Abstract
Objective
Evidence suggests that emotional stress can trigger acute coronary syndromes in patients with advanced coronary artery disease (CAD), although the mechanisms involved remain unclear. Hostility is associated with heightened reactivity to stress in healthy individuals, and with an elevated risk of adverse cardiac events in CAD patients. This study set out to test whether hostile individuals with advanced CAD were also more stress responsive.
Methods
Thirty-four men (aged 55.9±9.3 years) who had recently survived an acute coronary syndrome took part in laboratory testing. Trait hostility was assessed by the Cook Medley Hostility Scale, and cardiovascular activity, salivary cortisol, and plasma concentrations of interleukin-6 were assessed at baseline, during performance of two mental tasks, and during a 2-h recovery.
Results
Participants with higher hostility scores had heightened systolic and diastolic blood pressure (BP) reactivity to tasks (both P<.05), as well as a more sustained increase in systolic BP at 2 h post-task (P=.024), independent of age, BMI, smoking status, medication, and baseline BP. Hostility was also associated with elevated plasma interleukin-6 (IL-6) levels at 75 min (P=.023) and 2 h (P=.016) poststress and was negatively correlated with salivary cortisol at 75 min (P=.034).
Conclusion
Hostile individuals with advanced cardiovascular disease may be particularly susceptible to stress-induced increases in sympathetic activity and inflammation. These mechanisms may contribute to an elevated risk of emotionally triggered cardiac events in such patients.
Keywords: Acute coronary syndrome, Blood pressure, Hostility, Inflammation, Interleukin-6, Psychological stress
Introduction
Acute coronary syndromes (ACS), defined as ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation MI (NSTEMI), and unstable angina (UA), cause significant morbidity and mortality in industrialized societies [1]. Growing evidence from clinical studies using case cross-over analysis suggests that acute episodes of anger can trigger ACS in people with advanced coronary disease, with the period 1–2 h prior to symptom onset being particularly hazardous [2–5].
Hostility is an enduring personality trait that includes emotional (anger) as well as attitudinal (cynicism and mistrust of others) and behavioral (overt and repressed aggression) components [6], and numerous cross-sectional and prospective studies have highlighted hostility as a robust independent risk factor for coronary artery disease (CAD) and all-cause mortality in humans [7–11]. Findings relating hostility with adverse clinical outcomes in CAD patients have been somewhat less consistent [12]. However, a recent meta-analysis including 19 prospective studies of men and women with existing coronary disease found a significant independent association between hostility and risk of future cardiac events in these patients [11].
The pathophysiological mechanisms underlying emotional triggering of acute cardiac events are poorly understood. Atherosclerosis is a chronic inflammatory process involving the progressive recruitment and activation of leukocytes, lipid, platelets, and smooth muscle cells in the endothelial lining of coronary arteries, resulting in the formation of a fibrous plaque that protrudes into the arterial lumen. ACS occur when there is a rupture of vulnerable plaque and consequent activation and aggregation of platelets, leading to the formation of a blood clot (thrombus) that occludes the artery and prevents blood flow to the heart [13]. Emotional stress activates the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal axis, resulting in respective increases in blood pressure (BP) and circulating levels of catecholamines and glucocorticoids (cortisol) [14]. Acute elevations in BP can provoke plaque rupture by disrupting blood flow across the diseased vessel and increasing endothelial shear stress [15], and glucocorticoids regulate a number of processes involved in plaque stability including vascular endothelial function and inflammation [16]. Circulating levels of inflammatory cytokines are also up-regulated during stress, and these molecules play a pivotal role in plaque rupture and thrombosis [15,17].
There is considerable evidence that healthy hostile individuals have heightened or prolonged cardiovascular and neuroendocrine responses to acute emotional stress [18]. However, studies relating hostility and inflammatory stress responses are sparse. Furthermore, little is known about the role of hostility in acute stress responses in patients with advanced cardiovascular disease. We set out to address these issues by investigating the relationship between trait hostility and physiological responses to laboratory stress in a sample of patients who had recently survived an ACS. We predicted that patients with elevated hostility scores would be more responsive to stress.
Methods
Participants
Thirty-four men who had recently survived an ACS were included in this study, details of which are reported elsewhere [19]. Participants were a subsample from the ACCENT study, an investigation of 295 patients admitted with ACS to four hospitals in the London area [5]. Inclusion criteria for the ACCENT study were as follows: a diagnosis of ACS based on the presence of chest pain plus verification by diagnostic electrocardiogram (EKG) and/or cardiac enzyme changes; the ability to recall a specific time of symptom onset and to complete a research interview in English; age between 18 and 90 years; and the absence of comorbid conditions that could influence either symptom presentation, mood, or troponin positivity (including severe psychiatric illness, ongoing infection, or inflammatory conditions). Patients were classified on the basis of EKG and biochemical markers to the different forms of ACS. Primary treatments were classified as percutaneous coronary intervention (PCI), medical, or coronary artery bypass graft (CABG).
