Abstract
Estrogens rapidly regulate neuronal activity within seconds-to-minutes, yet it is unclear how estrogens interact with neural circuits to rapidly coordinate behavior. This study examines whether 17-beta-estradiol interacts with an opioidergic network to achieve rapid modulation of a vocal control circuit. Adult plainfin midshipman fish emit vocalizations that mainly differ in duration, and rhythmic activity of a hindbrain–spinal vocal pattern generator (VPG) directly establishes the temporal features of midshipman vocalizations. VPG activity is therefore predictive of natural calls, and ‘fictive calls’ can be elicited by electrical microstimulation of the VPG. Prior studies show that intramuscular estradiol injection rapidly (within 5 min) increases fictive call duration in midshipman. Here, we delivered opioid antagonists near the VPG prior to estradiol injection. Rapid estradiol actions on fictive calling were completely suppressed by the broad-spectrum opioid antagonist naloxone and the mu-opioid antagonist beta-funaltrexamine, but were unaffected by the kappa-opioid antagonist nor-binaltorphimine. Unexpectedly, prior to estradiol administration, all three opioid antagonists caused immediate, transient reductions in fictive call duration. Together, our results indicate that: (1) vocal activity is modulated by opioidergic networks, confirming hypotheses from birds and mammals, and (2) the rapid actions of estradiol on vocal patterning depend on interactions with a mu-opioid modulatory network.
Keywords: Nongenomic, Extranuclear, Membrane, Teleost, Opioid
Introduction
Estrogens rapidly regulate behavior within minutes in a variety of contexts, most notably the sexual behavior of male rats (Cross and Roselli 1999) and Japanese quail (Cornil et al. 2006b). Estrogens also act within seconds-to-minutes on the neurophysiological properties of striatal, hippocampal and hypothalamic neurons (Qiu et al. 2003; Woolley 2007). These actions on neuronal excitability occur via interactions with ion channels and/or neurotransmitter receptors, including those for opioids (reviews: Kelly and Ronnekleiv 2002; Cornil et al. 2006a). Taken together, these observations suggest that the mechanisms established for estrogen action on neuronal activity mediate the rapid behavioral effects of estrogens. However, it remains largely unknown how estrogens rapidly modulate discrete networks of neurons that pattern behavioral output. In principle, estrogen-dependent modulation could be via either direct effects on central pattern generators or indirect effects on afferent modulatory networks.
17-β-Estradiol (E2) modulates the activity of a vocal central pattern generator in a sound-producing teleost fish, the plainfin midshipman (Porichthys notatus) (Remage-Healey and Bass 2004, 2007). Male midshipman establish and defend nests they build under rocky shelters, and emit long-duration (>1 min) advertisement calls known as “hums” to attract females to their nests. Adults of both sexes emit short-duration (~100 ms) “grunts” during aggressive encounters (Brantley and Bass 1994; Bass and McKibben 2003). The rhythmic activity of hindbrain–spinal vocal pattern generator (VPG; see Fig. 1) directly establishes the fundamental frequency and duration of midshipman calls (Bass and Baker 1990). The output of the VPG (‘fictive calls’) can be evoked by excitation of midbrain and hindbrain sites (Bass and Baker 1990; Weeg et al. 2005; Kittelberger et al. 2006), and the temporal firing properties of the VPG directly establish the temporal features of natural calls (Bass and Baker 1990). Intramuscular E2 injection rapidly (within 5 min) increases the duration of fictive calls in adult male and female midshipman (Remage-Healey and Bass 2004, 2007), and these effects are both short-lived (lasting 30–45 min) and stereospecific. Androgens and glucocorticoids also cause rapid changes in fictive calling in this species and candidate receptor mechanisms have been identified by blocking these acute effects with cyproterone acetate (antiandrogen) and RU486 (antiglucocorticoid), respectively (Remage-Healey and Bass 2004). A similar candidate receptor mechanism has not yet been identified for the rapid effects of E2. The rapid actions of E2 are localized to the hindbrain–spinal region that contains the VPG (Remage-Healey and Bass 2004), but whether E2 acts directly on the VPG itself, or via indirect actions on neurons that modulate the VPG, is unknown. The midshipman model system therefore presents an opportunity to identify mechanism(s) of acute estrogen action on a defined neural circuit (shared by all major vertebrate lineages, see Bass et al. 2008) that patterns vocalizations.
Fig. 1.
