Abstract
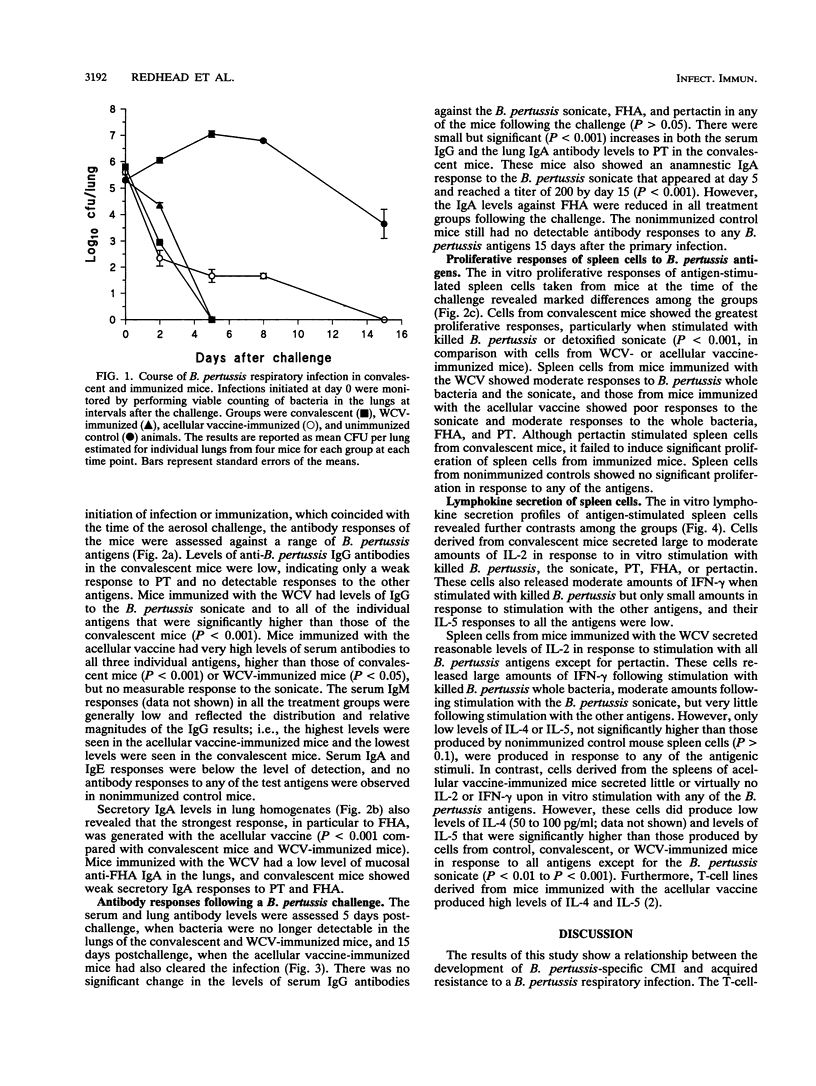
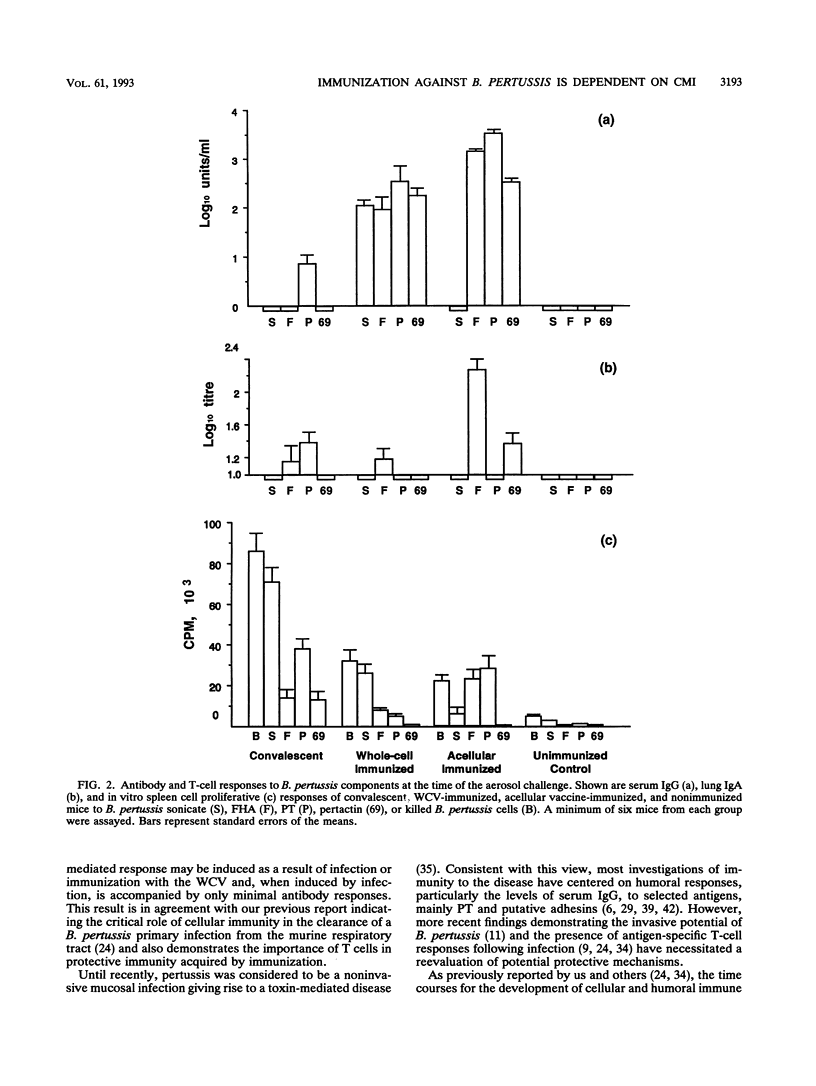
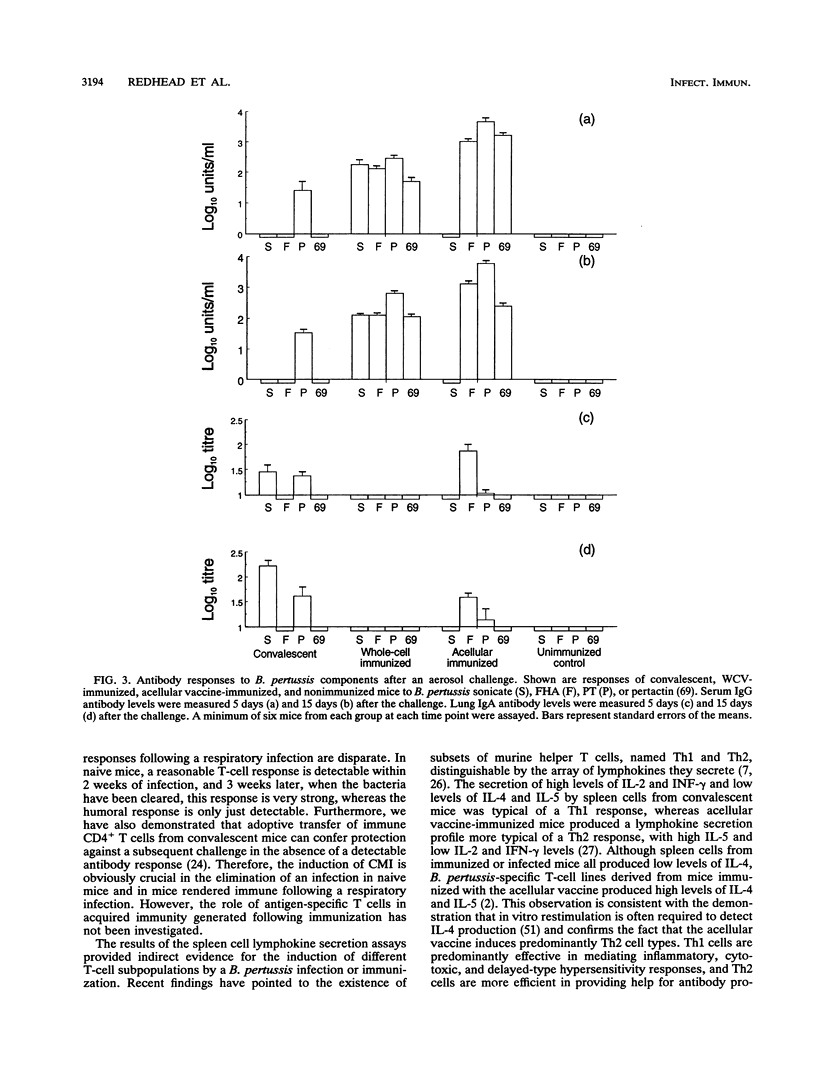
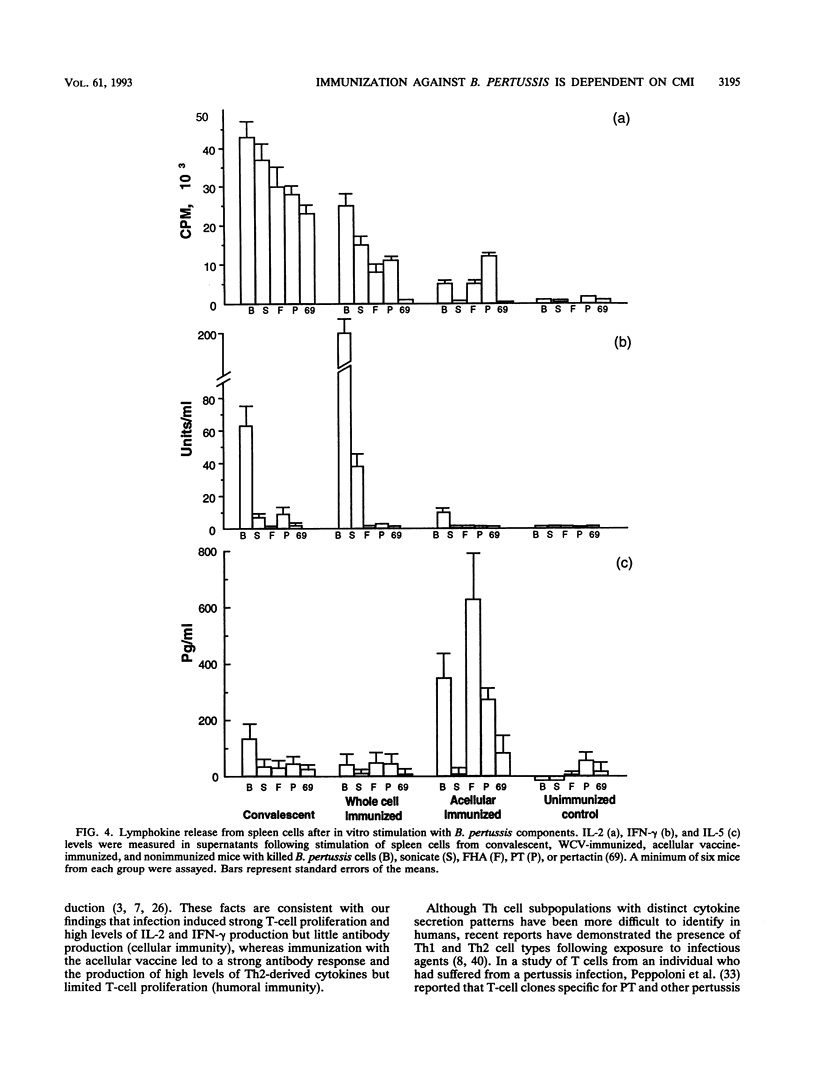
A murine respiratory challenge model was used to examine the induction of cellular and humoral immune responses and their role in protection against Bordetella pertussis following immunization or previous infection. Spleen cells from mice convalescing from a B. pertussis infection exhibited extensive in vitro T-cell proliferation and secreted high levels of interleukin-2 (IL-2) and gamma interferon but not IL-4 or IL-5, a cytokine profile typical of CD4+ Th1 cells. Serum from these mice had low or undetectable anti-B. pertussis antibody levels. In contrast, mice immunized with an acellular pertussis vaccine had high levels of B. pertussis antibodies and spleen cells secreting IL-5 but not gamma interferon, a profile characteristic of CD4+ Th2 cells. Immunization with an inactivated whole-cell vaccine induced both CD4+ Th1 and serum antibody responses. After exposure to a B. pertussis respiratory challenge, the convalescent mice and those immunized with the whole-cell vaccine eliminated the bacterial infection significantly faster than mice immunized with the acellular vaccine. These findings show that the selection of antigens and their form of presentation are important in determining whether the subsequent immune response is cellular, mediated by Th1 cells, or humoral, mediated by Th2 cells. In the murine model, the induction of a Th1-mediated cellular immune response appears to be a key element in acquired immunity to a B. pertussis infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Bromberg K., Tannis G., Steiner P. Detection of Bordetella pertussis associated with the alveolar macrophages of children with human immunodeficiency virus infection. Infect Immun. 1991 Dec;59(12):4715–4719. doi: 10.1128/iai.59.12.4715-4719.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles I. G., Li J. L., Roberts M., Beesley K., Romanos M., Pickard D. J., Francis M., Campbell D., Dougan G., Brennan M. J. Identification and characterization of a protective immunodominant B cell epitope of pertactin (P.69) from Bordetella pertussis. Eur J Immunol. 