Abstract
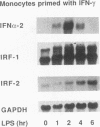
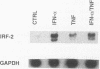
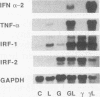
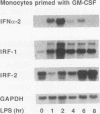
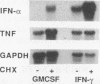
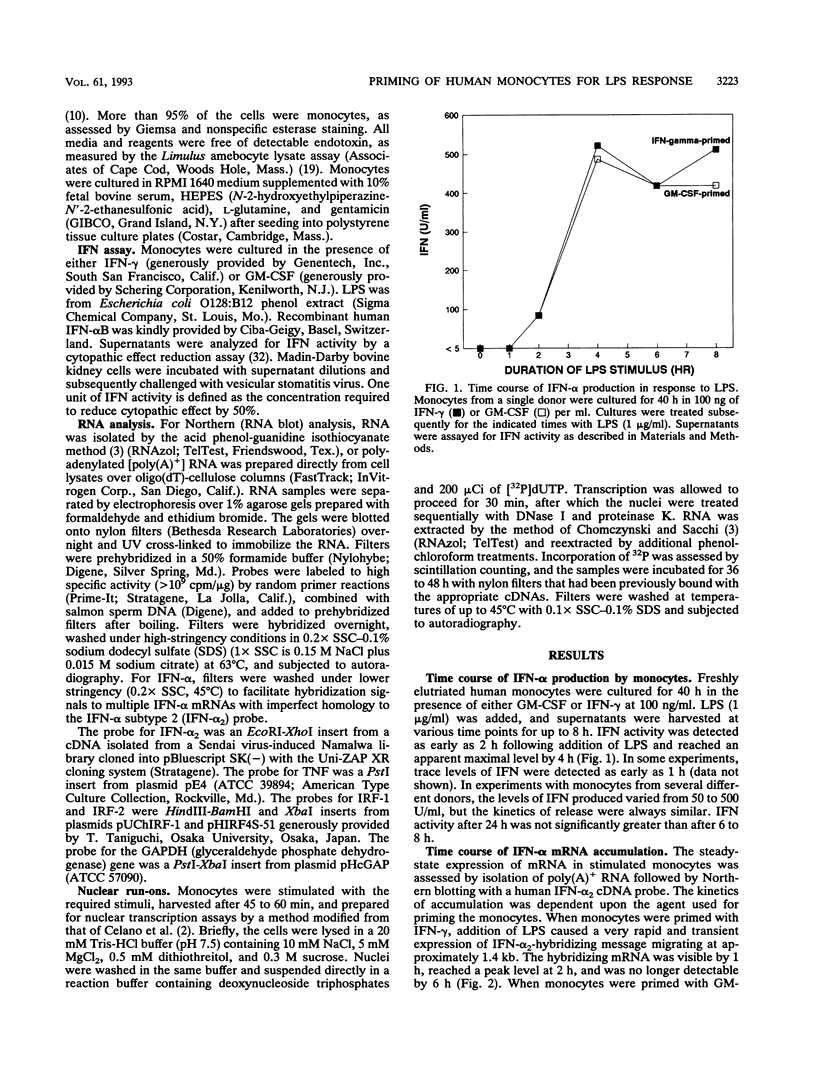
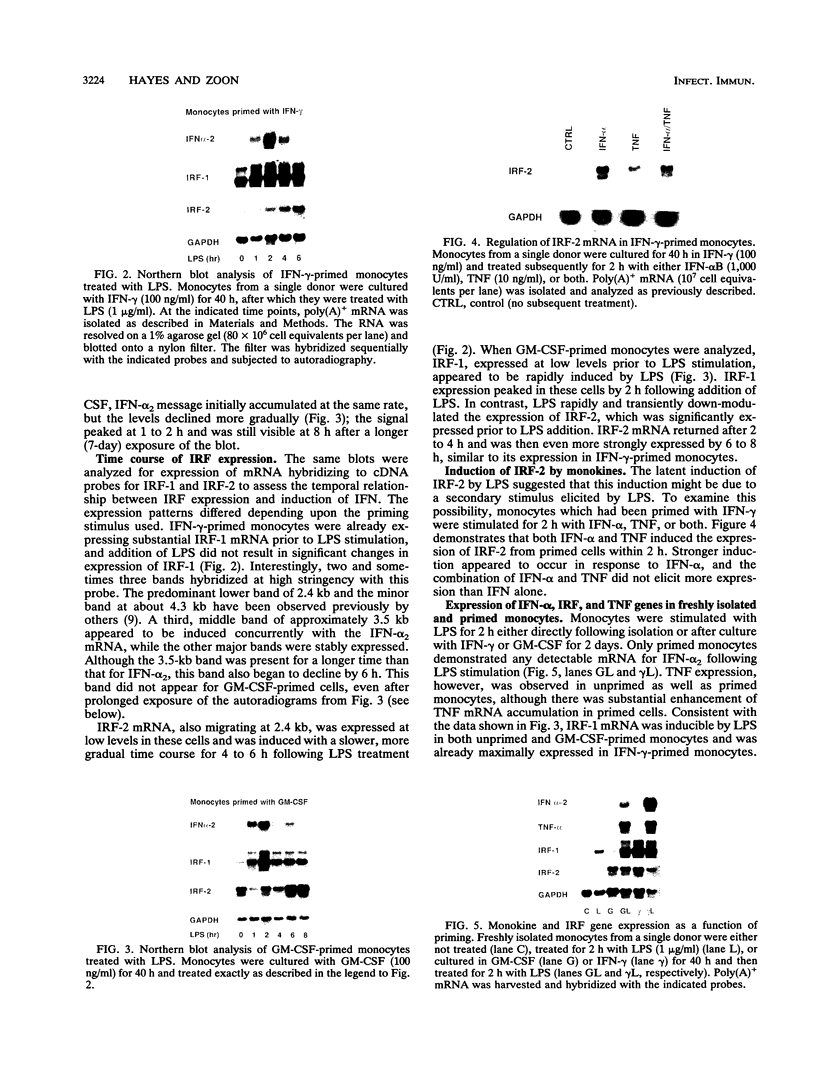
Culture of human monocytes with either granulocyte-macrophage colony-stimulating factor or gamma interferon (IFN-gamma) results in a primed state, during which these cells express heightened responses to bacterial lipopolysaccharide (LPS). The production of IFN-alpha in response to LPS by human monocytes has an absolute requirement for priming. Tumor necrosis factor (TNF) expression is also greatly enhanced in primed monocytes after LPS stimulation, but unlike IFN-alpha, TNF is readily expressed in unprimed monocytes as well. In an effort to determine the molecular events associated with IFN-alpha induction in this system, freshly isolated human monocytes were primed by culture with either IFN-gamma or granulocyte-macrophage colony-stimulating factor and then treated with LPS; expression of IFN-alpha subtype 2 (IFN-alpha 2), IFN regulatory factors (IRFs), and TNF was assessed by Northern (RNA blot) analysis. IRF-1 mRNA is expressed at high levels in monocytes and is regulated by both LPS and priming cytokines, but its expression alone does not correlate with the induction of IFN-alpha 2 expression. IRF-2 mRNA is expressed in a more gradual manner following LPS stimulation, implying a possible feedback mechanism for inhibiting IFN-alpha expression. However, nuclear run-on analysis indicates that IFN-alpha 2 is not transcriptionally modulated in this system, in striking contrast to TNF, which is clearly regulated at the transcriptional level. In addition, IFN-alpha 2 mRNA accumulation is superinduced when primed monocytes are treated with LPS plus cycloheximide, while TNF mRNA is relatively unaffected. The results demonstrate that priming can affect subsequent LPS-induced gene expression at different levels in human monocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celano P., Baylin S. B., Casero R. A., Jr Polyamines differentially modulate the transcription of growth-associated genes in human colon carcinoma cells. J Biol Chem. 1989 May 25;264(15):8922–8927. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dinter H., Hauser H. Superinduction of the human interferon-beta promoter. EMBO J. 1987 Mar;6(3):599–604. doi: 10.1002/j.1460-2075.1987.tb04796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleit H. B., Rabinovitch M. Interferon induction in marrow-derived macrophages: regulation by L cell conditioned medium. J Cell Physiol. 1981 Sep;108(3):347–352. doi: 10.1002/jcp.1041080308. [DOI] [PubMed] [Google Scholar]