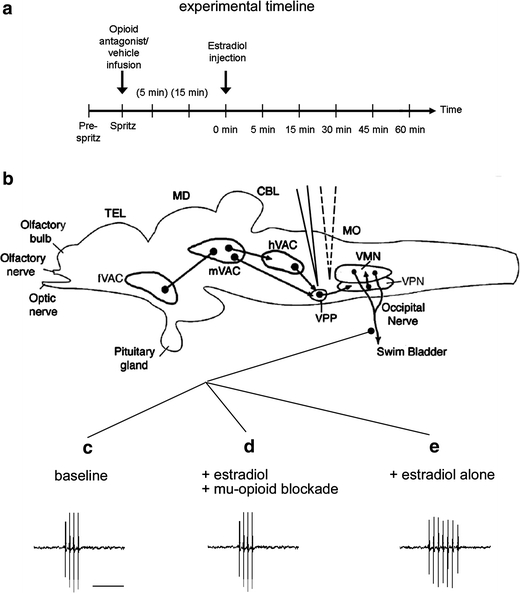
a Schematic of the experimental timeline for opioid antagonist infusion (‘spritz’) followed by estradiol (E2) injection, as indicated by vertical arrows. Each vertical line on the time axis represents an extracellular recording sample; parentheses indicate recording sample times during the post-spritz period. b Sagittal line drawing of the midshipman brain, showing the major nuclei in the descending vocal control system (Bass et al. 1994; Goodson and Bass 2002). The hindbrain–spinal vocal pattern generator (VPG) consists of a vocal motor nucleus (VMN) that innervates the vocal swimbladder muscles via occipital nerve roots, an adjacent column of vocal pacemaker neurons (VPN) which temporally pattern the activity of the VMN, and a rostral vocal prepacemaker nucleus (VPP). The VPG receives descending input from vocal–acoustic centers (VAC) in the hindbrain (h), midbrain (m), and forebrain (f). Electrical microstimulation of the VPP region (solid electrode) elicits patterned fictive calls (traces below; illustrations only and not real data) recorded via extracellular electrode adjacent to the occipital nerve root. Also depicted is the drug delivery electrode (dashed electrode) within the fourth ventricle. MO medulla oblongata, CBL cerebellum, MD midbrain, TEL telencephalon. c–e Illustrations of three modulatory effects on midshipman hindbrain–spinal VPG activity. Traces are 250 ms in duration (scale bar 100 ms) and are depicted for illustration purposes only. c Functional mu- and/or kappa-opioid circuits within the VPG region are required for baseline fictive call duration. d, e Rapid actions of E2 on fictive call duration require the presence of functional mu-opioidergic circuits. In the absence of functional mu-opioid circuits (d) fictive call duration remains at baseline. The combined rapid actions of E2 and mu-opioidergic circuits (e) result in increased fictive call duration
Here, we test the hypothesis that an opioidergic network provides a modulatory mechanism for the rapid actions of E2 in midshipman. Our focus on opioidergic mechanisms is related to three previous observations. First, rapid E2 actions on mammalian hypothalamic neurons occur via interactions with opioidergic systems (Kelly et al. 1992; Qiu et al. 2003). Second, a membrane-bound receptor that is proposed to mediate rapid corticosteroid suppression of clasping behavior in amphibians exhibits opioid-like binding properties (Evans et al. 2000). Lastly, longer-term (i.e., hours to days) interactions between steroids (estrogens and progestins) and opioidergic neurons have emerged in studies examining the regulation of sexual receptivity in rodents (Eckersell et al. 1998; Sinchak and Micevych 2001; Acosta-Martinez and Etgen 2002). We report results for three opioid antagonists: naloxone, a broad-spectrum opioid blocker; beta-funaltrexamine, a blocker specific to mu-opioid neurons in other vertebrates; and nor-binaltorphimine, a blocker specific to kappa-opioid neurons in other vertebrates (Bodnar and Klein 2006). We note that the receptor specificity of these antagonists has not been fully characterized for opioid receptors in teleosts, but that opioid receptor subtypes are broadly conserved among vertebrates (Brooks et al. 1994; Ebbesson et al. 1996; Khalap et al. 2005, see discussion).