1991 May;21(5):1147–1153. doi: 10.1002/eji.1830210509. [DOI] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLBY J. M., THOW D. C., STANDFAST A. F. The intranasal infection of mice with Bordetella pertussis. J Hyg (Lond) 1961 Jun;59:191–204. doi: 10.1017/s0022172400038857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Magistris M. T., Romano M., Nuti S., Rappuoli R., Tagliabue A. Dissecting human T cell responses against Bordetella species. J Exp Med. 1988 Oct 1;168(4):1351–1362. doi: 10.1084/jem.168.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Mastromauro C., Biagiotti R., Macchia D., Falagiani P., Ricci M., Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991 Jul;88(1):346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewanowich C. A., Melton A. R., Weiss A. A., Sherburne R. K., Peppler M. S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989 Sep;57(9):2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Nordensson K., Wilson L., Akporiaye E. T., Yocum D. E. Uptake and intracellular survival of Bordetella pertussis in human macrophages. Infect Immun. 1992 Nov;60(11):4578–4585. doi: 10.1128/iai.60.11.4578-4585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Galazka A. Control of pertussis in the world. World Health Stat Q. 1992;45(2-3):238–247. [PubMed] [Google Scholar]
- Gearing A. J., Bird C. R., Redhead K., Thomas M. Human cellular immune responses to Bordetella pertussis infection. FEMS Microbiol Immunol. 1989 Mar;1(4):205–211. doi: 10.1111/j.1574-6968.1989.tb02384.x. [DOI] [PubMed] [Google Scholar]
- Halperin S. A., Issekutz T. B., Kasina A. Modulation of Bordetella pertussis infection with monoclonal antibodies to pertussis toxin. J Infect Dis. 1991 Feb;163(2):355–361. doi: 10.1093/infdis/163.2.355. [DOI] [PubMed] [Google Scholar]
- Katsura Y. Cell-mediated and humoral immune responses in mice. III. Dynamic balance between delayed-type hypersensitivity and antibody response. Immunology. 1977 Mar;32(3):227–235. [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kimura A., Mountzouros K. T., Relman D. A., Falkow S., Cowell J. L. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect Immun. 