For the current experiment, patients were recruited on average 444.9±172 days post-ACS. This time interval was selected to ensure that the patients' clinical condition had stabilized. Patients who had experienced a second ACS or cardiac intervention during the previous 6 months or had severely impaired left ventricular function (<40%) or ongoing symptoms of chest pain or breathlessness were excluded. Patients who were aged over 80, were hypertensive, taking antidepressants, color blind, had a history of late ventricular dysrhythmia, atrial fibrillation, or developed new comorbidities that could affect hemodynamic or inflammatory measures were also excluded. Since the ACCENT study included an insufficient number of women, only men were included in the current investigation. There were 231 men in the ACCENT study, of whom three were >80 years old, and 21 experienced onset of ACS after they had been asleep for at least 2 h, so could not provide information about the circumstances surrounding ACS onset, an additional focus of our research [19]. Of the remainder, 12 lived outside the country or too far away for participation; 50 were hypertensive; five were deceased; 14 were lost to follow-up; 44 had relevant comorbidities, persisting symptoms, or very poor left ventricular function; and one was blind. Of the 82 subjects eligible for participation, 34 were tested in the time available to complete the study. Patients who participated in psychophysiological stress testing were younger on average than those who were excluded (P=.023) but did not differ in clinical presentation, medical management, number of diseased coronary arteries, or other cardiological or sociodemographic characteristics. All patients gave written consent, and the study was approved by the medical research ethics committees of University College London Hospital, St. George's Hospital, and Southend Hospital, in London.
Psychological measures
Hostility was measured using a 39-item abbreviated version of the Cook Medley Hostility Scale (CMHS) [6]. The CMHS is the most widely used self-reported measure of hostility and is reported to have good psychometric properties including adequate internal validity, good test–retest reliability, and construct validity [20,21]. Each item was scored on a four-point scale from 1=strongly disagree to 4=strongly agree, and measured trait tendencies towards cynical, mistrustful attitudes towards others, and, to a lesser extent, the individual's propensity for aggressive responding and experiencing hostile affects. Total scores could range from 39 to 156, with higher scores indicating greater hostility, and the Cronbach α in this sample was 0.90. Anxiety and depression were assessed using the Hospital Anxiety and Depression scale, a well-validated instrument specifically designed to assess mood in medical patients [22]. Each scale consists of seven items rated on four-point scales, and total scores can range from 0 to 21. Cronbach α scores were 0.80 and 0.77 for the anxiety and depression scales, respectively.
Laboratory stress testing procedure
Participants were tested individually in the morning, commencing at 9 a.m. They were instructed to avoid eating a high-fat or high-protein breakfast, and to refrain from caffeinated beverages, alcohol, and excessive exercise during the 12 h prior to the session. Following consultation with physicians, 18 patients were withdrawn from medication prior to the session and stopped taking aspirin 10 days before testing and β-blockers, statins, or angiotensin-converting enzyme (ACE) inhibitors 72 h before testing. The remaining 16 continued to take some form of medication at the time of testing (Table 1).
Table 1.
Participant characteristics (N=34)
| Age | 55.9±9.3 |
| Ethnicity (white European) | 29 (85.3 %) |
| Education level | |
| Less than high school | 22 (64.7%) |
| High school, college | 12 (35.3%) |
| Body mass index (kg/m2) | 29.4±4.1 |
| Waist–hip ratio | 1.01±0.51 |
| Cook Medley Hostility | 91.0±11.8 |
| Depression | 3.88±3.3 |
| Anxiety | 5.03±3.4 |
| Current smokers | 7 (20.6%) |
| Number of diseased vessels | 1.5±0.79 |
| ACS | |
| STEMI | 24 (70.6%) |
| NSTEMI and UA | 10 (29.4%) |
| Treatment | |
| Medical | 5 (15.2%) |
| PCI | 23 (69.7%) |
| CABG | 5 (15.2%) |
| β-Blockers at time of testing | 16 (47.1%) |
| Statins at time of testing | 11 (32.4%) |
| ACE inhibitors at time of testing | 8 (23.5%) |
| Aspirin at time of testing | 11 (32.4%) |
Values are means±standard deviations, and numbers (percentages) for categorical variables.
Anthropometric measures were obtained using standardized methods, then participants were seated comfortably and fitted with finger cuffs so that BP and heart rate could be continuously monitored using a Portapres-2 device (TNO-TPD Biomedical Instrumentation, Amsterdam, Holland) [23]. A venous cannula was inserted in the lower arm for blood sampling, and the participant rested for 30 min. Blood pressure and heart rate were recorded for the last 5 min of the rest period, after which participants provided a salivary cortisol sample, and a baseline blood sample was drawn. Participants then completed two 5-min behavioral tasks, administered in a fixed order. The first was a computerized color-word interference task, involving the successive presentation of target color words printed in an incongruous color. The task was to press a computer key that corresponded to the position at the bottom of the screen of the name of the color in which the target word was printed. The second task was presented after an interval of 5 min and involved simulated public speaking. Participants were presented with a hypothetical scenario in which they had been wrongly accused of shoplifting and were instructed to give a speech in their defense. They were told that their speech would be video recorded and later judged for efficacy and fluency. Five-minute recordings of BP and heart rate were made during each of the tasks, and a second cortisol and blood sample were obtained immediately after the second task. Participants then rested quietly for the remainder of the session. They were asked to rate subjective feelings of stress on a seven-point scale from 1=low to 7=high at baseline (towards the end of the rest period), following each of the tasks and then at 25, 70, and 115 min post-task. Ratings of task difficulty, controllability, and involvement were also made after each task on seven-point scales. Further recordings of BP and heart rate were made at 25–30, 70–75, and 115–120 min post-tasks, and blood samples were obtained at 30, 75, and 120 min post-tasks. Salivary cortisol was sampled at 15, 30, 75, and 120 min post-tasks.
