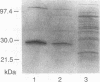
Abstract
The extracellular group B streptococcal enzyme described in numerous reports as a neuraminidase is really a hyaluronidase. Over the past 25 years, the enzyme was routinely assayed with bovine submaxillary mucin as the substrate and by the thiobarbituric acid procedure to measure released sialic acid. Characterization of the actual compound released by the enzyme revealed it to be an alpha,beta-unsaturated derivative of hyalobiuronic acid that was derived from hyaluronic acid contaminating the mucin preparation. Previous reports describing an association of elevated levels of extracellular neuraminidase with virulent strains of group B streptococci must be reevaluated with the recognition that the enzyme is really a hyaluronidase.
Full text
PDF

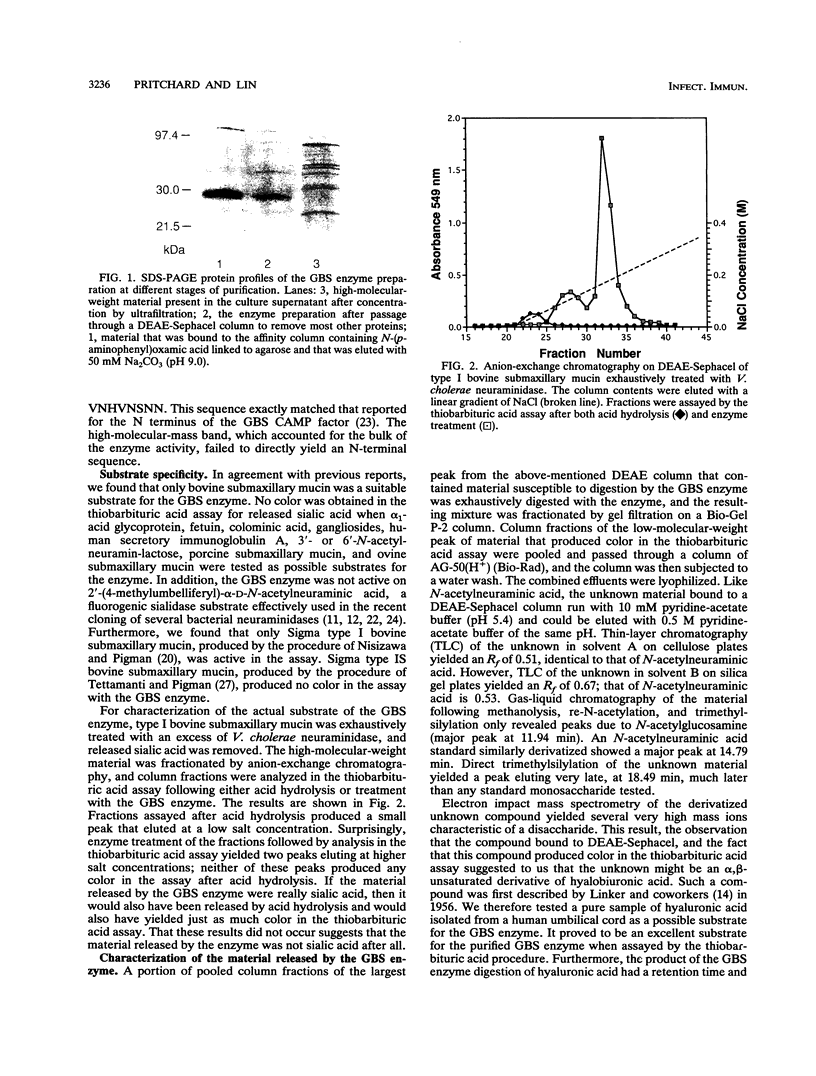
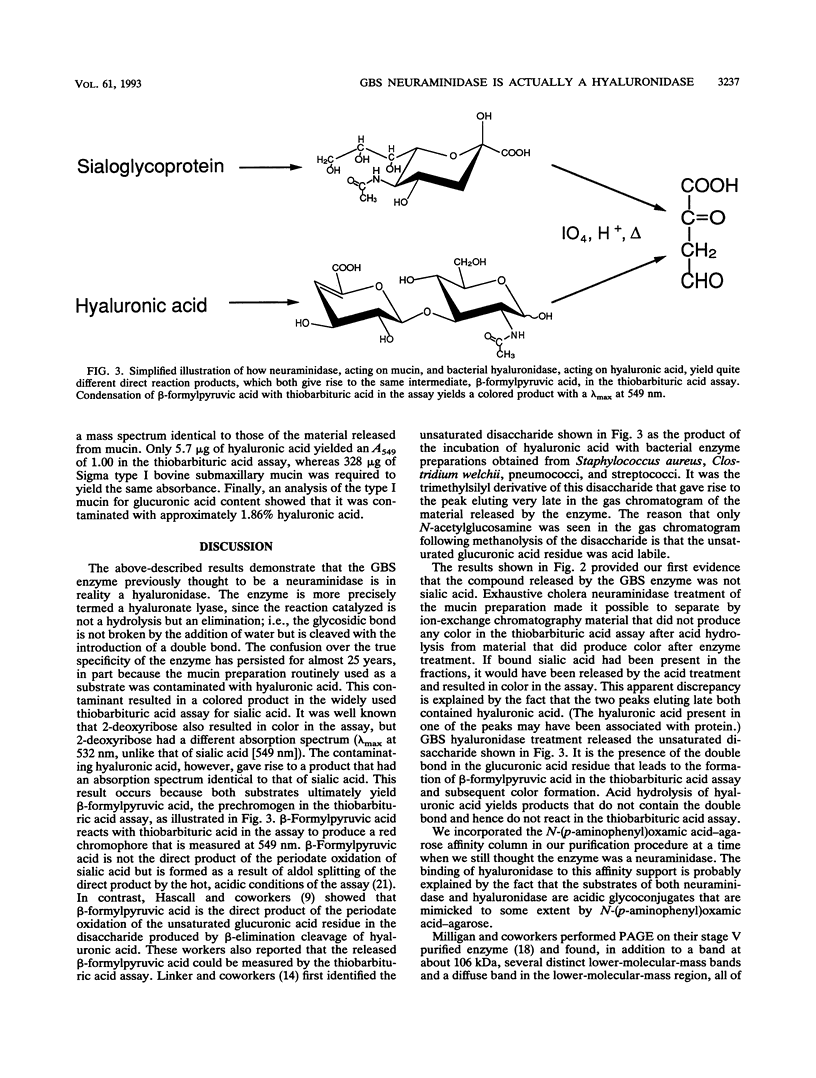


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown J. G., Straus D. C. Characterization of neuraminidases produced by various serotypes of group B streptococci. Infect Immun. 1987 Jan;55(1):1–6. doi: 10.1128/iai.55.1.1-6.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Illiano G. Purification of neuraminidases from Vibrio Cholerae, Clostridium Perfringens and influenza virus by affinity chromatography. Biochem Biophys Res Commun. 1971 Jul 2;44(1):178–184. doi: 10.1016/s0006-291x(71)80175-4. [DOI] [PubMed] [Google Scholar]
- Den H., Malinzak D. A., Rosenberg A. Cytotoxic contaminants in commercial Clostridium perfringens neuraminidase preparations purified by affinity chromatography. J Chromatogr. 1975 Aug 20;111(1):217–222. doi: 10.1016/s0021-9673(01)80167-9. [DOI] [PubMed] [Google Scholar]
- Edmond J. D., Johnston R. G., Kidd D., Rylance H. J., Sommerville R. G. The inhibition of neuraminidase and antiviral action. Br J Pharmacol Chemother. 1966 Aug;27(2):415–426. doi: 10.1111/j.1476-5381.1966.tb01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison J. R., Holland J. J. A procedure for the rapid, quantitative N-acetylation of amino sugar methyl glycosides. Anal Biochem. 1975 May 26;66(1):87–92. doi: 10.1016/0003-2697(75)90727-7. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Riolo R. L., Hayward J., Jr, Reynolds C. C. Treatment of bovine nasal cartilage proteoglycan with chondroitinases from Flavobacterium heparinum and Proteus vulgaris. J Biol Chem. 1972 Jul 25;247(14):4521–4528. [PubMed] [Google Scholar]
