Abstract
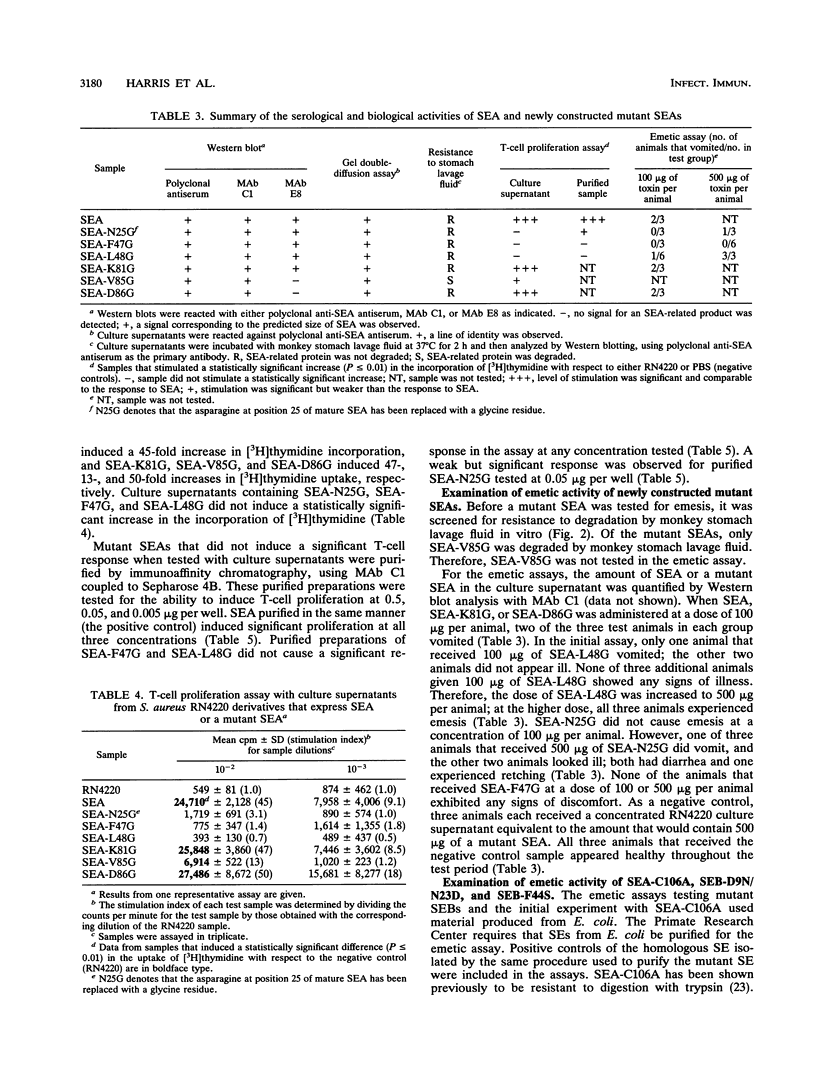
This study examined the emetic activity of several staphylococcal enterotoxin type A and B (SEA and SEB, respectively) mutants that had either one or two amino acid residue substitutions. New sea gene mutations were constructed by site-directed mutagenesis; gene products were obtained with glycine residues at position 25, 47, 48, 81, 85, or 86 of mature SEA. Culture supernatants from Staphylococcus aureus RN4220, or derivatives containing either sea or a sea mutation, were analyzed for the ability to stimulate proliferation of murine splenocytes, as determined by incorporation of [3H]thymidine. Culture supernatants containing SEA-N25G (a SEA mutant with a substitution of glycine for the asparagine residue at position 25), SEA-F47G, or SEA-L48G did not stimulate T-cell proliferation, unlike supernatants containing the other substitution mutants. Purified preparations of SEA-N25G had weak activity and those of SEA-F47G and SEA-L48G had essentially no activity in the T-cell proliferation assay. All mutants except SEA-V85G, which was degraded by monkey stomach lavage fluid in vitro, were tested for emetic activity. SEA-C106A and two SEB mutants, SEB-D9N/N23D and SEB-F44S (previously referred to as BR-257 and BR-358, respectively), whose construction and altered immunological properties have been reported previously, were also tested in the emetic assay. Each mutant was initially administered intragastrically at doses of 75 to 100 micrograms per animal; if none of the animals responded, the dose was increased four-to fivefold. SEA-F47G, SEA-C106A, and SEB-D9N/N23D were the only mutants that did not induce vomiting at either dose tested; these three mutants had reduced immunological activity. However, there was not a perfect correlation between immunological and emetic activities; SEA-L48G and SEB-F44S retained emetic activity, although they had essentially no T-cell-stimulatory activity. These studies suggest that these two activities can be dissociated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber G., Hammer D. K., Fleischer B. Relationship between enterotoxic- and T lymphocyte-stimulating activity of staphylococcal enterotoxin B. J Immunol. 1990 Jun 15;144(12):4501–4506. [PubMed] [Google Scholar]
- Bayles K. W., Iandolo J. J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J Bacteriol. 1989 Sep;171(9):4799–4806. doi: 10.1128/jb.171.9.4799-4806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley M. J., Borst D. W., Regassa L. B. Staphylococcal enterotoxins, toxic shock syndrome toxin and streptococcal pyrogenic exotoxins: a comparative study of their molecular biology. Chem Immunol. 1992;55:1–35. [PubMed] [Google Scholar]
- Betley M. J., Löfdahl S., Kreiswirth B. N., Bergdoll M. S., Novick R. P. Staphylococcal enterotoxin A gene is associated with a variable genetic element. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5179–5183. doi: 10.1073/pnas.81.16.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley M. J., Mekalanos J. J. Nucleotide sequence of the type A staphylococcal enterotoxin gene. J Bacteriol. 1988 Jan;170(1):34–41. doi: 10.1128/jb.170.1.34-41.