Abstract
Tuberous sclerosis is an autosomal dominant disorder characterized by the development of aberrant growths in many tissues and organs. Linkage analysis revealed two disease-determining genes on chromosome 9 and chromosome 16. The tuberous sclerosis complex gene-2 (TSC2) on chromosome 16 encodes the tumor suppressor protein tuberin. We have shown earlier that loss of TSC2 is sufficient to induce quiescent cells to enter the cell cycle. Here we show that TSC2-negative fibroblasts exhibit a shortened G1 phase. Although the expression of cyclin E, cyclin A, p21, or Cdc25A is unaffected, TSC2-negative cells express much lower amounts of the cyclin-dependent kinase (CDK) inhibitor p27 because of decreased protein stability. In TSC2 mutant cells the amount of p27 bound to CDK2 is diminished, accompanied with elevated kinase activity. Ectopic expression studies revealed that the aforementioned effects can be reverted by transfecting TSC2 in TSC2-negative cells. High ectopic levels of p27 have cell cycle inhibitory effects in TSC2-positive cells but not in TSC2-negative counterparts, although the latter still depend on CDK2 activity. Loss of TSC2 induces soft agar growth of fibroblasts, a process that cannot be inhibited by high levels of p27. Both phenotypes of TSC2-negative cells, their resistance to the activity of ectopic p27, and the instability of endogenous p27, could be explained by our observation that the nucleoprotein p27 is mislocated into the cytoplasm upon loss of TSC2. These findings provide insights into the molecular mechanism of how loss of TSC2 induces cell cycle entry and allow a better understanding of its tumor suppressor function.
Tuberous sclerosis complex (TSC) is an autosomal dominant disease characterized by mental retardation, epilepsy, and tumors of the skin, retina, heart, kidney, and brain (1, 2).
Linkage studies show that about 50% of TSC families are associated with the TSC2 locus, located on chromosome 16, whereas TSC1, which maps to chromosome 9, is implicated in the remainder (3–9). Mutations in the TSC2 gene have been described in patients with TSC (10, 11), and loss of heterozygosity at the TSC2 locus has been demonstrated in TSC patient lesions, as well as in sporadic tumors of non-TSC patients (12–14). The TSC2-encoded protein, designated tuberin, functions as a GTPase accelerating protein for the small molecular weight GTPases Rap1a and Rab5 (15–17). We recently have found that tuberin plays a critical role during neuronal differentiation (18). The Eker rat model has provided an animal system for the analysis of TSC2 function. The Eker mutation consists of a germ-line insertion in the rat homologue of the human TSC2 gene, resulting in premature truncation of TSC2 (19–21). Heterozygous carriers are characterized by the onset of renal tumors and subependymal hamartomas analogous to those seen in human TSC. These rats are also susceptible to carcinogens (22, 23). Fetuses homozygous for the Eker TSC2 mutation die in midgestation with apparent abnormalities in central nervous system development (22). Recently, direct evidence for the tumor suppressor function of TSC2 was obtained by introduction of the TSC2 cDNA into cell lines derived from Eker rat tumors. Overexpression of TSC2 inhibited the growth and suppressed the tumorigenicity of these lines (24, 25). All of these data strongly suggest that TSC2 functions as a tumor suppressor in humans and in the Eker rat, in accordance with Knudson′s “two-hit” hypothesis for tumor development (26). However, the molecular mechanism responsible for the cellular effects of loss of TSC2 has not been elucidated.
In the mammalian cell cycle, the transition from the quiescent state to proliferation has been shown to be regulated by cyclin-dependent kinases (CDKs). A current model of this regulation implicates cyclin D-CDK4/CDK6 complexes to phosphorylate the retinoblastoma protein, thereby activating the transcription factor E2F. Among the genes “turned on” by this mechanism are those for cyclin E and cyclin A, which when complexed with CDK2 promote entry into S phase. These G1 CDKs are negatively regulated by small inhibitory molecules. Two families of these inhibitors have been described, those that interact with all CDKs, including p21, p27, and p57, and those that specifically inhibit CDK4 and CDK6, p15, p16, p18, and p19. Recent data have identified p27 as a major gatekeeper of the quiescent status of mammalian cells (27–29). We have shown that down-regulation of tuberin expression induces quiescent Go-arrested cells to enter the cell cycle (30). Loss of TSC2 can prevent cells from entering a quiescent state after serum withdrawal and can induce cells to pass through the G1/S transition of the eukaryotic cell cycle. Entry into the cell cycle upon loss of TSC2 depends on the activity of the G1-CDKs (30). Here we show that (i) in TSC2-negative cells the CDK inhibitory protein p27 is unstable, and (ii) that high ectopic levels of p27 have lost cell cycle inhibitory effects in TSC2-negative cells. We find the nucleoprotein p27 to become mislocated into the cytoplasm upon loss of TSC2, what is likely the reason p27 cannot inhibit CDKs and why it becomes unstable. The relevance of these findings for the understanding of the tumor suppressor function of TSC2 is discussed.
MATERIALS AND METHODS
Cells, Cell Culture, Flow Cytometry, and Centrifugal Elutriation.
