Sublingual isosorbide dinitrate improves three-dimensional coronary MR imaging signal-to-noise ratios by 20% for targeted acquisitions and by 10% for whole-heart acquisitions.
Abstract
Purpose:
To quantify the effect of sublingual isosorbide dinitrate (ISDN) administration on coronary magnetic resonance (MR) imaging.
Materials and Methods:
Written informed consent was obtained from all participants, and the HIPAA-compliant protocol was approved by the Institutional Review Board. Coronary MR imaging was performed at 1.5 T before and after administration of ISDN (2.5 or 5 mg) in 25 healthy adult volunteers (mean age, 23 years ± 4; nine men, 16 women) with three-dimensional targeted (n = 20, randomized into four groups) or whole-heart (n = 5) acquisitions with gradient-recalled echo (GRE) or balanced steady-state free precession (SSFP) sequences. Image quality was assessed by two cardiologists on a four-point scale. Signal-to-noise ratio (SNR), vessel diameter, and vessel sharpness were characterized. A linear mixed-effects model was used for data analysis. A P value of less than .05 was considered to indicate a significant difference.
Results:
The maximum SNR enhancement with 5 mg of ISDN (GRE: 22.0% ± 10.7%; SSFP: 20.1% ± 6.0%) was similar (P > .05) to that with 2.5 mg (GRE: 21.9% ± 5.4%; SSFP: 19.1% ± 3.0%). However, the time to maximum SNR enhancement for the 5-mg dose (15.5 minutes ± 6.0) was earlier (P < .01) than that for 2.5 mg (23.5 minutes ± 6.7). The increase in vessel diameter with 5 mg of ISDN was greater than that with 2.5 mg (P < .05 for both GRE and SSFP sequences). The coronary images were sharper after ISDN administration (P < .03). Subjective image quality score significantly improved after ISDN administration for GRE images (P < .05 for both doses) but was similar for SSFP images (P = .24 for 2.5 mg; P = .27 for 5 mg). Whole-heart coronary SNR was improved about 10% after ISDN administration (P < .05).
Conclusion:
Sublingual ISDN improves coronary MR imaging SNR. Practitioners need to consider the dose and temporal effects of ISDN when performing coronary MR imaging.
© RSNA, 2010
Introduction
Despite substantial progress in the past decade, coronary artery magnetic resonance (MR) imaging still faces multiple challenges owing to the relatively small diameter, tortuous nature, and nearly constant motion of the vessels. The high spatial resolution required to image stenoses in the coronary arteries makes coronary MR imaging a signal-to-noise ratio (SNR)-limited application. Recent technical development of new sequences (1–3), contrast generation mechanisms (4–8), and 3.0-T coronary MR imaging (2,4,9–11) has enabled improvements in SNR and image contrast. An increase in SNR can generally be used to improve resolution or to reduce respiratory and cardiac motion artifacts by shortening acquisition time.
In the past decade, three-dimensional (3D) coronary artery MR imaging sequences have largely replaced two-dimensional approaches because they provide volumetric coverage of the coronary arteries with high SNR (12). In the 3D targeted approach, separate thin (∼30-mm) 3D slabs are prescribed along major axes of the coronary arteries, and imaging is performed during free-breathing with a navigator echo to compensate for respiratory motion (6,13,14). As an alternative, large 3D slab coronary MR imaging methods provide whole-heart coverage in a single volume (15–18). Two types of 3D coronary artery MR imaging sequences that have been typically used are gradient-recalled echo (GRE) and balanced steady-state free precession (SSFP) (3,6). Owing to their higher SNR and blood-myocardium contrast compared with GRE sequences, SSFP sequences are widely used in 3D coronary artery MR imaging at 1.5 T (3,17). However, GRE sequences are more frequently used at higher field strengths because of the sensitivity of SSFP sequences to B0 inhomogeneity (4,9). Similar accuracy for the diagnosis of coronary artery disease has been reported (19,20) between these two techniques.
Despite the advantages in SNR and volumetric coverage compared with two-dimensional techniques, 3D techniques suffer from inflow saturation of coronary blood signal (21). In two-dimensional imaging, inflowing “fresh” spins provide blood signal enhancement. This effect is reduced in 3D imaging because the inflowing spins experience more radiofrequency pulses while within the imaging volume and, consequently, the blood signal is saturated, although this inflow saturation effect is more complex in SSFP imaging owing to its fully refocused gradients and steady state in both longitudinal and transverse magnetization. Therefore, an increase in coronary blood flow induced by vasodilators may be exploited to reduce the inflow saturation and improve SNR (22).
Vasodilators, such as isosorbide dinitrate (ISDN) and nitroglycerin, enhance coronary artery blood flow by providing exogenous nitric oxide that leads to relaxation of smooth muscle cells and, hence, vasodilation (23). The use of ISDN for coronary MR imaging was first reported in a multicenter trial (24). In that study, sublingual ISDN (2.5 mg) was administered prior to imaging with a free-breathing targeted thin-slab 3D GRE sequence, but, to our knowledge, the effect of ISDN and its timing have not been studied. Accordingly, we sought to investigate the effect of sublingual ISDN in 3D coronary MR imaging on objective measures of coronary blood SNR, vessel diameter, and vessel sharpness and overall subjective image quality as functions of time, dose, and imaging sequence.