- Hayano S., Tanaka A. Sialidase-like enzymes produced by group A, B, C, G, and L streptococci and by Streptococcus sanguis. J Bacteriol. 1969 Mar;97(3):1328–1333. doi: 10.1128/jb.97.3.1328-1333.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsen M., Roggentin P., Schauer R. Cloning, sequencing and expression of the sialidase gene from Actinomyces viscosus DSM 43798. Biol Chem Hoppe Seyler. 1991 Dec;372(12):1065–1072. doi: 10.1515/bchm3.1991.372.2.1065. [DOI] [PubMed] [Google Scholar]
- Hoyer L. L., Hamilton A. C., Steenbergen S. M., Vimr E. R. Cloning, sequencing and distribution of the Salmonella typhimurium LT2 sialidase gene, nanH, provides evidence for interspecies gene transfer. Mol Microbiol. 1992 Apr;6(7):873–884. doi: 10.1111/j.1365-2958.1992.tb01538.x. [DOI] [PubMed] [Google Scholar]
- Kjems E., Perch B., Henrichsen J. Serotypes of group B streptococci and their relation to hyaluronidase production and hydrolysis of salicin. J Clin Microbiol. 1980 Feb;11(2):111–113. doi: 10.1128/jcm.11.2.111-113.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINKER A., MEYER K., HOFFMAN P. The production of unsaturated uronides by bacterial hyaluronidases. J Biol Chem. 1956 Mar;219(1):13–25. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mattingly S. J., Milligan T. W., Pierpont A. A., Straus D. C. Extracellular neuraminidase production by clinical isolates of group B streptococci from infected neonates. J Clin Microbiol. 1980 Oct;12(4):633–635. doi: 10.1128/jcm.12.4.633-635.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan T. W., Baker C. J., Straus D. C., Mattingly S. J. Association of elevated levels of extracellular neuraminidase with clinical isolates of type III group B streptococci. Infect Immun. 1978 Sep;21(3):738–746. doi: 10.1128/iai.21.3.738-746.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan T. W., Mattingly S. J., Straus D. C. Purification and partial characterization of neuraminidase from type III group B streptococci. J Bacteriol. 1980 Oct;144(1):164–171. doi: 10.1128/jb.144.1.164-171.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISIZAWA K., PIGMAN W. The composition and properties of the mucin clot from cattle submaxillary glands. Arch Oral Biol. 1959 Oct;1:161–170. doi: 10.1016/0003-9969(59)90008-1. [DOI] [PubMed] [Google Scholar]