EEF4 (TSC2-positive) and EEF8 (TSC2-negative) cells were derived from Eker rat embryos homozygous for the wild-type and the Eker-mutant TSC2 gene, respectively. Whole embryos were removed on day 10.5 before in utero deaths of the Eker homozygous mutants have occurred. Samples used in these experiments were derived from passage 42 of the immortalized cell lines. In addition, early passages (10–14) of primary embryonic fibroblasts derived from TSC2-positive (EEF-TSC2+/+) and TSC2-negative (EEF-TSC2−/−) Eker rats were used in this study. SKNSH human neuroblastoma cells were obtained from the American Type Culture Collection (ATCC HB11), and Rat1 and Rat1-MycER cells were kindly provided by M. Eilers (Marburg, Germany). All cells were grown either in DMEM or RPMI 1640 medium, both supplemented with 10% calf serum and antibiotics (30 mg/liter of penicillin, 50 mg/liter of streptomycin sulfate). All cultures were kept at 37°C and 7% CO2 and routinely screened for mycoplasma. For cytofluorometric analyses, cells were harvested by trypsinization and fixed by rapid submersion in 5 ml of ice-cold 85% ethanol. After at least 1-hr fixation at −20°C, cells were pelleted and stained in 1 ml of staining solution (0.25 mg/ml propidium iodide/0.05 mg/ml RNase/0.1% Triton X-100 in citrate buffer, pH 7.8). Stained cells were analyzed on a Becton-Dickinson FACScan. Separation of logarithmically growing cells into distinct cell cycle phases was accomplished by centrifugal elutriation as described (32).
Western Blot Analysis, Immunoprecipitation, and Immune Complex Kinase Assays.
Protein extracts were prepared in buffer containing 20 mM Hepes (pH 7.9), 0.4 M NaCl, 2.5% glycerol, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM NaF, 0.5 mM Na3VO4, 0.02 μg/ml leupeptin, 0.02 μg/ml aprotinin, 0.003 μg/ml benzamidinchloride, 0.1 μg/ml trypsin inhibitor, and 0.5 mM DTT. Cells were lysed by freeze and thaw. After 20 min on ice, the extracts were centrifuged, and supernatants were stored at −70°C. Protein concentrations were determined by using the Bio-Rad protein assay reagent with BSA as a standard. A total of 100 μg of protein was run on a 12.5% SDS-polyacrylamide gel and transferred to nitrocellulose. Blots were stained with Ponceau-S to confirm equal loaded amounts of protein. Immunodetection was performed by using specific antibodies, and the signals were developed by using the enhanced chemiluminescence method (Amersham). Immunoprecipitations and analyses of CDK activities were performed according to ref. 33. The following antibodies obtained from Santa Cruz Biotechnology were used: antituberin antibodies, SC-892 or SC-893; against cyclin E, SC-481; against cyclin A, SC-751; against p21, SC-397; against p27, SC-528; against CDK2, SC-163; against CDK4, SC-260; and against Cdc25A, SC-97. In addition, we used the antituberin antibody 5063, which was raised against amino acids 1387–1784, kindly provided by J. DeClue (National Cancer Institute, Bethesda, MD) (15, 30).
DNA Transfection and Antisense Treatment.
cDNAs encoding TSC2, wild-type p21, wild-type p27, the p27VPKK mutant, which cannot be phosphorylated by CDK2 (mutation of Thr-187 to valine compare with ref. 34), or a dominant-negative mutant of CDK2 (35) downstream of the constitutive cytomegalovirus (CMV) expression promoter in the selectable mammalian pcDNA3 vector was transfected into cells by the calcium-phosphate method as described (30). In the case of cytofluorometric analysis of transfectants, the cells were cotransfected with CMV-green fluorescence protein (GFP) cDNA and detected by their green fluorescence. For anti-TSC2 antisense treatment an antisense oligonucleotide specific for the GTPase accelerating protein 3 region of the TSC2 gene (AS) and a mismatch control oligonucleotide of the same length with the same proportion of the four base pairs but randomly organized were used. For a detailed description of the used oligonucleotides, their specificity and gene suppression potency and for the detailed antisense treatment protocol see refs. 18 and 30.
Soft Agar Growth Assay.
Cells (105) were mixed with 0.8% agar and poured onto a bed of 1.4% agar in culture plates. Both top and bottom agar were prepared in DMEM/10% fetal calf serum (FCS). Cells were fed every second day with DMEM/10% FCS and assayed for focus formation at different time points.
Immunocytochemistry.
For immunocytochemical detection of p27, EEF4 and EEF8 cells were grown on glass coverslips and fixed in cold methanol/acetone (1:1). Cells were incubated with anti-p27 antibody overnight at 4°C. Thereafter cells were washed, incubated with a biotinylated secondary antibody, washed again, and incubated with fluorescein isothiocyanate-conjugated streptavidin (details of the method are described in ref. 18).
RESULTS
Loss of TSC2 Affects Cell Cycle Regulation.