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Handley J. P., Schlievert P. M. Biological and immunological properties of the carboxyl terminus of staphylococcal enterotoxin C1. Infect Immun. 1989 Jan;57(1):23–28. doi: 10.1128/iai.57.1.23-28.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Schlievert P. M. Conservation of the biologically active portions of staphylococcal enterotoxins C1 and C2. Infect Immun. 1989 Jul;57(7):2249–2252. doi: 10.1128/iai.57.7.2249-2252.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Schlievert P. M. Nucleotide sequence of the staphylococcal enterotoxin C1 gene and relatedness to other pyrogenic toxins. Mol Gen Genet. 1987 Aug;209(1):15–20. doi: 10.1007/BF00329830. [DOI] [PubMed] [Google Scholar]
- Brunson K. W., Watson D. W. Pyrogenic specificity of streptococcal exotoxins, staphylococcal enterotoxin, and gram-negative endotoxin. Infect Immun. 1974 Aug;10(2):347–351. doi: 10.1128/iai.10.2.347-351.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelow R., O'Hehir R. E., Schreifels R., Kummerehl T. J., Riley G., Lamb J. R. Localization of the immunologic activity in the superantigen Staphylococcal enterotoxin B using truncated recombinant fusion proteins. J Immunol. 1992 Jan 1;148(1):1–6. [PubMed] [Google Scholar]
- CLARK W. G., BORISON H. L. PYROGENIC EFFECT OF PURIFIED STAPHYLOCOCCAL ENTEROTOXIN. J Pharmacol Exp Ther. 1963 Nov;142:237–241. [PubMed] [Google Scholar]
- Carlsson R., Sjögren H. O. Kinetics of IL-2 and interferon-gamma production, expression of IL-2 receptors, and cell proliferation in human mononuclear cells exposed to staphylococcal enterotoxin A. Cell Immunol. 1985 Nov;96(1):175–183. doi: 10.1016/0008-8749(85)90349-1. [DOI] [PubMed] [Google Scholar]
- Couch J. L., Betley M. J. Nucleotide sequence of the type C3 staphylococcal enterotoxin gene suggests that intergenic recombination causes antigenic variation. J Bacteriol. 1989 Aug;171(8):4507–4510. doi: 10.1128/jb.171.8.4507-4510.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch J. L., Soltis M. T., Betley M. J. Cloning and nucleotide sequence of the type E staphylococcal enterotoxin gene. J Bacteriol. 1988 Jul;170(7):2954–2960. doi: 10.1128/jb.170.7.2954-2960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast D. J., Schlievert P. M., Nelson R. D. Toxic shock syndrome-associated staphylococcal and streptococcal pyrogenic toxins are potent inducers of tumor necrosis factor production. Infect Immun. 1989 Jan;57(1):291–294. doi: 10.1128/iai.57.1.291-294.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs N. D., Pontzer C. H., Jarpe M. A., Johnson H. M. Mapping of multiple binding domains of the superantigen staphylococcal enterotoxin A for HLA. J Immunol. 1992 Apr 15;148(8):2516–2521. [PubMed] [Google Scholar]
- Grossman D., Cook R. G., Sparrow J. T., Mollick J. A., Rich R. R. Dissociation of the stimulatory activities of staphylococcal enterotoxins for T cells and monocytes. J Exp Med. 1990 Dec 1;172(6):1831–1841. doi: 10.1084/jem.172.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman D., Lamphear J. G., Mollick J. A., Betley M. J., Rich R. R. Dual roles for class II major histocompatibility complex molecules in staphylococcal enterotoxin-induced cytokine production and in vivo toxicity. Infect Immun. 1992 Dec;60(12):5190–5196. doi: 10.1128/iai.60.12.5190-5196.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman D., Van M., Mollick J. A., Highlander S. K., Rich R. R. Mutation of the disulfide loop in staphylococcal enterotoxin A. Consequences for T cell recognition. J Immunol. 1991 Nov 15;147(10):3274–3281. [PubMed] [Google Scholar]