Materials and Methods
Coronary MR imaging was performed in healthy adult volunteers before and after ISDN administration. Written informed consent was obtained from all participants, and the Health Insurance Portability and Accountability Act–compliant protocol was approved by our Institutional Review Board. All volunteers were imaged with a 1.5-T magnet (Achieva; Philips Healthcare, Best, the Netherlands) with a 32-channel cardiac array coil (Invivo, Gainesville, Fla) for which an electronic signal combiner was used to form a 16-channel signal (eight anterior and eight posterior) from the 32-channel coil array or with a five-channel synergy coil (Philips Healthcare). In each volunteer, the same coil was used for all studies. Blood pressure was noninvasively monitored every 15 minutes by using an automated sphygmomanometer (Dinamap; GE Healthcare, Milwaukee, Wis) with a cuff on the volunteer’s calf. Volunteers with systolic pressure lower than 100 mm Hg were given no more than 2.5 mg of ISDN to minimize the possibility of symptomatic hypotension.
Targeted 3D Study
A total of 20 adult clinically healthy volunteers (eight men [mean age, 24 years ± 3 {standard deviation}] and 12 women [mean age, 23 years ± 3]) without contraindications to MR imaging were included in our study. Scout images were acquired with an SSFP sequence with 3.12 × 3.12–mm in-plane resolution and 10-mm section thickness. A reference image set was acquired by using the body and phased-array coils so that each individual coil sensitivity map could be calculated (25). This was followed by an image set acquired with an axial breath-hold cine SSFP sequence (repetition time msec/echo time msec, 3.7/1.85; temporal resolution, 48 milliseconds; spatial resolution, 1.2 × 1.2 mm; acceleration rate, two) to visually identify (P.H., with 5 years experience) the quiescent period of the right coronary artery (RCA). The corresponding trigger delay was used in later sequences. A low-resolution coronary survey 3D volume was then acquired for localization and assignment of the appropriate imaging slab orientation (6).
Volunteers were randomly divided into four groups (A, B, C, and D), with five in each group, to investigate the effects of the imaging sequence and the ISDN dose. In groups A and B, RCA images were acquired with a 3D targeted thin-slab SSFP sequence with 2.5- or 5-mg doses of ISDN, respectively. In groups C and D, images were acquired with a GRE sequence with 2.5 or 5 mg of ISDN, respectively. For each group, baseline coronary imaging (GRE or SSFP) was performed after assignment of slab orientation, followed by sublingual administration of ISDN (2.5 or 5 mg). Imaging was started immediately after ISDN administration with the same sequence that was used for baseline imaging, and it was repeated five times with no time gap to study the time-course effect of ISDN. The initiation time for imaging session with respect to ISDN administration was recorded (P.H.). For groups C and D, a free-breathing 3D electrocardiographically gated GRE sequence was used (7.7/2.2; field of view, 270 × 270 × 30 mm; flip angle, 30°; spatial resolution, 0.7 × 1 × 1.5 mm reconstructed to 0.52 × 0.52 × 0.75 mm). The parameters for SSFP imaging in groups A and B were as follows: 4.6/2.3; field of view, 270 × 270 × 30 mm; flip angle, 90°; and spatial resolution, 1 × 1 × 1.5 mm reconstructed to 0.52 × 0.52 × 0.75 mm. A half-α preparation pulse was used with the SSFP imaging. For all groups, a two-dimensional spiral navigator echo, positioned on the right hemidiaphragm (6), was used for respiratory motion gating and tracking with a gating window of 5 mm. A T2 magnetization preparation sequence (echo time, 50 milliseconds) was also used to enhance blood-myocardium contrast (6,8). To obtain a more accurate SNR measurement, no parallel imaging was used for coronary MR imaging acquisitions.
Qualitative image measurements.—Qualitative analyses were performed by two cardiologists (W.J.M. and M.L.C., with more than 10 and 15 years experience with cardiac MR imaging, respectively) who were blinded to subject, ISDN dose, and sequence information. The images were randomized before presentation to the cardiologists, who graded the images during a single reading session. A four-point scale (24) was used to evaluate the diagnostic value of the images: 1 = poor, 2 = fair, 3 = good, and 4 = very good or excellent. For each image, separate scores were given for the proximal, middle, and distal segments of the RCA.
SNR measurements.—SNR was measured (P.H.) with the image analysis software on the imager console. To quantify SNR, a region of interest was selected outside of the chest wall, and the standard deviation of its pixel values was used as the noise. A polygonal region of interest was carefully drawn in the proximal RCA after four-fold magnification. The mean pixel value in the region of interest was used as the signal intensity of the coronary artery blood. SNR was calculated as the mean signal intensity from the coronary blood divided by the standard deviation of pixel values in the region of interest outside of the chest well (ie, noise). For consistency, the same regions of interest were used in all image sets for each patient, unless motion was detected between sets. The change in SNR was calculated according to the following equation: ΔSNR = (SNRISDN − SNRpre)/SNRpre, where SNRISDN is the SNR after administration of ISDN and SNRpre is the baseline SNR.
Because coronary MR imaging was performed five times after administration of ISDN, imaging was performed only in a targeted 3D slab along the long axis of the RCA. The left coronary artery (LCA) was partially visible in the RCA images. To study the effect of ISDN administration on the LCA system, the SNR in the proximal LCA was also measured by using a similar method.
