10.1128/jb.173.5.1738-1747.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Kido N., Arakawa Y., Mori M., Naito S., Ohta M., Kato N. Rapid small-scale preparation method of cell surface polysaccharides. Microbiol Immunol. 1990;34(7):635–641. doi: 10.1111/j.1348-0421.1990.tb01039.x. [DOI] [PubMed] [Google Scholar]
- WILKINSON J. F., DUDMAN W. F., ASPINALL G. O. The extracellular polysaccharide of Aerobacter aerogenes A3 (S1) (Klebsiella type 54). Biochem J. 1955 Mar;59(3):446–451. doi: 10.1042/bj0590446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacharotayankun R., Arakawa Y., Ohta M., Hasegawa T., Mori M., Horii T., Kato N. Involvement of rcsB in Klebsiella K2 capsule synthesis in Escherichia coli K-12. J Bacteriol. 1992 Feb;174(3):1063–1067. doi: 10.1128/jb.174.3.1063-1067.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C. Bacterial extracellular polysaccharides. Can J Microbiol. 1988 Apr;34(4):415–420. doi: 10.1139/m88-073. [DOI] [PubMed] [Google Scholar]
- Williams P., Lambert P. A., Haigh C. G., Brown M. R. The influence of the O and K antigens of Klebsiella aerogenes on surface hydrophobicity and susceptibility to phagocytosis and antimicrobial agents. J Med Microbiol. 1986 Mar;21(2):125–132. doi: 10.1099/00222615-21-2-125. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]