Materials and methods
Animals
Midshipman fish have two adult male morphs that diverge in a large suite of traits including reproductive tactics and vocal behaviors (Brantley and Bass 1994). Type I males build and guard nests in the intertidal zone, whereas smaller type II males sneak and satellite spawn in attempt to steal fertilizations from type I’s. Only type I males are known to produce the long-duration advertisement hums as well as extended trains of brief agonistic grunts and growls that are intermediate in duration between hums and grunts (Bass and McKibben 2003). Type II males and females are only known to generate grunts in non-spawning contexts. This study included 40 type I males (13–20 cm standard length) collected from nest sites in northern California and Washington state. While females have the highest plasma E2 levels, detectable levels of circulating E2 are found in close to 70% of type I males during pre-nesting and nesting periods (Sisneros et al. 2004). Also, aromatase-containing glial cells surround the dorsolateral aspect of the vocal motor nucleus (VMN) and have processes that ramify throughout the VMN which likely provide a local source of E2 via aromatization of circulating testosterone (Forlano et al. 2001; Sisneros et al. 2004).
Evoked activity of the vocal pattern generator (VPG)
Electrophysiology procedures closely followed those outlined in earlier studies with midshipman fish (Bass and Baker 1990; Goodson and Bass 2000; Remage-Healey and Bass 2004). The brain and rostral spinal cord were exposed by dorsal craniotomy under general anesthesia (0.025% benzocaine; Sigma) and long-lasting analgesia (0.25% bupivacaine; Abbott Laboratories, Chicago, IL; with 0.01 mg/mg epinephrine; International Medication Systems, El Monte, CA). Following surgery, fish were stabilized in a plexiglass tank during recovery and perfused through the mouth with fresh saltwater maintained at 17°C with a Peltier device. Exposed brain areas were covered with Flouroinert (3 M Corp., St. Paul, MN), and intramuscular injections of pancuronium bromide (0.5 mg/kg; Astra Pharmaceutical Products, Westborough MA) were used for immobilization. Brief electrical stimuli (pulse width = 0.1 ms, frequency = 250 Hz, duration = 25 ms) were delivered via an insulated tungsten electrode exposed at the tip (Fig. 1b) to a hindbrain–spinal vocal site VPP (Bass et al. 2008), formerly designated as VM (Bass et al. 1994), that is vocally active (Remage-Healey and Bass 2004; 2006). Electrically evoked vocal motor volleys were recorded with an extracellular electrode (Teflon-coated silver wire with exposed ball tip) placed on a ventral occipital nerve root that carries vocal motor axons to the ipsilateral vocal muscle attached to the lateral walls of the swim bladder (Fig. 1b). Activity recorded from either left- or right-side occipital nerve root reflects bilateral output (Bass and Baker 1990). Rhythmic vocal motor volleys occurred as discrete bursts (fictive calls, Fig. 1a–c) similar to natural grunts (see Brantley and Bass 1994) and were recorded at 1-s intervals. Recordings were digitized on a Macintosh using IGOR Pro software (Wavemetrics, Inc., Lake Oswego, OR). The duration of each fictive call was measured from the recordings and the mean burst duration was computed for each time point and normalized to 100% of baseline output.
Delivery of opioid antagonists
All opioid antagonists were obtained from Tocris Bioscience (Ellisville, MO) and dissolved in buffered teleost Ringer’s (Forster–Taggart) solution (all solutions had pH between 6.81 and 8.19). Fictive calls evoked from the hindbrain–spinal VPP region were considered stable when a single fictive call was entrained to each stimulus. Once stability was established, a series of ten fictive calls was evoked at 1-s intervals to determine baseline (pre-drug) vocal motor output. Immediately following the baseline period, either saline vehicle (n = 4 animals) or one of three opioid receptor antagonists was pressure injected (see below) into the hindbrain (fourth) ventricle that directly borders the VMN (see Fig. 1b) (Bass and Baker 1990; Bass et al. 1994). Antagonists included the broad-spectrum opioid receptor antagonist naloxone (1, 10, 25 mM; n = 6, 4, 4 animals each, respectively), the mu-specific opioid antagonist beta-funaltrexamine (1, 10 mM; n = 4 each), and the kappa-specific opioid antagonist nor-binaltorphimine (0.66, 6.6 mM; n = 3 each). Drug doses were based on previous studies of opioid–steroid interactions (Evans et al. 2000; Acosta-Martinez and Etgen 2002; Ceccarelli et al. 2004; Schmitt and Kaufman 2005). The standard dose sequence (1, 10 mM) was lowered slightly for nor-binaltorphimine due to limited reagent availability; it is an effective kappa-opioid antagonist in both mammalian and non-mammalian vertebrates (Bradford et al. 2005).