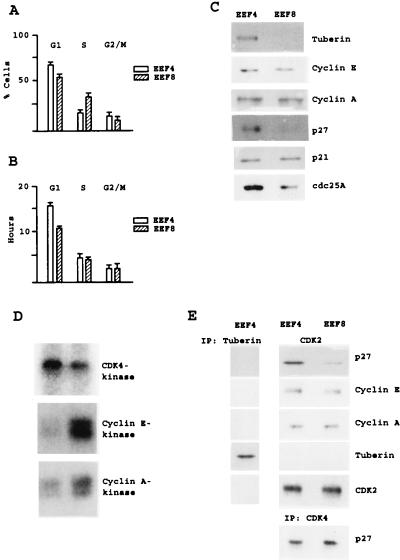
To explore potential effects of loss of TSC2 on cell cycle regulation, we generated immortalized cell lines derived from Eker rat embryos homozygous for the wild-type and the Eker-mutant TSC2 gene, respectively. Exponentially growing EEF4 cells (TSC2-positive) and their TSC2-negative counterparts (EEF8) were cytofluorometrically analyzed for DNA content. Both cell types showed a cell cycle distribution characteristic of growing cells. Quantitation of multiple experiments showed that loss of TSC2 caused a decrease in the number of G1 cells (from 67% ± 4% to 56% ± 3%) with a concomitant increase in the number of S phase cells (Fig. 1A). In parallel, determination of the cell doubling time by cell counting showed a reduction in the doubling time upon loss of TSC2 of 4.1 ± 0.3 hr mainly because of a shortened G1 phase (Fig. 1B). Next, we asked whether loss of TSC2 triggers deregulation of cell cycle-regulating molecules. Western blot analyses revealed that the expression of cyclin E, cyclin A, and p21 was unaffected and that the CDK-activating phosphatase Cdc25A was even slightly down-regulated in TSC2-negative cells. Loss of TSC2 caused a remarkable down-regulation of endogenous p27 levels (Fig. 1C). Analyses of cyclin E- and cyclin A-associated CDK2 activities showed that this down-regulation of the CDK-inhibitor p27 was accompanied by induced CDK2 activity. CDK4-associated kinase activity was not affected by loss of TSC2 (Fig. 1D). p27 inhibits CDK2 activity by directly binding the kinase. Accordingly, the amount of p27 bound to CDK2 reflects the pool of inactive CDK2 in the cells (36). Western blot analysis of CDK2 immunoprecipitates revealed that the amount of p27 bound to the kinase decreases upon loss of TSC2. The levels of CDK2-bound cyclin E or cyclin A and of CDK4-bound p27 were not affected upon loss of TSC2 (Fig. 1E). These data suggest that induction of CDK2 activity in TSC2-negative cells is a result of deregulated p27 expression. In addition, the results showed that under the conditions of these experiments tuberin does not bind to CDK2, cyclin E, cyclin A, or p27. (Fig. 1E).
Figure 1.
Cell cycle analysis of TSC2-positive and TSC2-negative rat embryonic fibroblasts. (A) Flow cytometry analysis of DNA content of logarithmically growing TSC2-negative rat embryonic fibroblasts (EEF8) and TSC2-positive counterparts (EEF4). (B) Doubling times of the cells analyzed in A were determined by cell counting, and the duration of the distinct cell cycle phases was calculated by relating doubling times and percentage of cell cycle phase distributions. The values of three independent experiments are presented. (C) Western blot analyses of tuberin, cyclin E, cyclin A, p27, p21, and Cdc25A expression in EEF4 and EEF8 cells. (D) Protein extracts of EEF4 and EEF8 cells were assayed for CDK4-, cyclin E- and cyclin A-associated kinase activities by using glutathione S-transferase-retinoblastoma protein or histone H1 as substrate, respectively. (E) Immunoprecipitates (IP) performed with antituberin or anti-CDK2 antibodies were investigated for p27, cyclin E, cyclin A, tuberin, and CDK2 protein by Western blot analyses. Immunoprecipitates performed with anti-CDK4 antibody were analyzed for p27 protein by Western blotting.
Abundance of p27 Depends on TSC2.
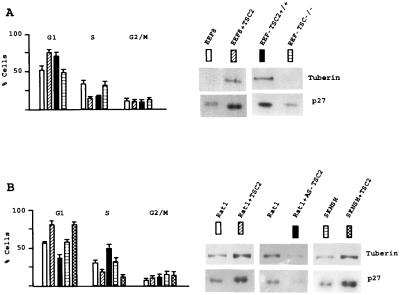
To exclude the possibility that the effects of TSC2 on cell cycle distribution and on p27 expression were caused by clonal effects of EEF4 and EEF8 cells, we transfected EEF8 cells with TSC2. Ectopic overexpression of TSC2 in TSC2-negative cells reverted the aforementioned effects, resulting in elongated G1 phase and up-regulated p27 levels (Fig. 2A). To further confirm that secondary, undefined changes associated with cell immortalization are not necessary for TSC2-negative EEF8 cells to exhibit altered cell cycle control we analyzed early passages of primary embryonic fibroblasts derived from TSC2-positive (EEF-TSC2+/+) or TSC2-negative (TSC2−/−) Eker rats. Also in these primary cells loss of tuberin triggered a decrease in G1 cells and down-regulation of p27 expression (Fig. 2A). These data allow the conclusion that the differences in cell cycle regulation between EEF4 and EEF8 cells are caused by the loss of TSC2. To determine whether altered levels of TSC2 lead to deregulation of cell cycle distribution and p27 expression in other cells, we transfected Rat1 immortalized fibroblasts and SKNSH human neuroblastoma cells with TSC2. In both cellular systems high levels of TSC2 triggered an elongation of the G1 phase and up-regulation of p27 expression (Fig. 2B). We recently have established the conditions to specifically down-regulate TSC2 in Rat1 cells via antisense oligonucleotides (18, 30). Now we found that TSC2-antisense treatment resulted in a shortened G1 phase and decreased levels of p27 (Fig. 2B). Taken together, these data demonstrate that cell cycle distribution and p27 expression depend on TSC2 expression in different cells.