1990 Jan;58(1):7–16. doi: 10.1128/iai.58.1.7-16.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaratilake L. M., Ferrante A., Rzepczyk C. The role of T lymphocytes in immunity to Plasmodium falciparum. Enhancement of neutrophil-mediated parasite killing by lymphotoxin and IFN-gamma: comparisons with tumor necrosis factor effects. J Immunol. 1991 Jan 15;146(2):762–767. [PubMed] [Google Scholar]
- Melville-Smith M. E., Seagroatt V. A., Watkins J. T. A comparison of enzyme-linked immunosorbent assay (ELISA) with the toxin neutralization test in mice as a method for the estimation of tetanus antitoxin in human sera. J Biol Stand. 1983 Apr;11(2):137–144. doi: 10.1016/s0092-1157(83)80038-9. [DOI] [PubMed] [Google Scholar]
- Mills K. H., Barnard A., Watkins J., Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993 Feb;61(2):399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. W., Vieira P., Fiorentino D. F., Trounstine M. L., Khan T. A., Mosmann T. R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990 Jun 8;248(4960):1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Mustafa A. S., Godal T. BCG induced CD4+ cytotoxic T cells from BCG vaccinated healthy subjects: relation between cytotoxicity and suppression in vitro. Clin Exp Immunol. 1987 Aug;69(2):255–262. [PMC free article] [PubMed] [Google Scholar]
- Oda M., Izumiya K., Sato Y., Hirayama M. Transplacental and transcolostral immunity to pertussis in a mouse model using acellular pertussis vaccine. J Infect Dis. 1983 Jul;148(1):138–145. doi: 10.1093/infdis/148.1.138. [DOI] [PubMed] [Google Scholar]
- Olander R. M., Muotiala A., Karvonen M., Kuronen T., Runeberg-Nyman K. Serum antibody response to B. pertussis Tn5 mutants, purified PT and FHA in two different mouse strains and passive protection in the murine intranasal infection model. Microb Pathog. 1990 Jan;8(1):37–45. doi: 10.1016/0882-4010(90)90006-c. [DOI] [PubMed] [Google Scholar]
- Oswald I. P., Gazzinelli R. T., Sher A., James S. L. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992 Jun 1;148(11):3578–3582. [PubMed] [Google Scholar]