Drugs and vehicle were delivered via a glass micropipette (microfilament 6010 1.0 mm OD, AM Systems, Carlsborg, WA) with broken tips (18–20 μm) using a picospritzer (Biomedical Engineering, Thornwood, NY). Only one dose of opioid antagonist or vehicle was used per experiment (i.e., in each animal). Injection parameters followed those well-established for this preparation (one pressure pulse per second at 30 psi and pulse duration of 10 ms for estimated infusion volume of approximately 0.16 nl; see Goodson and Bass 2000; Kittelberger et al. 2006). The picospritzer electrode was checked before and immediately following each experiment to verify that pressure delivery was functional. A simultaneous recording of ten fictive calls was obtained (as above) concurrent with pressure delivery of solutions to identify any instantaneous effects of either drug or saline delivery on vocal motor patterning (see also the experiment timeline in Fig. 1a). Two subsequent recordings of 15 fictive calls were obtained at 5 and 15 min following solution delivery (all treatments prior to E2 administration; see Fig. 1a) to determine whether drugs or saline altered vocal motor patterning. Five minutes after the 15-min post-drug recording, fish were administered an intramuscular injection (dorsal trunk muscle) of E2 (0.02 mg/kg) via a pre-inserted 23-G butterfly needle. This treatment dose rapidly increases fictive call duration (within 5 min and lasting up to 30 min) in type I male midshipman (Remage-Healey and Bass 2004, 2007). Immediately following the injection, a subsequent series of 15 fictive calls were obtained at 5, 15, 30, 45, and 60 min, and each time point was standardized to 100% of the baseline output. After the last recording, blood samples were obtained from the gill arch (Remage-Healey and Bass 2004, 2005; Remage-Healey and Bass 2006), and plasma was analyzed for E2 using enzyme immunoassay (Cayman Chemical, Ann Arbor, MI, techniques reported previously in Remage-Healey and Bass 2004, 2005, 2007). Plasma E2 levels were confirmed to be elevated (mean = 34.52 ng/ml ± 8.01 SEM; n = 16) for a subset of animals that received E2 injections (see also Remage-Healey and Bass 2004).
Analysis
As described above, fictive call durations were sampled and standardized to 100% of baseline. Statistical analyses of the effects of drugs on fictive call duration were performed using Statview 4.57. Repeated-measures ANOVA was performed for data from all treatments for analysis of treatment effects over time in a within- and between-subjects design. Tukey’s post hoc tests are reported for between-subject effects for comparisons of saline versus drug treatments for P < 0.05.
Results
Overview
We first show that each of the opioid antagonists used here—naloxone, beta-funaltrexamine, and nor-binaltorphimine—caused a rapid and brief (either immediate or within 5 min of infusion) suppression of fictive calling when delivered to the hindbrain VPP region. All antagonists had similarly brief effects when initially applied, but calling recovered to baseline quickly (within 5–10 min). In contrast, subsequent attenuation of rapid E2 effects on fictive calling was both antagonist-specific and prolonged, lasting up to 30–60 min. This encompasses the duration of E2’s actions on fictive calling in this species (Remage-Healey and Bass 2004, 2007). Only naloxone and the mu-opioid-specific antagonist beta-funaltrexamine, but not the kappa-opioid antagonist nor-binaltorphimine, inhibited E2’s effects on calling.
Antagonists alone
Naloxone
The broad-spectrum opioid antagonist naloxone (1, 10, and 25 mM) caused a transient reduction in fictive call duration, in a dose-dependent manner (Fig. 2a). There were significant effects of time-after-delivery (F = 8.74; df = 3,42; P = 0.0001), a dose × time interaction (F = 3.36; df = 9.42; P = 0.003), and a non-significant trend for the effect of dose (F = 3.12; df = 3,14; P = 0.06). Tukey’s post hoc test revealed that naloxone produced significant (P < 0.05) reductions in fictive call duration at 5 min after delivery for all three doses (1, 10, 25 mM) when compared to saline alone. Fictive call duration was indistinguishable from baseline at 15 min following naloxone treatment. For the 5-min time point, the response to naloxone was linearly dose-dependent; i.e., there was a larger reduction in fictive call duration as the doses of naloxone increased.
Fig. 2.