Vessel diameter.—The raw data were imported into a 3D coronary image analysis tool (SoapBubble; Philips Healthcare) for quantitative measurement and visualization (26). The coronary artery was identified by using the vessel tracking tool, and the mean vessel diameter in the proximal 4 cm of the RCA was automatically calculated. The change in vessel diameter was calculated as: ΔD = (D ISDN − D pre)/D pre, where D ISDN is the mean vessel diameter after administration of ISDN and D pre is the mean vessel diameter at baseline.
Vessel sharpness.—The SoapBubble tool (26) was used to quantitatively evaluate the vessel definition with a Deriche algorithm (27). Vessel sharpness scores were calculated for both sides of the vessel, and final sharpness was defined as the mean. Higher sharpness scores indicate sharper vessel borders. The percentage change in vessel sharpness was calculated for each volunteer.
Whole-Heart Study
To study the effect of ISDN on thick-slab (10–12-cm) whole-heart SSFP images, five additional healthy volunteers (one man [24 years] and four women [mean age, 22 years ± 3]) were recruited and underwent whole-heart coronary MR imaging before and twice after administration of 5 mg of ISDN. Images were acquired with a free-breathing SSFP sequence (4.8/2.4; flip angle, 90°, field of view, 300 × 300 × 120 mm; isotropic spatial resolution, 1.3 × 1.3 × 1.3 mm reconstructed to 0.65 × 0.65 × 0.65 mm with zero-padding) with T2 magnetization preparation (echo time, 50 milliseconds) and spectrally selective fat saturation. SNR and vessel diameter changes in the proximal RCA and LCA were measured with the aforementioned methods. Because parallel imaging with an acceleration factor of two was used in the whole-heart study to reduce acquisition duration, each acquisition was followed by a noise acquisition with identical parameters but with radiofrequency excitation pulses disabled. The region of interest used in coronary artery signal intensity measurements was copied to the noise acquisition image to compensate for spatially varying g factor (28). SNR was calculated as the mean signal intensity from the coronary blood divided by the standard deviation of this measured noise.
Statistical Analysis
All measurements are presented as the mean ± 1 standard deviation. Software (SAS, version 8.01; SAS Institute, Cary, NC) was used for all statistical analyses. Owing to the repeated-measures design of our study (one acquisition before and five after administration of ISDN), the correlation induced by within-subject variance had to be taken into account in the analysis. We used a linear mixed-effects model with compound symmetry variance-covariance structure for the error matrix and linear contrasts (29) for comparing the SNR, vessel diameter, vessel sharpness, and qualitative image scores before and after ISDN administration. The model was adjusted for reader and coronary segment in the qualitative image score analysis. A two-tailed P value less than .05 was considered to indicate significance.
Results
All volunteers completed the study without complications. There was no significant difference between the mean ages of male and female volunteers. One volunteer did not receive the 5-mg ISDN dose because of low blood pressure. Mean blood pressure decreased significantly after ISDN administration (systolic: 129 mm Hg ± 16 vs 104 mm Hg ± 8; diastolic: 58 mm Hg ± 7 vs 50 mm Hg ± 7; both P < .05). Heart rate did not change significantly (68 beats per minute ± 10 vs 70 beats per minutes ± 11).
Targeted 3D Study
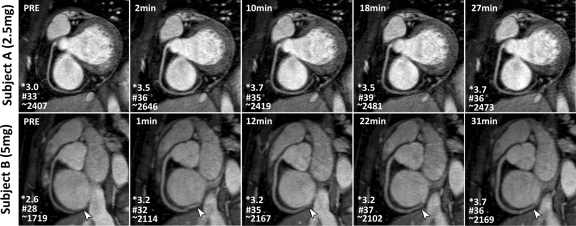
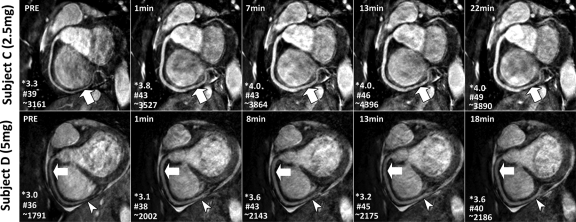
Image quality.—Figure 1 shows examples of RCA MR images obtained with a GRE sequence with two different doses of ISDN. All images are labeled with scan initiation time after ISDN administration. Corresponding images with an SSFP sequence in two other volunteers are shown in Figure 2. Vasodilation and image quality improvement versus baseline images can be observed for both ISDN doses with both GRE and SSFP sequences. In addition to improving the overall image quality, the SNR gain and vasodilation from ISDN administration also enabled better visualization of the distal segments of the coronary arteries (Figs 1, Figs 2).
Figure 1:
Multiplanar reconstruction RCA 3D free-breathing GRE MR images (7.7/2.2; flip angle, 30°) acquired before (PRE) and after administration of (top) 2.5 or (bottom) 5 mg of ISDN in two healthy volunteers. Time after ISDN administration appears on upper left of images. Increased vessel diameter and RCA signal enhancement were observed with both doses, especially distally (arrowheads). * = image quality score, # = SNR, ∼ = vessel sharpness score.