Cortisol measurement
Saliva samples were collected using Salivettes (Sarstedt, Leicester, UK) and stored at −80°C prior to analyses. Salivary cortisol was analyzed in randomized, duplicate samples using a commercially available time-resolved immunoassay with chemiluminescence detection (CLIA; IBL-Hamburg, Hamburg, Germany), at the Technical University of Dresden, Germany. This assay had a detection limit of 0.16 ng/ml and intra- and interassay coefficients of variation (CVs) of <10% and <12%, respectively. The experimenter performing the assay was blinded to participant details. Salivary cortisol levels are highly correlated with circulating levels of free cortisol and are routinely used as a biomarker of psychological stress [24].
Interleukin-6 measurement
Whole blood (10 ml) samples were drawn using 21-gauge Butterfly needles into Vacutainer tubes containing EDTA as anticoagulant, then centrifuged immediately at 1250×g for 10 min at room temperature. Plasma was removed, aliquoted, and frozen at −80°C prior to analysis. Plasma interleukin-6 (IL-6) concentrations were assessed in randomized, duplicate samples using a high-sensitivity two-site ELISA from R&D Systems (Oxford, UK). The detection limit of this assay was 0.09 pg/ml, with intra- and interassay CVs of 5.3% and 9.2%, respectively. The experimenter running this assay was blinded to participant details.
Statistical analyses
All analyses were performed using SPSS version 14.0 (SPSS, Chicago, IL, USA). Some data were lost due to equipment or assay failures, so analyses were performed on 32 patients for cortisol and 31 patients for BP, heart rate, and IL-6. Other variables were measured in all 34 participants. Responses to mental stress testing were analyzed using repeated measures analysis of variance, with post hoc comparisons using Tukey's LSD test. Cardiovascular variables were analyzed across five trials (baseline, task, 30 min, 75 min, and 120 min), IL-6 across four trials (baseline, 30 min, 75 min, and 120 min post-task), and cortisol across six trials (baseline; immediately post-task; and 15, 30, 75, and 120 min post-task). Associations with hostility were analyzed using multiple regression. For baseline measures, hostility was entered into the regression models along with age, BMI, smoking status, and medication status as covariates. The use of medications was overlapping, since all patients who were taking aspirin, statins, or ACE inhibitors were also taking β-blockers. Consequently, medication status was indexed by the use of β-blockers. Replacing BMI with waist/hip ratio as an indicator of adiposity did not alter the results. Associations of hostility with stress reactivity and recovery involved regressions onto changes between baseline and task or post-task values, and included the baseline level of the dependent variable as an additional covariate. All tests were two tailed.
Results
Participant characteristics
Participant characteristics are presented in Table 1. Men in this study had a mean age of 55.9 (S.D. 9.3). They were predominantly white, with a limited education level. A significant number were overweight and approximately one fifth smoked. Twenty-four participants (70.6%) were admitted with STEMI and the remainder with NSTEMI or UA. Several participants continued to take medication at the time of testing with just under half on β-blockers, around a quarter on ACE inhibitors, and approximately one third on statins or aspirin. Participants' mean score on the CMHS was 91.0 (S.D. 11.8). Hostility was not related to age, education level, ethnicity, BMI, waist–hip ratio, or smoking status, nor was it related to clinical variables including type of ACS, number of diseased vessels, treatment plan, or medication status at the time of testing. Hostility scores were positively correlated with depression (r=0.38, P=.029) but not with anxiety (r=0.22, P=.23).
Stress responses
Details of participants' subjective and physiological stress responses are presented in Table 2. Participants rated both tasks as difficult and involving, with mean ratings of 5.26±1.3 and 5.35±1.4, respectively, for the color-word and 4.50±1.4 and 4.38±1.9 for the speech task. There were significant main effects of trial for perceived stress levels [F(4,128)=63.6, P<.001], systolic BP [F(4,120)=32.3, P<.001], diastolic BP [F(4,120)=25.4, P<.001], heart rate [F(4,116)=50.4, P<.001], and cortisol [F(5,155)=19.1, P<.001]. Perceived stress levels increased during each of the tasks, returning to low levels during recovery. Participants' BP also increased significantly during tasks, with an average rise of 33.3±18.8 mmHg in systolic BP and 16.8±9.4 mmHg in diastolic BP, respectively. Both systolic and diastolic BP remained elevated above baseline levels during the 2-h recovery period. Similarly, heart rate increased substantially in response to tasks, with an average rise of 8.2±5.5 bpm. There were large individual differences in cardiovascular stress responses with changes in systolic BP ranging from −17.8 to +73.91 mmHg during tasks and from −19.8 to +29.9 mmHg at 2 h post-task. Similarly, diastolic BP responses ranged from −5.11 to +42.6 mmHg during tasks. Salivary cortisol levels increased following tasks, peaking at 15 min post-task. Although the average increase in plasma IL-6 was not significant, there were marked individual differences in this response, with changes in IL-6 ranging from −1.38 to +1.56 pg/ml at 75 min and −1.34 to +1.68 pg/ml at 120 min poststress. There were no significant associations between IL-6 measures and any of the cardiovascular responses. However, there was an inverse relationship between cortisol and IL-6 responses; the cortisol change at 75 min was inversely correlated with increases in IL-6 at 75 min (r=−0.49, P=.009) and 120 min (r=−0.51, P=.006).