Figure 2.
p27 protein levels depend on the TSC2 status. (A) TSC2-negative cells (EEF8) were transfected with the empty control vector or with an expression vector containing TSC2 cDNA. After 14 days of antibiotic selection, cells were analyzed for DNA distribution on the flow cytometer and for tuberin and p27 protein expression by Western blotting. Early passages of primary embryonic fibroblasts derived from TSC2-positive (EEF-TSC2+/+) and tuberin-negative (EEF-TSC2−/−) Eker rats were analyzed for DNA distribution and tuberin and p27 expression. (B) Rat1 immortalized fibroblasts and SKNSH human neuroblastoma cells were transfected with the empty control vector or with an expression vector containing TSC2 cDNA. After 14 days of antibiotic selection cells were analyzed for DNA distribution and for tuberin and p27 protein expression. Rat1 cells also were treated with TSC2 antisense oligonucleotides for 24 hr and analyzed as described above.
TSC2 Affects the Regulation of p27 Stability.
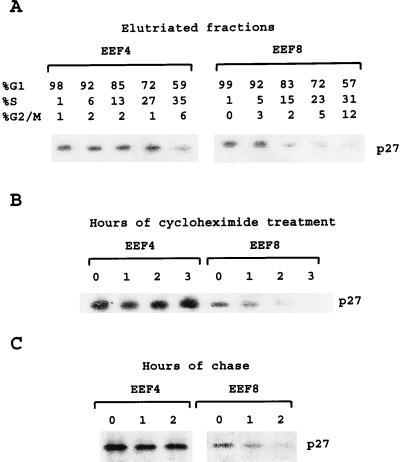
It had been shown earlier that the abundance of p27 is mainly controlled by regulation of protein stability. It has been reported that the half-life of p27 is long in Go/G1 cells and sharply decreases when cells enter S phase. This cell cycle regulation of p27 has been suggested to occur via the ubiquitin-proteasome pathway and, compared with S phase cells, Go/G1 cells contain a far lower amount of p27 ubiquitinating activity (36). If p27 stability is affected in TSC2-negative cells, this observation would predict that the elimination of p27 during the G1/S transition would be deregulated in these cells and that the half-life of p27 is shortened upon loss of TSC2. We first separated EEF4 and EEF8 cells according to their different cell cycle phases by centrifugal elutriation and analyzed the fractions representative for the G1/S transition for p27 expression. This experiment revealed that in TSC2-negative cells p27 is degraded earlier compared with their TSC2-positive counterparts (Fig. 3A). We next inhibited translation by cycloheximide treatment of EEF4 and EEF8 cells and analyzed degradation of p27. In agreement with earlier reports on the half-life of p27 (reviewed in ref. 36), p27 started to decrease at 3 hr of cyclohexmide treatment of EEF4 cells (data not shown). In EEF8 cells p27 levels already heavily decreased after 1 hr (Fig. 3B). These results were confirmed by analyzing p27 stability via a pulse–chase experiment (Fig. 3C). These findings provide strong evidence that TSC2 affects the process of p27 degradation.
Figure 3.
TSC2 affects the regulation of p27 stability. (A) Logarithmically growing EEF4 and EEF8 cells were separated according to the different cell cycle phases by centrifugal elutriation. The obtained cell fractions were cytofluorometrically analyzed for DNA distribution after staining DNA with propidium iodide (Upper). Protein extracts of the obtained fractions were analyzed for p27 protein expression by Western blot analysis. (B) Logarithmically growing EEF4 and EEF8 cells were incubated in 100 μg/ml of cycloheximide for the indicated time periods. p27 protein levels were compared by Western blot analysis. (C) Logarithmically growing EEF4 and EEF8 cells were incubated for 4 hr with [35S]methionine and then chased in medium containing unlabeled methionine for the indicated times. Radiolabeled p27 was immunoprecipitated, separated by gel electrophoresis, and detected by autoradiography. To be able to detect p27 degradation in EEF8 cells on the same Western blot with EEF4 cells it was necessary to perform long exposures (compare with Fig. 1C). The presented blots are overexposed in respect to p27 expression in EEF4 cells. p27 protein started to decrease after 3-hr cycloheximide treatment of EEF4 cells (data not shown).
Cell Cycle Inhibitory Effects of p27 Are Inactivated in TSC2-Negative Cells.