Figure 2:
RCA 3D free-breathing SSFP MR images (4.6/2.3; flip angle, 90°) acquired before (PRE) and after administration of (top) 2.5 or (bottom) 5 mg of ISDN in two healthy volunteers. Time after ISDN administration appears on upper left of images. Improved RCA vasodilation and signal enhancement (arrows) were observed on all images obtained after ISDN administration. Owing to enhanced SNR, distal segments were visualized better with ISDN (arrowheads). * = image quality score, # = SNR, ∼ = vessel sharpness score.
Subjective image quality scores improved significantly for GRE MR images after ISDN administration (P < .05 for both 2.5 [group C] and 5 [group D] mg), but the changes were not significant for SSFP MR images (P = .24 for 2.5 mg [group A] and P = .27 for 5 mg [group B]). The mean difference between scores before and those after administration of ISDN, adjusted for reader and coronary segment, was 0.32 (95% confidence interval [CI]: 0.07, 0.57) for group C, 0.33 (95% CI: 0.05, 0.61) for group D, 0.20 (95% CI: −0.08, 0.48) for group A, and 0.07 (95% CI: −0.4, 0.54) for group B. The effects of vessel segment on improvement of image quality score after ISDN administration were not significant (P = .16 for group C, P = .43 for group D, P = .16 for group A, and P = .97 for group B).
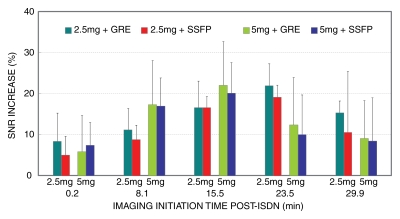
SNR measurements.—Figure 3 shows the percentage change in coronary blood SNR after ISDN administration. Coronary SNR significantly improved after ISDN administration (P < .05). The maximum SNR enhancement in the RCA with 5 mg of ISDN (GRE: 22.0% ± 10.7%; SSFP 20.1% ± 6.0%) was similar (P > .05) to that with 2.5 mg (GRE: 21.9% ± 5.4%; SSFP: 19.1% ± 3.0%). The maximum SNR enhancement in the proximal LCA with 5 mg of ISDN (GRE: 17.3% ± 12.6%; SSFP: 23.9% ± 41.3%) was similar (P > .05) to that with 2.5 mg (GRE: 17.2% ± 13.6%; SSFP: 20.5% ± 32.3%). The imaging initiation time for maximum SNR enhancement for both the RCA and proximal LCA was shorter for the 5-mg dose than for the 2.5-mg dose (15.5 minutes ± 6.0 vs 23.5 minutes ± 6.7 minutes; P < .01). The individual SNR data are summarized in Table 1.
Figure 3:
Bar graph shows time course of SNR enhancement change after ISDN administration in four groups studied. Maximum (∼20%) increase in SNR was observed on image sets initiated at 15 (5 mg) and 25 (2.5 mg) minutes after ISDN administration. Individual imaging initiation times were averaged for each of five sets. Error bars = standard deviations.
Table 1.
Percentage Changes in Individual RCA SNRs after ISDN Administration
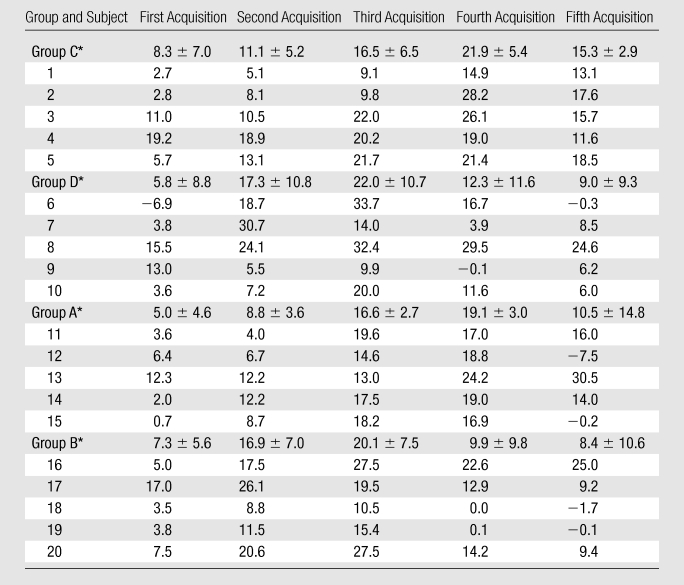
Note.—Data are percentages. Group A = SSFP sequence with 2.5 mg of ISDN, group B = SSFP sequence with 5 mg of ISDN, group C = GRE sequence with 2.5 mg of ISDN, group D = GRE sequence with 5 mg of ISDN.
Data are means ± standard deviations.
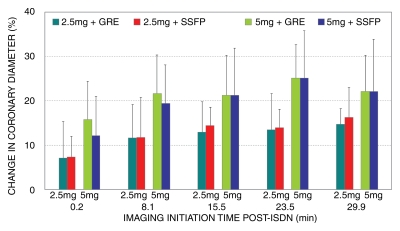
Vessel diameter.—Figure 4 shows the time course of coronary vessel diameter increase during the first 30 minutes after ISDN administration. Vessel diameter was significantly increased after ISDN administration (P < .02). Vasodilation with 5 mg (GRE: 23.7% ± 8.9%; SSFP: 26.5% ± 10.4%) was greater (P < .03) than that with 2.5 mg (GRE: 14.7% ± 3.6%; SSFP: 16.3% ± 6.8%). The vasodilations measured from GRE and SSFP imagers were similar (P > .05). The individual vessel diameter data are summarized in Table 2.