- Fleit H. B., Rabinovitch M. Production of interferon by in vitro derived bone marrow macrophages. Cell Immunol. 1981 Jan 15;57(2):495–504. doi: 10.1016/0008-8749(81)90107-6. [DOI] [PubMed] [Google Scholar]
- Fujita T., Kimura Y., Miyamoto M., Barsoumian E. L., Taniguchi T. Induction of endogenous IFN-alpha and IFN-beta genes by a regulatory transcription factor, IRF-1. Nature. 1989 Jan 19;337(6204):270–272. doi: 10.1038/337270a0. [DOI] [PubMed] [Google Scholar]
- Fujita T., Reis L. F., Watanabe N., Kimura Y., Taniguchi T., Vilcek J. Induction of the transcription factor IRF-1 and interferon-beta mRNAs by cytokines and activators of second-messenger pathways. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9936–9940. doi: 10.1073/pnas.86.24.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard T. L., Jurgensen C. H., Fauci A. S. Differential effect of monoclonal anti-DR antibody on monocytes in antigen- and mitogen-stimulated responses: mechanism of inhibition and relationship to interleukin 1 secretion. Cell Immunol. 1983 Dec;82(2):394–402. doi: 10.1016/0008-8749(83)90172-7. [DOI] [PubMed] [Google Scholar]
- Gogos J. A., Hsu T., Bolton J., Kafatos F. C. Sequence discrimination by alternatively spliced isoforms of a DNA binding zinc finger domain. Science. 1992 Sep 25;257(5078):1951–1955. doi: 10.1126/science.1290524. [DOI] [PubMed] [Google Scholar]
- Harada H., Fujita T., Miyamoto M., Kimura Y., Maruyama M., Furia A., Miyata T., Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989 Aug 25;58(4):729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- Harada H., Willison K., Sakakibara J., Miyamoto M., Fujita T., Taniguchi T. Absence of the type I IFN system in EC cells: transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell. 1990 Oct 19;63(2):303–312. doi: 10.1016/0092-8674(90)90163-9. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Spitalny G. L. Endotoxin-induced interferon synthesis in macrophage cultures. J Reticuloendothel Soc. 1983 May;33(5):369–380. [PubMed] [Google Scholar]
- Hayes M. P., Enterline J. C., Gerrard T. L., Zoon K. C. Regulation of interferon production by human monocytes: requirements for priming for lipopolysaccharide-induced production. J Leukoc Biol. 1991 Aug;50(2):176–181. doi: 10.1002/jlb.50.2.176. [DOI] [PubMed] [Google Scholar]
- Hiscott J., Cantell K., Weissmann C. Differential expression of human interferon genes. Nucleic Acids Res. 1984 May 11;12(9):3727–3746. doi: 10.1093/nar/12.9.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T., Gogos J. A., Kirsh S. A., Kafatos F. C. Multiple zinc finger forms resulting from developmentally regulated alternative splicing of a transcription factor gene. Science. 1992 Sep 25;257(5078):1946–1950. doi: 10.1126/science.1411512. [DOI] [PubMed] [Google Scholar]