Table 2.
Cardiovascular, inflammatory, and subjective stress responses
| Baseline | Tasks | Immediately post-task | 15 min post-task | 30 min post-task | 75 min post-task | 120 min post-task | |
|---|---|---|---|---|---|---|---|
| Systolic BP (mmHg) | 118.6±3.8a | 151.9±4.3b | 133.0±4.3c | 135.0±4.3c | 128.0±4.1c | ||
| Diastolic BP (mmHg) | 74.7±2.0a | 91.5±2.2b | 84.0±2.1c | 85.3±2.2c | 82.5±2.2c | ||
| Heart rate (bpm) | 62.8±1.9a | 71.0±2.2b | 61.1±1.8c | 61.4±1.7c | 60.6±1.7c | ||
| IL-6 (pg/ml) | 1.96±0.21a | 2.16± 0.23a | 2.30±0.28a | 2.23±0.24a | |||
| Cortisol (nmol/l) | 7.99±0.81a | 10.38±1.1b | 13.36±1.6c | 8.98±0.95a,b | 5.30±0.42d | 5.36±0.62d | |
| Subjective stress rating | 1.61±0.16a | 3.77±0.17b | 1.61±0.16a | 1.30±0.13a | 1.39±0.17a |
Values are means±S.E. Stress responses were analyzed using repeated measures analysis of variance, with post hoc comparisons using Tukey's LSD test. Trials in rows with different superscripts are significantly different from one another (P<.05).
Relationship between trait hostility and subjective and physiological parameters
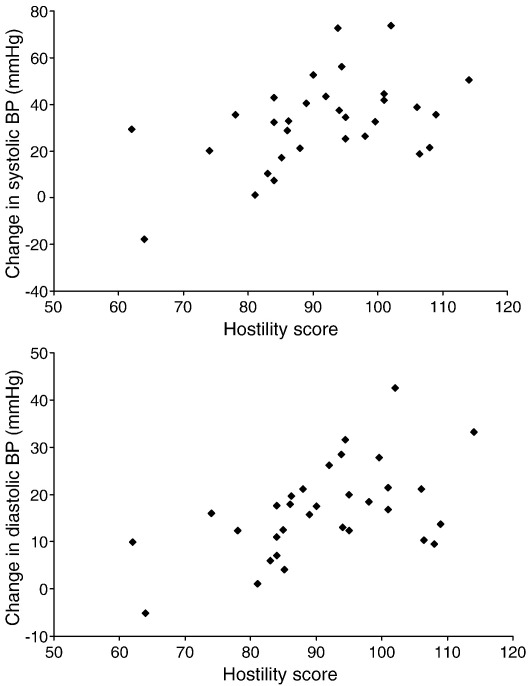
There was no association between hostility and baseline levels of subjective stress, BP, heart rate, salivary cortisol, or plasma IL-6 concentrations. However, multiple regression analysis revealed a significant relationship between trait hostility and systolic BP reactivity to tasks (β=0.358, P=.048). The variance accounted for by the complete model (r2) was 0.32, and the variance accounted for by hostility (Δr2) was 0.11. A similar relationship was found between hostility and diastolic BP reactivity to tasks (β=0.476, P=.007, r2=0.37, Δr2=0.21). These associations, illustrated in Fig. 1, were independent of age, BMI, smoking status, medication at the time of testing, and baseline levels of the respective dependent variable. Hostility was not related to either heart rate reactivity or cortisol reactivity.
Fig. 1.
Changes in systolic BP (upper panel) and diastolic BP (lower panel) between baseline and task trials in relation to hostility scores.