- Harris T. O., Hufnagle W. O., Betley M. J. Staphylococcal enterotoxin type A internal deletion mutants: serological activity and induction of T-cell proliferation. Infect Immun. 1993 May;61(5):2059–2068. doi: 10.1128/iai.61.5.2059-2068.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A., Kappler J. W., Marrack P., Pullen A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- Hovde C. J., Hackett S. P., Bohach G. A. Nucleotide sequence of the staphylococcal enterotoxin C3 gene: sequence comparison of all three type C staphylococcal enterotoxins. Mol Gen Genet. 1990 Jan;220(2):329–333. doi: 10.1007/BF00260504. [DOI] [PubMed] [Google Scholar]
- Hufnagle W. O., Tremaine M. T., Betley M. J. The carboxyl-terminal region of staphylococcal enterotoxin type A is required for a fully active molecule. Infect Immun. 1991 Jun;59(6):2126–2134. doi: 10.1128/iai.59.6.2126-2134.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordănescu S. Recombinant plasmid obtained from two different, compatible staphylococcal plasmids. J Bacteriol. 1975 Nov;124(2):597–601. doi: 10.1128/jb.124.2.597-601.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M. J., Hudson K. R., Fraser J. D., Gascoigne N. R. Enterotoxin residues determining T-cell receptor V beta binding specificity. Nature. 1992 Oct 29;359(6398):841–843. doi: 10.1038/359841a0. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Magazine H. I. Potent mitogenic activity of staphylococcal enterotoxin A requires induction of interleukin 2. Int Arch Allergy Appl Immunol. 1988;87(1):87–90. doi: 10.1159/000234654. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Russell J. K., Pontzer C. H. Staphylococcal enterotoxin microbial superantigens. FASEB J. 1991 Sep;5(12):2706–2712. doi: 10.1096/fasebj.5.12.1916093. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Stanton G. J., Baron S. Relative ability of mitogens to stimulate production of interferon by lymphoid cells and to induce suppression of the in vitro immune response. Proc Soc Exp Biol Med. 1977 Jan;154(1):138–141. [PubMed] [Google Scholar]
- Jones C. L., Khan S. A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986 Apr;166(1):29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Herman A., Clements J., Marrack P. Mutations defining functional regions of the superantigen staphylococcal enterotoxin B. J Exp Med. 1992 Feb 1;175(2):387–396. doi: 10.1084/jem.175.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Langford M. P., Stanton G. J., Johnson H. M. Biological effects of staphylococcal enterotoxin A on human peripheral lymphocytes. Infect Immun. 1978 Oct;22(1):62–68. doi: 10.1128/iai.22.1.62-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Misfeldt M. L. Microbial "superantigens". Infect Immun. 1990 Aug;58(8):2409–2413. doi: 10.1128/iai.58.8.2409-2413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Gillis Z. A., Pier G. B. Induction of interleukin-1 by strains of Staphylococcus aureus from patients with nonmenstrual toxic shock syndrome. J Infect Dis. 1986 Jul;154(1):55–63. doi: 10.1093/infdis/154.1.55. [DOI] [PubMed] [Google Scholar]