Figure 4:
Bar graph shows time course of coronary vessel diameter change after ISDN administration in four groups studied. Significant vasodilation was observed immediately after ISDN administration and persisted throughout time course (P < .02). Vasodilation was greater (P < .03) with 5-mg than with 2.5-mg ISDN dose. Vasodilation between GRE and SSFP sequences was similar. Error bars = standard deviations.
Table 2.
Percentage Changes in Individual RCA Vessel Diameters after ISDN Administration
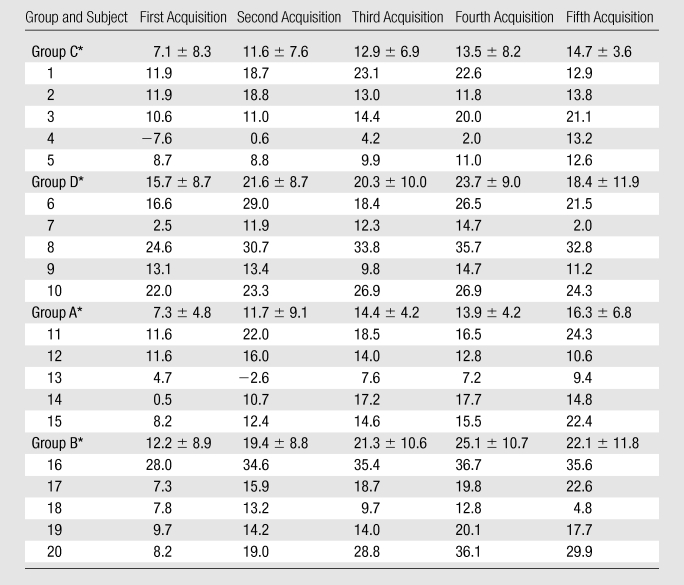
Note.—Data are percentages. Group A = SSFP sequence with 2.5 mg of ISDN, group B = SSFP sequence with 5 mg of ISDN, group C = GRE sequence with 2.5 mg of ISDN, group D = GRE sequence with 5 mg of ISDN.
Data are means ± standard deviations.
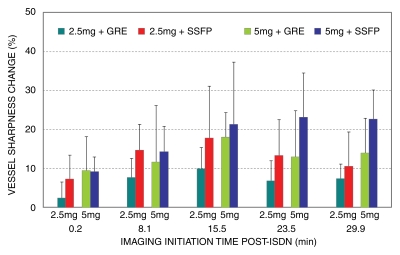
Vessel sharpness.—Figure 5 shows the time course of vessel sharpness changes. The images from all four groups were sharper after ISDN administration (P < .03). The maximum vessel sharpness increase occurred 16–23 minutes after ISDN administration (P < .05 for all four groups). There was a trend for increased sharpness with the SSFP sequence as opposed to the GRE sequence (P = .057). The individual vessel sharpness data are summarized in Table 3.
Figure 5:
Bar graph shows time course of coronary vessel sharpness score change after ISDN administration in four groups studied. Coronary images were sharper after ISDN administration (P < .03). Error bars = standard deviations.
Table 3.
Percentage Changes in Individual RCA Vessel Sharpness Scores after ISDN Administration
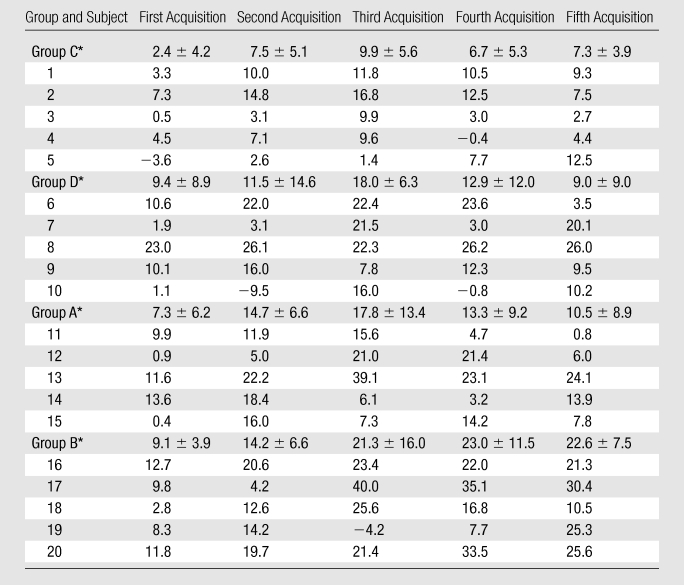
Note.—Data are percentages. Group A = SSFP sequence with 2.5 mg of ISDN, group B = SSFP sequence with 5 mg of ISDN, group C = GRE sequence with 2.5 mg of ISDN, group D = GRE sequence with 5 mg of ISDN.
Data are means ± standard deviations.