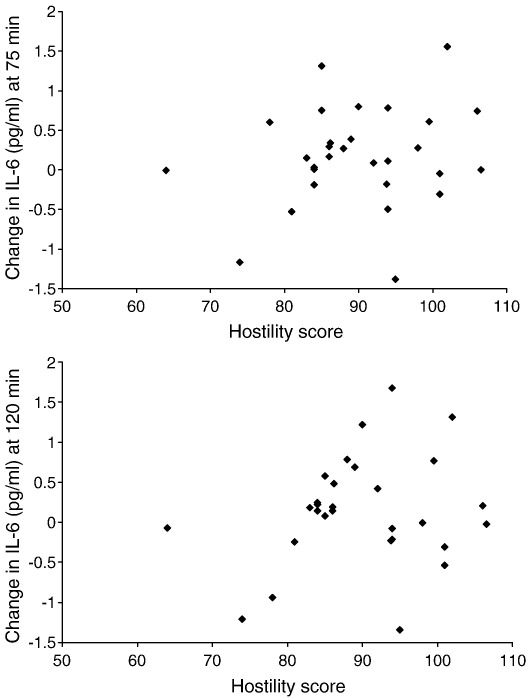
Regression analyses also revealed a significant association between hostility and prolonged increases in systolic BP at 2 h post-task (β=0.400, P=.024, r2=.38, Δr2=0.15) and between hostility and elevated plasma IL-6 concentrations at both 75 min post-task (β=0.309, P=.023, r2=.63, Δr2=0.09) and 2 h post-task (β=0.327, P=.016, r2=.64, Δr2=0.10). By contrast, hostility was negatively correlated with cortisol levels at 75 min post-task (β=−0.325, P=.034, r2=.64, Δr2=0.10) and was unrelated to heart rate recovery. As above, all associations were independent of age, BMI, smoking status, medication at the time of testing, and baseline levels of the respective dependent variable. The relationship between hostility and IL-6 responses is illustrated in Fig. 2, showing larger increases in IL-6 at 75 and 120 min post-task in more hostile individuals.
Fig. 2.
Changes in plasma IL-6 concentrations between baseline and 75 min (upper panel) and 2 h (lower panel) in relation to hostility scores.
There was no association between hostility and subjective task appraisal, or ratings of perceived stress, or between subjective stress responses and BP or cytokine responses, indicating that heightened/prolonged stress reactivity in high-hostile men was not due to differences in the subjective experience of the tasks. Similarly, depression and anxiety had no impact on the associations between hostility and biological responses (data not shown).
Discussion
One of the main findings of our study was that ACS patients scoring higher on the CMHS had larger increases in systolic and diastolic BP during acute laboratory stress, as well as a sustained increase in systolic BP following cessation of the stress. These results add to the large body of previous research demonstrating excessive and prolonged cardiovascular reactivity to stressful situations among healthy hostile individuals. Specifically, studies have shown that high hostile men and women display larger increases in BP and heart rate as well as elevated circulating levels of catecholamines, during and following exposure to acute laboratory stressors [18,25–27]. Our study extends these observations to a diseased patient sample.
The other main study finding was that hostile ACS patients had heightened poststress levels of IL-6. This suggests that hostility may promote inflammation, although the cross-sectional design prevents causal conclusions from being drawn. A number of previous reports have found positive correlations between hostility and basal circulating levels of IL-6 in healthy humans. Total CMHS scores were associated with elevated plasma IL-6 concentrations in a small study of healthy men [28]. More recently, a larger cross-sectional analysis of 6814 adults aged 45–84 years found a positive graded relationship between serum IL-6 and scores of cynical distrust, a component of hostility assessed using a subscale of the CMHS [29], and comparable associations were observed between circulating IL-6 and scores on the hostile affect, cynicism, and aggressive responding subscales of the CMHS in samples of middle-aged men and women [30,31]. However, results have been variable, with some studies reporting no association between hostility and IL-6 levels in healthy adults [32], and others reporting an association only in individuals with concurrently high or low levels of depressive symptoms [33–35]. In the present investigation, hostility was unrelated to basal IL-6 concentrations and the association between hostility and IL-6 stress responses was independent of depressive symptoms. Differences between study findings may be due to heterogeneity in the hostility measure used, the specific population studied and/or the presence of other moderating psychosocial factors. To our knowledge, only one previous investigation has examined the relationship between hostility and inflammatory stress responses. Kiecolt-Glaser et al. [36] found that couples with a high frequency of hostile marital interactions, ‘high-hostile couples’, developed larger increases in plasma levels of IL-6 and TNFα the morning after a conflictual interaction task, compared with low-hostile couples. Supporting our findings, these results suggest that hostile individuals may be more susceptible to stress-induced inflammation.
The mechanisms driving the association between hostility and acute stress responses are unclear. Excessive sympathetic nervous activity has been linked to β-adrenergic receptor (β-AR) down-regulation in animals, and a number of studies have related hostility to blunted cardiac and vascular β-AR sensitivity in humans [37,38]. Since β-AR activation plays a central role in the healthy vasodilatory response of blood vessels to stress, a blunted adrenergic response, promoting vasoconstriction, would result in an increase in total peripheral resistance and elevated BP. There is also evidence for diminished stress-induced parasympathetic nervous activity in hostile individuals, and this ‘autonomic imbalance’ likely contributes to heightened cardiovascular reactivity, due to a reduction in cardiac autonomic control [27,39]. Lastly, glucocorticoids inhibit inflammatory cytokine production by monocytes [16] and evidence suggests that high cortisol responders have smaller inflammatory cytokine responses to acute psychological stress [40]. In the current investigation, hostile patients had lower cortisol levels at 75 min poststress, and cortisol responses were inversely related to IL-6 stress responses. Thus diminished cortisol levels in hostile patients might also facilitate a heightened inflammatory response.