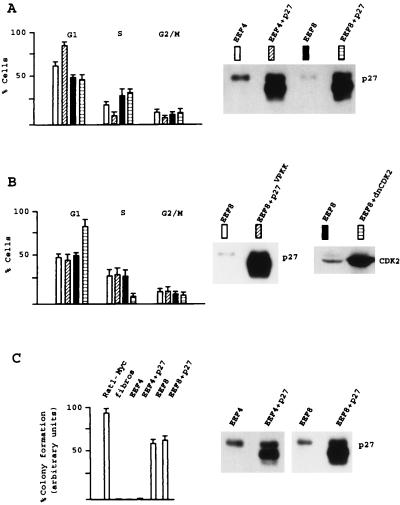
In all normal cells, forced expression of p27 causes the cell cycle to arrest in G1, an effect that can be visualized by an increase of G1 cells upon ectopic p27 expression in logarithmically growing cells. We confirmed this potency of p27 by overexpressing this CDK inhibitor in TSC2-positive EEF4 cells. Quantitation of multiple experiments revealed that overexpression of p27 triggers an increase of G1 cells of 18% ± 3% (Fig. 4A). Strikingly, no effect on cell cycle distribution was observed when p27 was overexpressed in EEF8 cells to the same levels as in EEF4 cells (Fig. 4A). High levels of ectopic p21 arrested both EEF4 and EEF8 cells in G1 (data not shown). From our data on p27 stability in TSC2-negative cells (described above) one could speculate that p27 cannot trigger cell cycle inhibitory effects because of its increased degradation in these cells. However, our observation that p27 was expressible to the same levels in EEF8 cells as in EEF4 cells made that unlikely (Fig. 4A). It had been shown earlier that degradation of p27 is induced by phosphorylation on Thr-187 in the p27 C terminus via CDK2 (34, 36, 37). To address the role of CDK2-dependent p27 degradation in growth rescue by loss of TSC2, we overexpressed the nonphosphorylatable p27VPKK mutant (mutation of Thr-187 to valine, compare to ref. 34) in TSC2-negative EEF8 cells. p27VPKK behaved exactly as wild-type p27 in the transfection assay, indicating that CDK2-dependent p27 degradation is not the mechanism used by TSC2-negative cells to overcome p27-dependent growth arrest (Fig. 4B). It is noteworty that we found the p27VPKK mutant to be expressible to the same amounts in both EEF4 cells and EEF8 cells without mediating cell cycle effects (data not shown). The resistance of TSC2-negative cells against p27-dependent arrest could be explained by two different mechanisms: p27 could be inactive in inhibiting CDKs in EEF8 cells, or loss of TSC2 could trigger cell proliferation independently of CDK2 activity (proliferation despite inactive CDK2). To further investigate this issue we tested whether TSC2-negative cells can grow independently of active CDK2. Overexpression of a dominant-negative mutant of CDK2, which was shown earlier to inhibit CDK2 activity (35), clearly arrested TSC2-negative fibroblasts (Fig. 4B). These data show that loss of TSC2 does not mediate CDK2-independent cell proliferation and strongly suggest that p27 cannot efficiently inhibit CDK2 in EEF8 cells.
Figure 4.
High ectopic levels of p27 lose their cell cycle inhibitory effects in TSC2-negative cells. (A) Logarithmically growing EEF4 cells (TSC2-positive) and EEF8 cells (TSC2-negative) were cotransfected either with the empty control vector and an expression vector containing GFP or the expression plasmid containing p27 cDNA and GFP. GFP-positive cells were cytofluorometrically analyzed for DNA content. The cells were further analyzed for p27 protein expression by Western blotting. The transfected p27 protein migrates faster than the endogenous p27 protein. (B) EEF8 cells were transfected with empty control vectors, with the expression vector containing the p27VPKK mutant or a dominant-negative CDK2 mutant together with a GFP-expressing plasmid. GFP-positive cells were analyzed for DNA distribution on the flow cytometer, and cell extracts were analyzed for p27 expression by Western blot detection. (C) Rat1 cells expressing high levels of Myc (positive control), primary fibroblasts (fibros, negative control), EEF4 cells, and EEF8 cells, both transfected either with the empty expression vector or with p27 were analyzed for soft agar growth, and colonies were scored after 1 week. The experiment was repeated three times, and the obtained data are presented in percentage relative to the highest value (set 100%). EEF4 and EEF8 cell extracts were analyzed for p27 expression by Western blotting.
Transformation of primary cells by oncogenes is multistep, requiring the cooperation of two genes. c-Myc alone, however, is capable of transforming specific rat cell lines, associated with anchorage-independent growth (reviewed in ref. 38) as confirmed by the experiment presented in Fig. 4C. Soft agar assays further demonstrated that loss of TSC2 induces growth independent of cell adhesion, a process that could not be inhibited by high ectopic levels of p27 (Fig. 4C). Taken together, these data demonstrate that loss of TSC2 mediates growth advantage and that p27 cannot exert its cell cycle inhibitory function in cells, which lost TSC2 expression.
Loss of TSC2 Affects p27 Localization.
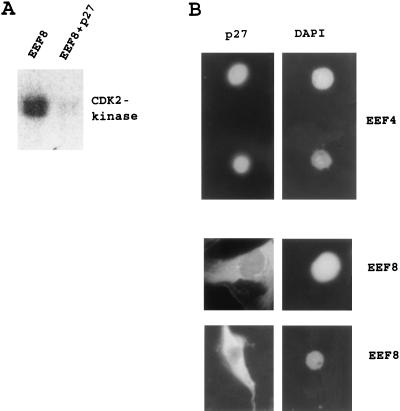
So far we have described two phenotypes induced by loss of TSC2: (i) p27 is unstable in TSC2-negative cells, leading to elevated CDK2 activity and shortened G1 phase, and (ii) TSC2 mutant cells are resistant to ectopic p27. Our finding, that these cells depend on functional CDK2, strongly suggests that p27 cannot inhibit CDK2 in these cells. To further elucidate the mechanism of the affects of TSC2 on p27 we first tested whether the CDK2 complex is, per se, resistant to p27 in TSC2-negative cell extracts. Kinase assays revealed that CDK2 precipitated from EEF8 cells can be inhibited in vitro by the addition of recombinant p27 protein (Fig. 5A). These data strongly suggest that p27 is functionally disabled in vivo in TSC2-negative cells. We next investigated the subcellular localization of p27 in EEF4 and in EEF8 cells. p27 has been reported to exclusively localize into the nucleus (reviewed in ref. 28). While EEF4 cells exhibit nuclear localization of p27, this protein is mislocated into the cytoplasm upon loss of TSC2 (Fig. 5B). Although it appeared that the nuclei of EEF8 cells also weakly stained p27 positive, a major portion of p27 was detectable in the cytoplasm. This mislocation of p27 upon loss of TSC2 could be an explanation for both the instability of endogenous p27 in TSC2-negative cells and the resistance of TSC2-mutant cells to the cell cycle inhibitory function of ectopic p27.