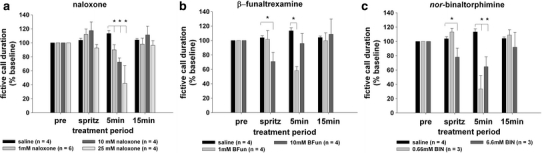
Interocerebroventricular microinjection of opioid antagonists rapidly reduce fictive call duration in midshipman. The broad-spectrum opioid antagonist naloxone (a) the mu-specific-opioid antagonist beta-funaltrexamine (b) and the kappa-specific-opioid antagonist nor-binaltorphimine (c) each transiently suppress fictive call duration. All treatment effects recover to baseline at 15 min. *P < 0.05 for Tukey’s post hoc test for between-subject comparisons of fictive calling during infusion of antagonists versus vehicle (saline)
Beta-funaltrexamine
The mu-specific opioid antagonist beta-funaltrexamine (1, 10 mM) also caused a transient reduction in fictive call duration (Fig. 2b). There were significant effects of time-after-delivery (F = 3.69; df = 3.27; P = 0.04), a dose × time interaction (F = 5.55; df = 6.27; P = 0.0007), and no significant effect of dose (F = 1.21; df = 2.9; P = 0.34). Tukey’s post hoc test revealed that beta-funaltrexamine produced significant (P < 0.05) reductions in fictive call duration immediately upon delivery (‘spritz’; 10 mM dose) and at 5 min after delivery (1 mM dose) when compared to saline alone. While the time-dependence of the response to beta-funaltrexamine differed slightly for the two doses, they were similar in temporal pattern. That is, the larger dose (10 mM) produced only an immediate reduction in fictive calling and the lower dose (1 mM) produced a reduction only at 5 min after delivery of the drug; each recovered to baseline by the next time point tested, i.e., within at least 5 min (10 mM) or 10 min (1 mM). Fictive call duration was indistinguishable from baseline at 15 min following beta-funaltrexamine treatment.
Nor-binaltorphimine
The kappa-specific opioid antagonist nor-binaltorphimine (0.66, 6.6 mM) also caused a transient reduction in fictive call duration that was, like naloxone, dose-dependent (Fig. 2c). There were significant effects of time-after-delivery (F = 11.43; df = 3.21; P = 0.0001), a dose × time interaction (F = 11.91; df = 6.21; P < 0.0001), and a significant effect of dose (F = 29.7; df = 2.7; P = 0.0004). Tukey’s post hoc test revealed that nor-binaltorphimine produced significant (P < 0.05) reductions in fictive call duration immediately upon delivery (0 min; 6.6 mM dose) and at 5 min after delivery (0.66 and 6.6 mM doses) when compared to saline alone. Fictive call duration was indistinguishable from baseline at 15 min following nor-binaltorphimine treatment. Similar to beta-funaltrexamine, the dose-dependence of the response to nor-binaltorphimine was apparent in that only the highest dose (6.6 mM) produced an immediate reduction in fictive calling. Unlike beta-funaltrexamine, both low and high doses produced reductions in fictive calling at the 5-min time point. Similar to both naloxone and beta-funaltrexamine, fictive call suppression that was present at 5 min recovered to baseline at the next time point tested, i.e., 15 min after picospritzing the drug.
Antagonists together with E2
Naloxone
The broad-spectrum opioid antagonist naloxone (1, 10, and 25 mM) caused suppression of E2’s rapid effects on fictive call duration that was dose-dependent (Fig. 3a). There were significant effects of time-after-injection (F = 2.43; df = 5.60; P = 0.04), a dose × time interaction (F = 2.23; df = 15.60; P = 0.01), and a significant effect of naloxone dose (F = 5.33; df = 3.12; P = 0.01) when comparing naloxone + E2 treatments versus saline + E2 treatment. Tukey’s post hoc test revealed that naloxone + E2 treatments produced significantly shorter fictive call duration from saline + E2 treatment at 5 min (10 and 25 mM), 15 min (25 mM), and 30 min (10 and 25 mM). Therefore, only the intermediate (10 mM) and high (25 mM) doses of naloxone were effective in suppressing the rapid actions of E2.
Fig. 3.