- Parish C. R. The relationship between humoral and cell-mediated immunity. Transplant Rev. 1972;13:35–66. doi: 10.1111/j.1600-065x.1972.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Peppoloni S., Nencioni L., Di Tommaso A., Tagliabue A., Parronchi P., Romagnani S., Rappuoli R., De Magistris M. T. Lymphokine secretion and cytotoxic activity of human CD4+ T-cell clones against Bordetella pertussis. Infect Immun. 1991 Oct;59(10):3768–3773. doi: 10.1128/iai.59.10.3768-3773.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J. W., Ibsen P. H., Hasløv K., Heron I. Proliferative responses and gamma interferon and tumor necrosis factor production by lymphocytes isolated from tracheobroncheal lymph nodes and spleen of mice aerosol infected with Bordetella pertussis. Infect Immun. 1992 Nov;60(11):4563–4570. doi: 10.1128/iai.60.11.4563-4570.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M., Furman B. L., Wardlaw A. C. Bordetella pertussis respiratory tract infection in the mouse: pathophysiological responses. J Infect Dis. 1980 Jul;142(1):56–66. doi: 10.1093/infdis/142.1.56. [DOI] [PubMed] [Google Scholar]
- Pittman M. Pertussis toxin: the cause of the harmful effects and prolonged immunity of whooping cough. A hypothesis. Rev Infect Dis. 1979 May-Jun;1(3):401–412. doi: 10.1093/clinids/1.3.401. [DOI] [PubMed] [Google Scholar]
- Redhead K., Das R. E. A collaborative assay of the proposed third British Reference Preparation for Pertussis Vaccine and of the relative potencies of the second International Standard and the second British Reference Preparation for Pertussis Vaccine. Biologicals. 1991 Apr;19(2):107–111. doi: 10.1016/1045-1056(91)90008-8. [DOI] [PubMed] [Google Scholar]
- Robinson A., Ashworth L. A., Baskerville A., Irons L. I. Protection against intranasal infection of mice with Bordetella pertussis. Dev Biol Stand. 1985;61:165–172. [PubMed] [Google Scholar]
- Robinson A., Irons L. I., Ashworth L. A. Pertussis vaccine: present status and future prospects. Vaccine. 1985 Mar;3(1):11–22. doi: 10.1016/0264-410x(85)90004-0. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991 Aug;12(8):256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- Ruuskanen O., Noel A., Putto-Laurila A., Pêtre J., Capiau C., Delem A., Vandevoorde D., Simoen E., Teuwen D. E., Bogaerts H. Development of an acellular pertussis vaccine and its administration as a booster in healthy adults. Vaccine. 1991 Feb;9(2):117–121. doi: 10.1016/0264-410x(91)90267-a. [DOI] [PubMed] [Google Scholar]