Figure 5.
Loss of TSC2 affects p27 localization. (A) Protein extracts of EEF8 cells were assayed for CDK2-associated kinase activity by using histone H1 as substrate. CDK2 activity in EEF8 extracts could be inhibited by the addition of 20nM recombinant p27 protein directly to the kinase assay’s reaction. (B) Immunocytochemical detection of the subcellular localization of p27 in EEF4 and in EEF8 cells. The signals in EEF8 cells have been enhanced to visualize p27 localization. Nuclei were identified by 4′,6-diamidino-2-phenylindole (DAPI) staining.
DISCUSSION
Forced expression of p27 causes the cell cycle to arrest in G1 (39, 40), and conversely, inhibition of p27 expression by antisense oligonucleotides drives quiescent cells into the cell cycle (41). It is widely accepted that the latter is the result of activation of G1-CDKs upon loss of p27 (27–29). In this report we have shown that loss of TSC2 inactivates p27’s property to inhibit cell cycle progression. We further found that loss of TSC2 triggers down-regulation of p27 expression caused by decreased protein stability. This down-regulation is accompanied by less p27 bound to CDK2 protein and by an induction of CDK2 activity upon loss of TSC2. Earlier data of our laboratory demonstrated that loss of tuberin expression is sufficient to drive a quiescent cell into the cell cycle (30). These results suggest that cells lacking functional tuberin, such as the cells that give rise to aberrant growths of TSC patients, also may fail to arrest normally because of inactivation of the CDK inhibitor p27.
Our data suggest that TSC2 affects two aspects of p27 biology: (i) high levels of ectopic p27 cannot trigger cell cycle arrest of TSC2-negative cells, and (ii) by using different approaches we have shown that the stability of p27 decreases upon loss of TSC2. The former phenotype is independent of CDK2-dependent p27 degradation, because the nonphosphorylatable p27VPKK mutant also was unable to affect cell cycle progression of TSC2-negative cells. On the other hand, it could be that the elevated degradation of p27 in TSC2-negative cells is a secondary effect associated with the inhibition of p27’s activity in these cells.
Two different models could explain the resistance of TSC2-negative cells against high levels of p27. p27 could be unable to inhibit CDK activities or loss of TSC2 could trigger proliferation independently of CDK2 activity. Inhibition of CDK2 activity by overexpression of a dominant-negative CDK2 mutant revealed that TSC2-negative cells cannot grow without active CDK2. Although p27 obviously cannot inhibit CDK2 in vivo in TSC2-mutant cells, we found that recombinant p27 protein can inhibit CDK2 in vitro in TSC2-negative extracts. These data strongly suggest that ectopic p27 is functionally disabled to efficiently inhibit CDK2 in TSC2-negative cells. Our finding that the nucleoprotein p27 becomes mislocated into the cytoplasm upon loss of TSC2 might be the explanation for these observations. Immunoprecipitations showed that tuberin does not associate directly with p27, CDK2, cyclin E, or cyclin A, suggesting that its effect is mediated by a distinct cellular protein(s).
How does TSC2 affect p27 stability? Two different pathways could lead to decreased p27 protein stability in TSC2-negative cells. It had been shown earlier that degradation of p27 is induced by phosphorylation on Thr-187 in the p27 C terminus via CDK2 (34, 36, 37). Therefore, it is possible that TSC2 is involved in regulating CDK2 directly and that the decreased level of p27 in TSC2-negative cells is only an indirect effect of elevated CDK activity. Such elevation of CDK2 activity could be mediated by up-regulation of the expression of the CDK-activating phosphatase CDC25A or of CDK2-interacting cyclin E and/or cyclin A. Down-regulation of another CDK2-inhibitor, such as p21, could be involved. The assembly of CDK2 with its activating cyclins could be deregulated. The CDK-activating kinase (CAK) could be affected. CDK2 could be activated further by deregulated cyclin D/CDK4 complexes titrating p27 away from CDK2. On the other hand, tuberin could be directly involved in modulating p27 turnover. Although at the moment we cannot clarify which pathway leads to p27 degradation in TSC2-negative cells, we favor the latter. We did not find any evidence that tuberin could be directly involved in deregulating CDK2 (compare Fig. 1). Tuberin does not bind to CDK2, cyclin E, or cyclin A. Loss of TSC2 has no positive effects on the expression of cyclin E, cyclin A, or Cdc25A, and no negative effects on p21 expression. Neither binding of cyclin E or A to CDK2 nor the ratio of CAK-phosphorylated to unphosphorylated CDK2 is affected upon loss of TSC2. Tuberin has no effect on CDK4 activity or on the assembly of CDK4 and p27. On the other hand, we found p27 to become mislocated into the cytoplasm upon loss of TSC2, which could be a reason p27 becomes unstable.