Prospective evidence in healthy humans has shown that heightened and/or prolonged BP reactivity to acute laboratory stress is predictive of future hypertension and progression of preclinical cardiovascular disease states [41,42]. However, the data relating acute stress reactivity with clinical CAD are less convincing, and most studies in healthy individuals have found no relationship between BP responses and future cardiac events or mortality [41]. Acute cardiovascular and inflammatory stress responses may be more relevant in people with advanced cardiovascular disease. Evidence suggests that, whereas healthy coronary vessels dilate in response to stress-induced SNS activation, atherosclerotic vessels constrict, leading to increased peripheral vascular resistance and myocardial ischemia [43]. In addition, IL-6 stimulates a number of key processes that contribute to plaque rupture and thrombosis including macrophage production of matrix metalloproteinases (enzymes that degrade the plaque's protective fibrous cap), monocyte production of tissue factor (a key initiator of blood coagulation in ACS), and hepatic synthesis of fibrinogen (an acute phase protein central to blood coagulation and platelet aggregation) [13,44]. Plasma IL-6 levels are significantly elevated in ACS patients compared to patients with stable CAD or healthy age-matched controls [45,46], and blood IL-6 levels assessed at or near to the time of hospital admission are a significant independent predictor of subsequent coronary events and mortality [46,47].
The number of patients suitable for this investigation was limited and the sample size was small. We therefore decided not to randomize patients to stress and control conditions but to apply the stressors to all participants. We and others have previously shown that repeated measurements or the passage of time in the absence of behavioral tasks does not lead to elevations of cardiovascular and inflammatory markers [15,17]. Nevertheless, it would have been preferable to include a comparison control group of healthy individuals without ACS, to test whether our observed associations between hostility and stress responses were specific to (or strengthened by) the presence of advanced CAD. The majority of participants were poorly educated men of white European origin, and results may not apply to other groups. Several were overweight and current smokers, and the analysis was complicated by the inclusion of patients whose medications had been withdrawn and those individuals who continued to take medication at the time of testing. A number of previous studies examining the effects of medication on physiological stress reactivity in humans found no effect of aspirin or β-blockers on either cardiovascular or cortisol responses to stress [48–50]. However, one recent study showed that aspirin attenuated IL-6 stress responses [49] and an effect of medication on stress reactivity cannot be excluded. Due to the cross-sectional nature of our study, the directionality of the relationship between hostility and cardiovascular and cytokine responses cannot be determined. The laboratory tasks used were not optimal for evoking anger, and it would have been useful to include a measure of state anger, given the evidence relating anger and emotional triggering of ACS. Lastly, the CMHS is a self-report measure and there is evidence that other hostility measures such as behavioral ratings or ratings by significant others may have more predictive utility in studies relating personality and cardiovascular risk [9,51].
To our knowledge, this is the first study to directly associate hostility with inflammatory and cardiovascular stress responses in ACS patients. Our findings indicate that hostile individuals with advanced cardiovascular disease are particularly susceptible to stress-induced increases in sympathetic activity and inflammation. These mechanisms may contribute to an elevated risk of emotionally triggered cardiac events in such patients.
Acknowledgments
We are grateful to Professor John Martin (Centre for Cardiovascular Biology and Medicine, UCL) for his support for the study and to the staff and patients of University College Hospital, St. George's Hospital, and Southend Hospital.
Footnotes
This research was funded by the British Heart Foundation.
References
- 1.Sheridan PJ, Crossman DC. Critical review of unstable angina and non-ST elevation myocardial infarction. Postgrad Med J. 2002;78:717–726. doi: 10.1136/pmj.78.926.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, Friedman R, Benson H, Muller JE. Triggering of acute myocardial infarction onset by episodes of anger. Determinants of Myocardial Infarction Onset Study Investigators. Circulation. 1995;92:1720–1725. doi: 10.1161/01.cir.92.7.1720. [DOI] [PubMed] [Google Scholar]
- 3.Mittleman MA, Maclure M, Nachnani M, Sherwood JB, Muller JE. Educational attainment, anger, and the risk of triggering myocardial infarction onset. The Determinants of Myocardial Infarction Onset Study Investigators. Arch Intern Med. 1997;157:769–775. [PubMed] [Google Scholar]