- Paerels G. B., Schut J. The mechanism of the periodate-thiobarbituric acid reaction of sialic acids. Biochem J. 1965 Sep;96(3):787–792. doi: 10.1042/bj0960787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe B., Rothe B., Roggentin P., Schauer R. The sialidase gene from Clostridium septicum: cloning, sequencing, expression in Escherichia coli and identification of conserved sequences in sialidases and other proteins. Mol Gen Genet. 1991 Apr;226(1-2):190–197. doi: 10.1007/BF00273603. [DOI] [PubMed] [Google Scholar]
- Russo T. A., Thompson J. S., Godoy V. G., Malamy M. H. Cloning and expression of the Bacteroides fragilis TAL2480 neuraminidase gene, nanH, in Escherichia coli. J Bacteriol. 1990 May;172(5):2594–2600. doi: 10.1128/jb.172.5.2594-2600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühlmann J., Wittmann-Liebold B., Jürgens D., Fehrenbach F. J. Complete amino acid sequence of protein B. FEBS Lett. 1988 Aug 1;235(1-2):262–266. doi: 10.1016/0014-5793(88)81275-4. [DOI] [PubMed] [Google Scholar]
- Skoza L., Mohos S. Stable thiobarbituric acid chromophore with dimethyl sulphoxide. Application to sialic acid assay in analytical de-O-acetylation. Biochem J. 1976 Dec 1;159(3):457–462. doi: 10.1042/bj1590457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettamanti G., Pigman W. Purification and characterization of bovine and ovine submaxillary mucins. Arch Biochem Biophys. 1968 Mar 20;124(1):41–50. doi: 10.1016/0003-9861(68)90301-9. [DOI] [PubMed] [Google Scholar]
- Varki A., Diaz S. The release and purification of sialic acids from glycoconjugates: methods to minimize the loss and migration of O-acetyl groups. Anal Biochem. 1984 Feb;137(1):236–247. doi: 10.1016/0003-2697(84)90377-4. [DOI] [PubMed] [Google Scholar]
- Zangwill K. M., Schuchat A., Wenger J. D. Group B streptococcal disease in the United States, 1990: report from a multistate active surveillance system. MMWR CDC Surveill Summ. 1992 Nov 20;41(6):25–32. [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980 Feb;27(2):444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]