Whole-Heart Study
The imaging initiation times for whole-heart acquisitions were 0.2 minutes ± 0.1 and 17 minutes ± 5 after ISDN administration. Coronary SNR improved during the first whole-heart acquisition after ISDN administration (RCA: 11.7% ± 8.5%; LCA: 6.9% ± 2.1%; both P < .05), but only the SNR in the LCA significantly improved during the second acquisition (RCA: 1.8% ± 4.4%, P > .05; LCA: 7.5% ± 4.2%, P < .05). Vessel diameter increased significantly during both the first (RCA: 13.1% ± 2.0%; LCA: 11.7% ± 3.1%; both P < .05) and second (RCA: 17.7% ± 7.9%; LCA: 19.3% ± 4.2%; both P < .05) whole-heart acquisitions.
Discussion
We have demonstrated that SNR, vessel sharpness, and image quality improve owing to inflow enhancement on 3D targeted coronary MR images obtained with both GRE and SSFP sequences and that SNR increases on whole-heart SSFP MR images. Our data suggest that maximum SNR enhancement for both sequences is similar for both 2.5- and 5-mg ISDN doses. However, with the 5-mg dose, maximum SNR enhancement occurred earlier, and vasodilation was greater. Commonly, sublingual ISDN is administered followed immediately by initiation of coronary MR imaging (24). Our data suggest that coronary MR imaging should be performed 15 minutes after administration of a 5-mg dose of ISDN.
Researchers in previous studies have evaluated the vasodilation effect of shorter acting nitrates. Terashima et al (22) reported use of a two-dimensional multisection spiral coronary MR imaging sequence to evaluate the vasodilation effect of sublingual nitroglycerin and found that coronary cross-sectional area increased 23%, with an SNR increase of 31%. The difference in SNR increase between their study and our data may be owing to the different techniques and vasodilators used. Simonetti et al (30) investigated the dose- and time-related response to intracoronary ISDN by using conventional coronary angiography. They found a coronary diameter increase of 18%–26% in response to administration of intracoronary ISDN, which is similar with our findings.
We selected ISDN dose on the basis of several factors. First, 2.5–5 mg is commonly used for anginal prophylaxis prior to physical activity, indicating that physiologically meaningful coronary vasodilation can be achieved with this dose. Second, although all healthy subjects completed our study without complications from ISDN administration, the potential side effects of ISDN (eg, blood pressure drop) had to be considered. We used relatively low doses to minimize possible side effects. Third, as ISDN is readily available in 2.5- and 5-mg doses, we elected to use these doses for convenience, subject to the preceding two concerns.
We have shown that ISDN administration significantly improves quality scores for images obtained with a GRE sequence but not for those obtained with an SSFP sequence, despite similar SNR improvements. A potential explanation is that SSFP coronary artery MR images have higher baseline SNR and image quality than do GRE MR images (20). For GRE images, the same quality improvement after ISDN administration may be more conspicuous owing to lower baseline image quality and SNR.
The tradeoff between spatial resolution and acquisition time to maintain a sufficient SNR is a major limiting factor in coronary MR imaging. Currently, submillimeter resolution is commonly achieved in coronary MR imaging, which is adequate for visualizing the course of coronary arteries. However, coronary MR imaging has inferior spatial resolution compared with coronary computed tomographic angiography (31), for which vasodilators are commonly used. The significant SNR gain we found by using ISDN may enable higher spatial resolution and shorter imaging time with parallel imaging. The recent progress of coronary MR imaging at 3.0 T (4,10,11,32) has the potential to improve SNR and spatial resolution. We expect ISDN administration to provide additional SNR enhancement for coronary MR imaging at 3.0 T.
Contrast agent–enhanced coronary MR imaging has been reported as a promising alternative to unenhanced techniques (7,33). In contrast-enhanced coronary MR imaging, the major source of signal enhancement is from the T1-shortening effect of the contrast agent rather than inflow of fresh spins. Therefore, ISDN administration may not provide significant SNR improvements in contrast-enhanced coronary MR imaging. However, the significant and sustained vasodilation with ISDN may be useful in contrast-enhanced coronary MR imaging, especially in coronary imaging methods with slow infusion of the contrast agent (4).
Three-dimensional thick-slab whole-heart coronary MR imaging has been described (17,34) as an alternative to the thin-slab targeted approach. Although the whole-heart approach provides higher SNR compared with the targeted approach, it suffers from higher inflow saturation of signal and reduction in SNR and contrast-to-noise ratio (21). Therefore, only SSFP sequences are typically used in the whole-heart approach (17), although both GRE and SSFP sequences have been used in targeted acquisitions (3,24). Compared with GRE, SSFP sequences generate a high intrinsic contrast between blood and myocardium and are less dependent on inflow of “fresh” spins to generate image contrast. Our data with the whole-heart approach showed the SNR gain from ISDN administration in whole-heart acquisitions was less than that in the targeted 3D method, which may be explained by the inflow saturation effect.
Owing to the individually varying imaging time in our study depending on the volunteer’s heart rate and navigator efficiencies, the data we have presented are means of data acquired at different times after ISDN administration. However, the goal of our time-course study was to obtain an approximate time of maximum benefits. The averaging appears to be sufficient to achieve the goal, given that it was impractical to image each individual at exactly the same time after ISDN administration.
Our study had limitations. Only young healthy adult volunteers were studied. The physiology of blood flow in the coronary arteries may be different in older subjects and in patients with coronary artery disease. The number of volunteers in our study (five in each group) was relatively small. Further research is needed to study the effect of sublingual ISDN on coronary MR imaging in a larger patient population.