- Kornbluth R. S., Edgington T. S. Tumor necrosis factor production by human monocytes is a regulated event: induction of TNF-alpha-mediated cellular cytotoxicity by endotoxin. J Immunol. 1986 Oct 15;137(8):2585–2591. [PubMed] [Google Scholar]
- Levin J., Tomasulo P. A., Oser R. S. Detection of endotoxin in human blood and demonstration of an inhibitor. J Lab Clin Med. 1970 Jun;75(6):903–911. [PubMed] [Google Scholar]
- Mangan D. F., Wahl S. M. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J Immunol. 1991 Nov 15;147(10):3408–3412. [PubMed] [Google Scholar]
- Neumann C., Sorg C. Sequential expression of functions during macrophage differentiation in murine bone marrow liquid cultures. Eur J Immunol. 1980 Nov;10(11):834–840. doi: 10.1002/eji.1830101107. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Kellum M., Kelley K. A., Antrobus S., Pitha P. M. Differential regulation of interferon synthesis in lymphoblastoid cells. J Interferon Res. 1985 Summer;5(3):493–510. doi: 10.1089/jir.1985.5.493. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Dieckmann B., Vannice J. L., Trahey M., McCormick F. Inhibition of protein synthesis stimulates the transcription of human beta-interferon genes in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3964–3968. doi: 10.1073/pnas.81.13.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Chirigos M. A. Selective neutralization by antiinterferon globulin of macrophage activation by L-cell interferon, Brucella abortus ether extract, Salmonella typhimurium lipopolysaccharide, and polyanions. Cell Immunol. 1979 Nov;48(1):52–58. doi: 10.1016/0008-8749(79)90098-4. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Torres B. A., Johnson H. M. Lipopolysaccharide and polyribonucleotide activation of macrophages: implications for a natural triggering signal in tumor cell killing. Biochem Biophys Res Commun. 1985 Aug 30;131(1):395–401. doi: 10.1016/0006-291x(85)91815-7. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., Fertsch D. Endogenous interferon production by endotoxin-responsive macrophages provides an autostimulatory differentiation signal. Infect Immun. 1984 Aug;45(2):417–423. doi: 10.1128/iai.45.2.417-423.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M. K., Ralph P. Macrophage growth factor CSF-1 stimulates human monocyte production of interferon, tumor necrosis factor, and colony stimulating activity. J Immunol. 1986 Oct 1;137(7):2281–2285. [PubMed] [Google Scholar]
- Weidle U., Weissmann C. The 5'-flanking region of a human IFN-alpha gene mediates viral induction of transcription. Nature. 1983 Jun 2;303(5916):442–446. doi: 10.1038/303442a0. [DOI] [PubMed] [Google Scholar]
- Zoon K. C., Buckler C. E., Bridgen P. J., Gurari-Rotman D. Production of human lymphoblastoid interferon by Namalva cells. J Clin Microbiol. 1978 Jan;7(1):44–51. doi: 10.1128/jcm.7.1.44-51.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]