In S. cerevisiae, p40Sic1, an inhibitor of yeast CDKs, is degraded by the ubiquitin-proteasome pathway, including the assembly of a ubiquitin ligase complex known as SCFCDC4 (for SKP1, Cullin, F-box protein CDC4). The functional analogy between p27 and p40Sic1 induced experiments indicating that mammalian SCF complexes also might be part of a p27-specific ubiquitination pathway (reviewed in ref. 36). Although data have been provided indicating that functional SCF ubiquitin ligase complexes exist in mammalian cells (42, 43), it is not clear at the moment to what extent they are involved in the degradation of p27. Tuberous sclerosis usually is classified as one of the phakomatoses or neurocutaneous syndromes, a group including neurofibromatosis I and II, von Hippel-Lindau syndrome, and Sturge-Weber disease (1, 2, 6). Recently, the von Hippel-Lindau tumor suppressor gene (VHL) had been shown to affect p27 stability (44) and to form a stable complex with the human cullin CUL-2 (31). However, our knowledge of how cancer syndrome genes, such as VHL or TSC2, affect the regulation of p27 turnover can only increase hand in hand with the elucidation of the mechanisms responsible for p27 degradation at the G1/S transition of the mammalian cell cycle.
Recently, p27 has attracted much attention among tumor biologists. Unlike traditional tumor suppressor genes, the p27 gene shows no homozygous deletions or mutations in a wide variety of analysed tumors. However, p27 is inactivated by the adenoviral E1A and the human papilloma viral E7 oncoproteins, as well as by c-Myc. It has further been shown that enhanced degradation of p27 is a prognostic marker in colorectal, breast, and nonsmall cell lung carcinomas (36). We now propose that p27 also may play a role in the pathogenesis of tuberous sclerosis through its regulation by the TSC2 gene.
Acknowledgments
We thank T. Littlewood, G. Evan, J. DeClue, D. Müller, M. Eilers, S. Van den Heuvel, E. Harlow, J. Vlach, and B. Amati for providing cells and reagents. We are particularly grateful to Bruno Amati (Institut Suisse de Recherches Experimentales sur le Cancer) for helpful discussion and critically reading the manuscript. Work in M.H.’s laboratory is supported by the Herzfelder′sche Familienstiftung, the Anton Dreher-Gedächtnisschenkung für Medizinische Forschung, the Austrian Nationalbank, and the Austrian Fonds zur Förderung der Wissenschaftlichen Forschung, P13127-GEN. R.S.Y. is partly supported by grants from the National Institutes of Health National Cancer Institute (CA 61889 and CA 71538) and the American Cancer Society.
ABBREVIATIONS
- TSC
tuberous sclerosis complex
- CDK
cyclin-dependent kinase
- GFP
green fluorescence protein
Footnotes
A Commentary on this article begins on page 15158.
References
- 1.Gomez M R. Tuberous Sclerosis. New York: Raven; 1988. [Google Scholar]
- 2.Osborn J P, Fryer A, Webb D. Ann NY Acad Sci. 1991;615:125–127. doi: 10.1111/j.1749-6632.1991.tb37754.x. [DOI] [PubMed] [Google Scholar]
- 3.Sampson J R, Janssen L A J, Sandkuijl L A the Tuberous Sclerosis Collaborative Group. J Med Genet. 1992;29:861–866. doi: 10.1136/jmg.29.12.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwiatkowski D J, Armour J, Bale A E, Fountain J W, Goudie D, Haines J L, Knowles M A, Pilz A, Slaugenhaupt S, Povey S. Cytogenet Cell Genet. 1993;64:94–106. doi: 10.1159/000133566. [DOI] [PubMed] [Google Scholar]
- 5.The European Chromosome 16 Tuberous Sclerosis Consortium. Cell. 1993;75:1305–1313. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 6.Povey S, Burley M W, Attwood J, Benham F, Hunt D, Jeremiah S J, Franklin D, Gillet G, Malas S, Robson E B, et al. Ann Hum Genet. 1994;58:107–127. doi: 10.1111/j.1469-1809.1994.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 7.Janssen B, Sampson J, van der Est M, Deelen W, Verhoef S, Daniels I, Hesseling A, Brook-Carter P, Nellist M, Lindhout D, et al. Hum Genet. 1994;94:437–440. doi: 10.1007/BF00201608. [DOI] [PubMed] [Google Scholar]
- 8.Sampson J R, Harris P C. Hum Mol Gen. 1994;3:1477–1480. doi: 10.1093/hmg/3.suppl_1.1477. [DOI] [PubMed] [Google Scholar]
- 9.The TSC1 Consortium. Science. 1997;277:805–808. [Google Scholar]
- 10.Kumar A, Wolpert C, Kandt R S, Segal J, Pufky J, Roses A D, Pericak-Vance M A, Gilbert J R. Hum Mol Genet. 1995;4:1471–1472. doi: 10.1093/hmg/4.8.1471. [DOI] [PubMed] [Google Scholar]
- 11.Wilson P J, Ramesh V, Kristiansen A, Bove C, Jozwiak S, Kwiatkowski D J, Short M P, Haines J L. Hum Mol Genet. 1996;5:249–256. doi: 10.1093/hmg/5.2.249. [DOI] [PubMed] [Google Scholar]