In conclusion, sublingual ISDN improves the 3D coronary MR imaging SNR by 20% for targeted acquisitions and by 10% for whole-heart acquisitions. Increases in vessel diameter are dose dependent. Maximum SNR enhancement occurs from 15 to 25 minutes after ISDN administration for 5- and 2.5-mg doses, respectively. Cardiac MR practitioners should consider these dose and temporal effects of ISDN when performing coronary MR imaging.
Advances in Knowledge.
We demonstrate that sublingual isosorbide dinitrate (ISDN) administration improves coronary MR imaging signal-to-noise ratios (SNRs) by 20% for thin-slab targeted acquisitions and by 10% for thick-slab whole-heart acquisitions, increases vessel diameter by 10%–15% (2.5-mg dose) or 20%–25% (5-mg dose), and improves image quality for gradient-recalled echo coronary MR imaging.
The maximum SNR enhancement occurs from 15 (5-mg dose) to 25 (2.5-mg dose) minutes after sublingual ISDN administration.
Implication for Patient Care.
Practitioners should consider the potential benefits of sublingual ISDN administration when performing coronary MR imaging.
Received March 19, 2009; revision requested April 21; revision received July 31; accepted August 26; final version accepted September 9.
Funding: This research was supported by National Institutes of Health (grant NIBIB/1R01EB008743-01A2).
Supported in part by grants from American Heart Association (AHA SDG-0730339N) and Harvard Catalyst, Harvard Clinical and Translational Science Center.
Authors stated no financial relationship to disclose.
Abbreviations:
- GRE
- gradient-recalled echo
- ISDN
- isosorbide dinitrate
- LCA
- left coronary artery
- RCA
- right coronary artery
- SNR
- signal to noise ratio
- SSFP
- steady-state free precession
- 3D
- three-dimensional
References
- 1.Meyer CH, Hu BS, Nishimura DG, Macovski A. Fast spiral coronary artery imaging. Magn Reson Med 1992;28(2):202–213 [DOI] [PubMed] [Google Scholar]
- 2.Bi X, Park J, Larson AC, Zhang Q, Simonetti O, Li D. Contrast-enhanced 4D radial coronary artery imaging at 3.0 T within a single breath-hold. Magn Reson Med 2005;54(2):470–475 [DOI] [PubMed] [Google Scholar]
- 3.Deshpande VS, Shea SM, Laub G, Simonetti OP, Finn JP, Li D. 3D magnetization-prepared true-FISP: a new technique for imaging coronary arteries. Magn Reson Med 2001;46(3):494–502 [DOI] [PubMed] [Google Scholar]
- 4.Bi X, Carr JC, Li D. Whole-heart coronary magnetic resonance angiography at 3 Tesla in 5 minutes with slow infusion of Gd-BOPTA, a high-relaxivity clinical contrast agent. Magn Reson Med 2007;58(1):1–7 [DOI] [PubMed] [Google Scholar]
- 5.Green JD, Omary RA, Schirf BE, Tang R, Li D. Catheter-directed contrast-enhanced coronary MR angiography in swine using magnetization-prepared True-FISP. Magn Reson Med 2003;50(6):1317–1321 [DOI] [PubMed] [Google Scholar]
- 6.Botnar RM, Stuber M, Danias PG, Kissinger KV, Manning WJ. Improved coronary artery definition with T2-weighted, free-breathing, three-dimensional coronary MRA. Circulation 1999;99(24):3139–3148 [DOI] [PubMed] [Google Scholar]
- 7.Goldfarb JW, Edelman RR. Coronary arteries: breath-hold, gadolinium-enhanced, three-dimensional MR angiography. Radiology 1998;206(3):830–834 [DOI] [PubMed] [Google Scholar]
- 8.Brittain JH, Hu BS, Wright GA, Meyer CH, Macovski A, Nishimura DG. Coronary angiography with magnetization-prepared T2 contrast. Magn Reson Med 1995;33(5):689–696 [DOI] [PubMed] [Google Scholar]
- 9.Bi X, Li D. Coronary arteries at 3.0 T: contrast-enhanced magnetization-prepared three-dimensional breathhold MR angiography. J Magn Reson Imaging 2005;21(2):133–139 [DOI] [PubMed] [Google Scholar]
- 10.Nezafat R, Stuber M, Ouwerkerk R, Gharib AM, Desai MY, Pettigrew RI. B1-insensitive T2 preparation for improved coronary magnetic resonance angiography at 3 T. Magn Reson Med 2006;55(4):858–864 [DOI] [PubMed] [Google Scholar]
- 11.Stuber M, Botnar RM, Fischer SE, et al. Preliminary report on in vivo coronary MRA at 3 Tesla in humans. Magn Reson Med 2002;48(3):425–429 [DOI] [PubMed] [Google Scholar]
- 12.Stuber M, Botnar RM, Danias PG, et al. Double-oblique free-breathing high resolution three-dimensional coronary magnetic resonance angiography. J Am Coll Cardiol 1999;34(2):524–531 [DOI] [PubMed] [Google Scholar]