- 4.Moller J, Hallqvist J, Diderichsen F, Theorell T, Reuterwall C, Ahlbom A. Do episodes of anger trigger myocardial infarction? A case-crossover analysis in the Stockholm Heart Epidemiology Program (SHEEP) Psychosom Med. 1999;61:842–849. doi: 10.1097/00006842-199911000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Strike PC, Perkins-Porras L, Whitehead DL, McEwan J, Steptoe A. Triggering of acute coronary syndromes by physical exertion and anger: clinical and sociodemographic characteristics. Heart. 2006;92:1035–1040. doi: 10.1136/hrt.2005.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook WW, Medley DM. Proposed hostility and pharisaic virtue scales for the MMPI. J Appl Psychol. 1954;38:414–418. [Google Scholar]
- 7.Miller TQ, Smith TW, Turner CW, Guijarro ML, Hallet AJ. A meta-analytic review of research on hostility and physical health. Psychol Bull. 1996;119:322–348. doi: 10.1037/0033-2909.119.2.322. [DOI] [PubMed] [Google Scholar]
- 8.Everson SA, Kauhanen J, Kaplan GA, Goldberg DE, Julkunen J, Tuomilehto J, Salonen JT. Hostility and increased risk of mortality and acute myocardial infarction: the mediating role of behavioral risk factors. Am J Epidemiol. 1997;146:142–152. doi: 10.1093/oxfordjournals.aje.a009245. [DOI] [PubMed] [Google Scholar]
- 9.Matthews KA, Gump BB, Harris KF, Haney TL, Barefoot JC. Hostile behaviors predict cardiovascular mortality among men enrolled in the Multiple Risk Factor Intervention Trial. Circulation. 2004;109:66–70. doi: 10.1161/01.CIR.0000105766.33142.13. [DOI] [PubMed] [Google Scholar]
- 10.Niaura R, Todaro JF, Stroud L, Spiro A, Ward KD, Weiss S. Hostility, the metabolic syndrome, and incident coronary heart disease. Health Psychol. 2002;21:588–593. doi: 10.1037//0278-6133.21.6.588. [DOI] [PubMed] [Google Scholar]
- 11.Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. J Am Coll Cardiol. 2009;53:936–946. doi: 10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 12.Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis of coronary heart disease. Systematic review of prospective cohort studies. BMJ. 1999;318:1460–1467. doi: 10.1136/bmj.318.7196.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 14.Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res. 2002;52:1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 15.Steptoe A, Brydon L. Psychosocial factors and coronary heart disease: the role of psychoneuroimmunological processes. In: Ader R, editor. 4th ed. Vol 2. Elsevier Academic Press; San Diego: 2007. pp. 945–974. (Psychoneuroimmunology). [Google Scholar]
- 16.Girod JP, Brotman DJ. Does altered glucocorticoid homeostasis increase cardiovascular risk? Cardiovasc Res. 2004;64:217–226. doi: 10.1016/j.cardiores.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Smith TW, Glazer K, Ruiz JM, Gallo LC. Hostility, anger, aggressiveness, and coronary heart disease: an interpersonal perspective on personality, emotion, and health. J Pers. 2004;72:1217–1270. doi: 10.1111/j.1467-6494.2004.00296.x. [DOI] [PubMed] [Google Scholar]
- 19.Strike PC, Magid K, Whitehead DL, Brydon L, Bhattacharyya MR, Steptoe A. Pathophysiological processes underlying emotional triggering of acute cardiac events. Proc Natl Acad Sci U S A. 2006;103:4322–4327. doi: 10.1073/pnas.0507097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contrada RJ, Jussim L. What does the Cook-Medley hostility scale measure? In search of an adequate measurement model. J Appl Psychol. 1992;22:615–627. [Google Scholar]
- 21.Smith TW, Frohm KD. What's so unhealthy about hostility? Construct validity and psychosocial correlates of the Cook and Medley Ho scale. Health Psychol. 1985;4:503–520. doi: 10.1037//0278-6133.4.6.503. [DOI] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 23.Imholz BP, Langewouters GJ, van Montfrans GA, Parati G, van Goudoever J, Wesseling KH, Wieling W, Mancia G. Feasibility of ambulatory, continuous 24-hour finger arterial pressure recording. Hypertension. 1993;21:65–73. doi: 10.1161/01.hyp.21.1.65. [DOI] [PubMed] [Google Scholar]
- 24.Hellhammer DH, Wust S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Suarez EC, Kuhn CM, Schanberg SM, Williams RB, Zimmermann EA. Neuroendocrine, cardiovascular, and emotional responses of hostile men: the role of interpersonal challenge. Psychosom Med. 1998;60:78–88. doi: 10.1097/00006842-199801000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Smith TW. Hostility and health: current status of a psychosomatic hypothesis. Health Psychol. 1992;11:139–150. doi: 10.1037//0278-6133.11.3.139. [DOI] [PubMed] [Google Scholar]
- 27.Brosschot JF, Thayer JF. Anger inhibition, cardiovascular recovery, and vagal function: a model of the link between hostility and cardiovascular disease. Ann Behav Med. 1998;20:326–332. doi: 10.1007/BF02886382. [DOI] [PubMed] [Google Scholar]
- 28.Suarez EC. Plasma interleukin-6 is associated with psychological coronary risk factors: moderation by use of multivitamin supplements. Brain Behav Immun. 2003;17:296–303. doi: 10.1016/s0889-1591(03)00059-x. [DOI] [PubMed] [Google Scholar]