- Sato H., Sato Y. Bordetella pertussis infection in mice: correlation of specific antibodies against two antigens, pertussis toxin, and filamentous hemagglutinin with mouse protectivity in an intracerebral or aerosol challenge system. Infect Immun. 1984 Nov;46(2):415–421. doi: 10.1128/iai.46.2.415-421.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Aerosol infection of mice with Bordetella pertussis. Infect Immun. 1980 Jul;29(1):261–266. doi: 10.1128/iai.29.1.261-266.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saukkonen K., Cabellos C., Burroughs M., Prasad S., Tuomanen E. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J Exp Med. 1991 May 1;173(5):1143–1149. doi: 10.1084/jem.173.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J. H., O'Garra A., Shrader B., van Kimmenade A., Bond M. W., Mosmann T. R., Coffman R. L. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. J Immunol. 1988 Sep 1;141(5):1576–1581. [PubMed] [Google Scholar]
- Scott P., Pearce E., Cheever A. W., Coffman R. L., Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989 Dec;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Sher A., Fiorentino D., Caspar P., Pearce E., Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991 Oct 15;147(8):2713–2716. [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Swain S. L., McKenzie D. T., Weinberg A. D., Hancock W. Characterization of T helper 1 and 2 cell subsets in normal mice. Helper T cells responsible for IL-4 and IL-5 production are present as precursors that require priming before they develop into lymphokine-secreting cells. J Immunol. 1988 Nov 15;141(10):3445–3455. [PubMed] [Google Scholar]
- Taylor P. M., Meager A., Askonas B. A. Influenza virus-specific T cells lead to early interferon gamma in lungs of infected hosts: development of a sensitive radioimmunoassay. J Gen Virol. 1989 Apr;70(Pt 4):975–978. doi: 10.1099/0022-1317-70-4-975. [DOI] [PubMed] [Google Scholar]