- Patel A. H., Foster T. J., Pattee P. A. Physical and genetic mapping of the protein A gene in the chromosome of Staphylococcus aureus 8325-4. J Gen Microbiol. 1989 Jul;135(7):1799–1807. doi: 10.1099/00221287-135-7-1799. [DOI] [PubMed] [Google Scholar]
- Pontzer C. H., Russell J. K., Johnson H. M. Localization of an immune functional site on staphylococcal enterotoxin A using the synthetic peptide approach. J Immunol. 1989 Jul 1;143(1):280–284. [PubMed] [Google Scholar]
- Ranelli D. M., Jones C. L., Johns M. B., Mussey G. J., Khan S. A. Molecular cloning of staphylococcal enterotoxin B gene in Escherichia coli and Staphylococcus aureus. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5850–5854. doi: 10.1073/pnas.82.17.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck B., Scheuber P. H., Londong W., Sailer-Kramer B., Bartsch K., Hammer D. K. Protection against the staphylococcal enterotoxin-induced intestinal disorder in the monkey by anti-idiotypic antibodies. Proc Natl Acad Sci U S A. 1988 May;85(9):3170–3174. doi: 10.1073/pnas.85.9.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins R., Gould S., Bergdoll M. Detecting the enterotoxigenicity of Staphylococcus aureus strains. Appl Microbiol. 1974 Dec;28(6):946–950. doi: 10.1128/am.28.6.946-950.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGIYAMA H., MCKISSIC E. M., Jr, BERGDOLL M. S., HELLER B. ENHANCEMENT OF BACTERIAL ENDOTOXIN LETHALITY BY STAPHYLOCOCCAL ENTEROTOXIN. J Infect Dis. 1964 Apr;114:111–118. doi: 10.1093/infdis/114.2.111. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz E. J., Roessler W. G., Woodburn M. J., Lynch J. M., Jacoby H. M., Silverman S. J., Gorman J. C., Spero L. Purification and some chemical and physical properties of staphylococcal enterotoxin A. Biochemistry. 1972 Feb 1;11(3):360–366. doi: 10.1021/bi00753a009. [DOI] [PubMed] [Google Scholar]
- Scheuber P. H., Golecki J. R., Kickhöfen B., Scheel D., Beck G., Hammer D. K. Skin reactivity of unsensitized monkeys upon challenge with staphylococcal enterotoxin B: a new approach for investigating the site of toxin action. Infect Immun. 1985 Dec;50(3):869–876. doi: 10.1128/iai.50.3.869-876.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B. R., Betley M. J. Comparative structural analysis of staphylococcal enterotoxins A and E. J Biol Chem. 1989 Mar 15;264(8):4404–4411. [PubMed] [Google Scholar]
- Smith B. G., Johnson H. M. The effect of staphylococcal enterotoxins on the primary in vitro immune response. J Immunol. 1975 Aug;115(2):575–578. [PubMed] [Google Scholar]
- Spero L., Morlock B. A. Biological activities of the peptides of staphylococcal enterotoxin C formed by limited tryptic hydrolysis. J Biol Chem. 1978 Dec 25;253(24):8787–8791. [PubMed] [Google Scholar]
- Stelma G. N., Jr, Bergdoll M. S. Inactivation of staphylococcal enterotoxin A by chemical modification. Biochem Biophys Res Commun. 1982 Mar 15;105(1):121–126. doi: 10.1016/s0006-291x(82)80019-3. [DOI] [PubMed] [Google Scholar]
- Swaminathan S., Furey W., Pletcher J., Sax M. Crystal structure of staphylococcal enterotoxin B, a superantigen. Nature. 1992 Oct 29;359(6398):801–806. doi: 10.1038/359801a0. [DOI] [PubMed] [Google Scholar]