- 13.Keegan J, Gatehouse P, Yang GZ, Firmin D. Coronary artery motion with the respiratory cycle during breath-holding and free-breathing: implications for slice-followed coronary artery imaging. Magn Reson Med 2002;47(3):476–481 [DOI] [PubMed] [Google Scholar]
- 14.Keegan J, Gatehouse PD, Yang GZ, Firmin DN. Non-model-based correction of respiratory motion using beat-to-beat 3D spiral fat-selective imaging. J Magn Reson Imaging 2007;26(3):624–629 [DOI] [PubMed] [Google Scholar]
- 15.Sakuma H, Ichikawa Y, Suzawa N, et al. Assessment of coronary arteries with total study time of less than 30 minutes by using whole-heart coronary MR angiography. Radiology 2005;237(1):316–321 [DOI] [PubMed] [Google Scholar]
- 16.Niendorf T, Hardy CJ, Giaquinto RO, et al. Toward single breath-hold whole-heart coverage coronary MRA using highly accelerated parallel imaging with a 32-channel MR system. Magn Reson Med 2006;56(1):167–176 [DOI] [PubMed] [Google Scholar]
- 17.Weber OM, Martin AJ, Higgins CB. Whole-heart steady-state free precession coronary artery magnetic resonance angiography. Magn Reson Med 2003;50(6):1223–1228 [DOI] [PubMed] [Google Scholar]
- 18.Stehning C, Bornert P, Nehrke K, Eggers H, Stuber M. Free-breathing whole-heart coronary MRA with 3D radial SSFP and self-navigated image reconstruction. Magn Reson Med 2005;54(2):476–480 [DOI] [PubMed] [Google Scholar]
- 19.Maintz D, Aepfelbacher FC, Kissinger KV, et al. Coronary MR angiography: comparison of quantitative and qualitative data from four techniques. AJR Am J Roentgenol 2004;182(2):515–521 [DOI] [PubMed] [Google Scholar]
- 20.Ozgun M, Hoffmeier A, Kouwenhoven M, et al. Comparison of 3D segmented gradient-echo and steady-state free precession coronary MRI sequences in patients with coronary artery disease. AJR Am J Roentgenol 2005;185(1):103–109 [DOI] [PubMed] [Google Scholar]
- 21.Nezafat R, Herzka D, Stehning C, Peters DC, Nehrke K, Manning WJ. Inflow quantification in three-dimensional cardiovascular MR imaging. J Magn Reson Imaging 2008;28(5):1273–1279 [DOI] [PubMed] [Google Scholar]
- 22.Terashima M, Meyer CH, Keeffe BG, et al. Noninvasive assessment of coronary vasodilation using magnetic resonance angiography. J Am Coll Cardiol 2005;45(1):104–110 [DOI] [PubMed] [Google Scholar]
- 23.Abrams J. Beneficial actions of nitrates in cardiovascular disease. Am J Cardiol 1996;77(13):31C–37C [DOI] [PubMed] [Google Scholar]
- 24.Kim WY, Danias PG, Stuber M, et al. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med 2001;345(26):1863–1869 [DOI] [PubMed] [Google Scholar]
- 25.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med 1999;42(5):952–962 [PubMed] [Google Scholar]
- 26.Etienne A, Botnar RM, Van Muiswinkel AM, Boesiger P, Manning WJ, Stuber M. “Soap-bubble” visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magn Reson Med 2002;48(4):658–666 [DOI] [PubMed] [Google Scholar]
- 27.Deriche R. Fast algorithms for low-level vision. IEEE Trans Pattern Anal Mach Intell 1990;12(1):78–87 [Google Scholar]
- 28.Pruessmann KP, Weiger M, Boesiger P. Sensitivity encoded cardiac MRI. J Cardiovasc Magn Reson 2001;3(1):1–9 [DOI] [PubMed] [Google Scholar]
- 29.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38:(4)963–974 [PubMed] [Google Scholar]
- 30.Simonetti I, Michelassi C, De Caterina R, Marzilli M, L’Abbate A. Dose- and time-related vasodilator response of conduit coronary arteries to intracoronary isosorbide dinitrate in human beings. Am Heart J 1989;117(2):323–331 [DOI] [PubMed] [Google Scholar]
- 31.Maintz D, Ozgun M, Hoffmeier A, et al. Whole-heart coronary magnetic resonance angiography: value for the detection of coronary artery stenoses in comparison to multislice computed tomography angiography. Acta Radiol 2007;48(9):967–973 [DOI] [PubMed] [Google Scholar]
- 32.Bi X, Deshpande V, Simonetti O, Laub G, Li D. Three-dimensional breathhold SSFP coronary MRA: a comparison between 1.5T and 3.0T. J Magn Reson Imaging 2005;22(2):206–212 [DOI] [PubMed] [Google Scholar]
- 33.Stuber M, Botnar RM, Danias PG, et al. Contrast agent-enhanced, free-breathing, three-dimensional coronary magnetic resonance angiography. J Magn Reson Imaging 1999;10(5):790–799 [DOI] [PubMed] [Google Scholar]
- 34.Sakuma H, Ichikawa Y, Chino S, Hirano T, Makino K, Takeda K. Detection of coronary artery stenosis with whole-heart coronary magnetic resonance angiography. J Am Coll Cardiol 2006;48(10):1946–1950 [DOI] [PubMed] [Google Scholar]