- 29.Ranjit N, ez-Roux AV, Shea S, Cushman M, Seeman T, Jackson SA, Ni H. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2007;167:174–181. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- 30.Marsland AL, Prather AA, Petersen KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain Behav Immun. 2008;22:753–761. doi: 10.1016/j.bbi.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sjogren E, Leanderson P, Kristenson M, Ernerudh J. Interleukin-6 levels in relation to psychosocial factors: studies on serum, saliva, and in vitro production by blood mononuclear cells. Brain Behav Immun. 2006;20:270–278. doi: 10.1016/j.bbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Graham JE, Robles TF, Kiecolt-Glaser JK, Malarkey WB, Bissell MG, Glaser R. Hostility and pain are related to inflammation in older adults. Brain Behav Immun. 2006;20:389–400. doi: 10.1016/j.bbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Suarez EC. Joint effect of hostility and severity of depressive symptoms on plasma interleukin-6 concentration. Psychosom Med. 2003;65:523–527. doi: 10.1097/01.psy.0000062530.94551.ea. [DOI] [PubMed] [Google Scholar]
- 34.Stewart JC, Janicki-Deverts D, Muldoon MF, Kamarck TW. Depressive symptoms moderate the influence of hostility on serum interleukin-6 and C-reactive protein. Psychosom Med. 2008;70:197–204. doi: 10.1097/PSY.0b013e3181642a0b. [DOI] [PubMed] [Google Scholar]
- 35.Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Cynical hostility, depressive symptoms, and the expression of inflammatory risk markers for coronary heart disease. J Behav Med. 2003;26:501–515. doi: 10.1023/a:1026273817984. [DOI] [PubMed] [Google Scholar]
- 36.Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 37.Suarez EC, Sherwood A, Hinderliter AL. Hostility and adrenergic receptor responsiveness: evidence of reduced beta-receptor responsiveness in high hostile men. J Psychosom Res. 1998;44:261–267. doi: 10.1016/s0022-3999(97)00201-8. [DOI] [PubMed] [Google Scholar]
- 38.Sherwood A, Hughes JW, Kuhn C, Hinderliter AL. Hostility is related to blunted beta-adrenergic receptor responsiveness among middle-aged women. Psychosom Med. 2004;66:507–513. doi: 10.1097/01.psy.0000132876.95620.04. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz JM, Uchino BN, Smith TW. Hostility and sex differences in the magnitude, duration, and determinants of heart rate response to forehead cold pressor: parasympathetic aspects of risk. Int J Psychophysiol. 2006;60:274–283. doi: 10.1016/j.ijpsycho.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain Behav Immun. 2003;17:373–383. doi: 10.1016/s0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 41.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Steptoe A, Marmot M. Impaired cardiovascular recovery following stress predicts 3-year increases in blood pressure. J Hypertens. 2005;23:529–536. doi: 10.1097/01.hjh.0000160208.66405.a8. [DOI] [PubMed] [Google Scholar]
- 43.Strike PC, Steptoe A. Systematic review of mental stress-induced myocardial ischaemia. Eur Heart J. 2003;24:690–703. doi: 10.1016/s0195-668x(02)00615-2. [DOI] [PubMed] [Google Scholar]
- 44.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 45.Biasucci LM, Vitelli A, Liuzzo G, Altamura S, Caligiuri G, Monaco C, Rebuzzi AG, Ciliberto G, Maseri A. Elevated levels of interleukin-6 in unstable angina. Circulation. 1996;94:874–877. doi: 10.1161/01.cir.94.5.874. [DOI] [PubMed] [Google Scholar]
- 46.Biasucci LM, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, Dinarello CA, Maseri A. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99:2079–2084. doi: 10.1161/01.cir.99.16.2079. [DOI] [PubMed] [Google Scholar]
- 47.Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. JAMA. 2001;286:2107–2113. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- 48.Mills PJ, Dimsdale JE. Cardiovascular reactivity to psychosocial stressors. A review of the effects of beta-blockade. Psychosomatics. 1991;32:209–220. doi: 10.1016/S0033-3182(91)72094-X. [DOI] [PubMed] [Google Scholar]
- 49.von Kanel R, Kudielka BM, Metzenthin P, Helfricht S, Preckel D, Haeberli A, Stutz M, Fischer JE. Aspirin, but not propranolol, attenuates the acute stress-induced increase in circulating levels of interleukin-6: a randomized, double-blind, placebo-controlled study. Brain Behav Immun. 2008;22:150–157. doi: 10.1016/j.bbi.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Kudielka BM, Fischer JE, Metzenthin P, Helfricht S, Preckel D, von Kanel R. No effect of 5-day treatment with acetylsalicylic acid (aspirin) or the beta-blocker propranolol (Inderal) on free cortisol responses to acute psychosocial stress: a randomized double-blind, placebo-controlled study. Neuropsychobiology. 2007;56:159–166. doi: 10.1159/000115783. [DOI] [PubMed] [Google Scholar]
- 51.Smith TW, Uchino BN, Berg CA, Florsheim P, Pearce G, Hawkins M, Henry NJ, Beveridge RM, Skinner MA, Hopkins PN, Yoon HC. Associations of self-reports versus spouse ratings of negative affectivity, dominance, and affiliation with coronary artery disease: where should we look and who should we ask when studying personality and health? Health Psychol. 2008;27:676–684. doi: 10.1037/0278-6133.27.6.676. [DOI] [PubMed] [Google Scholar]