- 12.Green A J, Smith M, Yates J R W. Nat Genet. 1994;6:193–196. doi: 10.1038/ng0294-193. [DOI] [PubMed] [Google Scholar]
- 13.Henske E P, Neumann H P H, Scheithauer B W, Herbst E W, Short M P, Kwiatkowski D J. Genes Chromosomes Cancer. 1995;13:295–298. doi: 10.1002/gcc.2870130411. [DOI] [PubMed] [Google Scholar]
- 14.Carbonara C, Longa L, Mazzuco G, Borrone C, Garre M L, Brisigotti M, Filippi G, Scabar A, Gianotti A, Falzoni P, et al. Genes Chromosomes Cancer. 1996;15:18–25. doi: 10.1002/(SICI)1098-2264(199601)15:1<18::AID-GCC3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Wienecke R, König A, DeClue J. J Biol Chem. 1995;270:16409–16414. doi: 10.1074/jbc.270.27.16409. [DOI] [PubMed] [Google Scholar]
- 16.Wienecke R, Maize J C, Shoarinejad F, Vass W C, Reed J, Bonafico J S, Resau J H, de Gunzburg J, Yeung R S, DeClue J E. Oncogene. 1996;13:913–923. [PubMed] [Google Scholar]
- 17.Xiao G-H, Shoarinejad F, Jin F, Golemis E A, Yeung R S. J Biol Chem. 1997;272:6097–6100. doi: 10.1074/jbc.272.10.6097. [DOI] [PubMed] [Google Scholar]
- 18.Soucek T, Hölzl G, Bernaschek G, Hengstschläger M. Oncogene. 1998;16:2197–2204. doi: 10.1038/sj.onc.1201743. [DOI] [PubMed] [Google Scholar]
- 19.Yeung R S, Xiao G-H, Jin F, Lee W-C, Testa J R, Knudson A G. Proc Natl Acad Sci USA. 1994;91:11413–11416. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi T, Hirayama Y, Kobayashi E, Kubo Y, Hino O. Nat Genet. 1995;9:70–74. doi: 10.1038/ng0195-70. [DOI] [PubMed] [Google Scholar]
- 21.Xiao G-H, Jin F, Yeung R S. Oncogene. 1995;11:81–87. [PubMed] [Google Scholar]
- 22.Hino O, Klein-Szanto A J P, Freed J J, Testa J R, Brown D Q, Vilensky M, Yeung R S, Tartof K D, Knudson A G. Proc Natl Acad Sci USA. 1993;90:327–331. doi: 10.1073/pnas.90.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker C, Goldsworthy T L, Wolf D C, Everitt J. Science. 1992;255:1693–1695. doi: 10.1126/science.1553556. [DOI] [PubMed] [Google Scholar]
- 24.Jin F, Wienecke R, Xiao G-H, Maize J C, DeClue J E, Yeung R S. Proc Natl Acad Sci USA. 1996;93:9154–9159. doi: 10.1073/pnas.93.17.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orimoto K, Tsuchiya H, Kobayashi T, Matsuda T, Hino O. Biochem Biophys Res Commun. 1996;219:70–75. doi: 10.1006/bbrc.1996.0183. [DOI] [PubMed] [Google Scholar]
- 26.Knudson A G. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinberg R A. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 28.Sherr C J, Roberts J M. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 29.Beijeersbergen R L, Bernards R. Biochim Biophys Acta. 1996;1287:103–120. doi: 10.1016/0304-419x(96)00002-9. [DOI] [PubMed] [Google Scholar]
- 30.Soucek T, Pusch O, Wienecke R, DeClue J E, Hengstschläger M. J Biol Chem. 1997;272:29031–29038. doi: 10.1074/jbc.272.46.29301. [DOI] [PubMed] [Google Scholar]
- 31.Pause A, Lee S, Worrell R A, Chen D Y T, Burgess W H, Linehan W M, Klausner R D. Proc Natl Acad Sci USA. 1997;94:2156–2161. doi: 10.1073/pnas.94.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pusch O, Bernaschek G, Eilers M, Hengstschläger M. Oncogene. 1997;15:649–656. doi: 10.1038/sj.onc.1201236. [DOI] [PubMed] [Google Scholar]
- 33.Dulic V, Lees E, Reed S I. Science. 1992;257:1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- 34.Vlach J, Hennecke S, Amati B. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van den Heuvel S, Harlow E. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 36.Alessandrini A, Chiaur D S, Pagano M. Leukemia. 1997;11:342–345. doi: 10.1038/sj.leu.2400581. [DOI] [PubMed] [Google Scholar]
- 37.Sheaff R J, Groudine M, Gordon M, Roberts J M, Clurman B E. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 38.Henriksson M, Lüscher B. Adv Cancer Res. 1996;68:109–182. doi: 10.1016/s0065-230x(08)60353-x. [DOI] [PubMed] [Google Scholar]
- 39.Polyak K, Kato J, Solomon M, Sherr C, Massague J, Roberts J M, Koff A. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 40.Toyoshima H, Hunter T. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 41.Coats S, Flannagan W M, Nourse J, Roberts J M. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- 42.Lisztwan J, Marti A, Sutterlüty H, Gstaiger M, Wirbelbauer C, Krek W. EMBO J. 1998;17:368–383. doi: 10.1093/emboj/17.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyapina S A, Correll C C, Kipreos E T, Deshaies R J. Proc Natl Acad Sci USA. 1998;95:7451–7456. doi: 10.1073/pnas.95.13.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pause A, Lee S, Lonergan K M, Klausner R D. Proc Natl Acad Sci USA. 1998;95:993–998. doi: 10.1073/pnas.95.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]