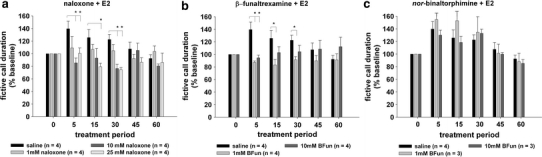
Pre-treatment with mu-opioid antagonists suppresses the rapid actions of 17-β-estradiol (E2) on fictive calling in midshipman. The broad-spectrum opioid antagonist naloxone (a) and the mu-opioid-specific antagonist beta-funaltrexamine (b) each significantly suppress the acute increase in fictive call duration following E2 injection at 5, 15 and 30 min. In contrast, the kappa-opioid-specific antagonist nor-binaltorphimine (c) produces no effect on the rapid actions of E2, indicating that the observed actions are mu-opioid-specific. *P < 0.05 for Tukey’s post hoc test for between-subject comparisons of fictive calling during infusion of antagonists versus vehicle (saline)
Beta-funaltrexamine
The mu-specific opioid antagonist beta-funaltrexamine (1, 10 mM) also suppressed E2’s rapid effects on fictive call duration (Fig. 3b). There was no significant effect of time-after-injection (F = 0.68; df = 5.45; P = 0.64), but there was a significant dose × time interaction (F = 3.44; df = 10.45; P = 0.002) and a significant effect of beta-funaltrexamine dose (F = 4.26; df = 2.9; P = 0.05) when comparing beta-funaltrexamine + E2 treatments versus saline + E2 treatment. Like naloxone, Tukey’s post hoc test revealed that beta-funaltrexamine + E2 treatments produced significantly shorter fictive call duration from saline + E2 treatment at 5 min (1 and 10 mM), 15 min (1 mM), and 30 min (1 mM). Thus, unlike naloxone, only the low (1 mM) dose of beta-funaltrexamine was effective in suppressing the entire time-course of rapid E2 actions, while the response to the high (10 mM) dose was no longer significantly different from the saline + E2 treatment after 15 min.
Nor-binaltorphimine
Unlike naloxone and beta-funaltrexamine, the kappa-specific antagonist nor-binaltorphimine (0.66, 6.6 mM) had no significant effect on the rapid increase in fictive call duration induced by E2 (Fig. 3c). There was a significant effect of time-after-injection (response to E2; F = 10.70; df = 5.35; P < 0.0001), but there was no time × dose interaction (F = 0.57; df = 10.35; P = 0.83), and no significant effect of binaltorphimine dose (F = 0.42; df = 2.7; P = 0.67) when comparing nor-binaltorphimine + E2 treatments versus saline + E2 treatment. Tukey’s post hoc test did not detect significant differences between nor-binaltorphimine + E2 and saline + E2 treatment for any time point at any dose. It is possible that a higher dose of nor-binaltorphimine would have caused suppression of fictive calling. However, the current dose regimen matched the order of magnitude for the other opioid antagonists used in this study (micro- to milli-molar range) and is within the high-range of doses for effective kappa-opioid blockade as reported in the literature (Gogas et al. 1996; Mitchell et al. 2005; Bodnar and Klein 2006; Pascoe et al. 2008).
Discussion
This study reports that intracerebroventricular microinjection of opioid antagonists can acutely inhibit fictive calling in midshipman fish. We also report that the rapid neuromodulation of fictive calling by E2 is suppressed during specific inhibition of a central mu-opioidergic network. Our findings are therefore consistent with the hypothesis that rapid E2 actions on hindbrain–spinal VPG activity occur via interactions with a central mu-opioidergic network whose activation, in turn, modulates the VPG (as summarized in Fig. 1).
Opioidergic network and vocal mechanisms
This study provides evidence that opioids are neuromodulatory in contexts outside of their well-established role in regulating nociception and reinforcement/reward via actions in the forebrain (Bodnar and Klein 2006). The current observation that opioidergic neurons are involved in modulating vocal motor mechanisms may not be unique to midshipman fish, since opioids and their receptors are expressed throughout the song-control pathway in oscine (Deviche et al. 1993) and non-oscine (Durand et al. 1998) songbirds. Furthermore, opioids regulate vocal behaviors in both songbirds and mammals (Riters et al. 2005; Bodnar and Klein 2006), and, consistent with these earlier observations, the current study provides neurophysiological evidence that opioidergic neurons participate in the patterning of vocalizations.
Both the mu-specific (beta-funaltrexamine) and the kappa-specific (nor-binaltorphimine) opioid antagonists caused immediate suppression of fictive call duration during infusion (‘spritz’; latency < 1 min), whereas the broad-spectrum opioid antagonist naloxone caused vocalization suppression after a latency of 5 min. Therefore, suppression was common to all three antagonists as was a rapid recovery to baseline. Relative to the other antagonists, the naloxone effect was delayed but similarly short-lived, which may relate to differences in the ability of each drug to penetrate neural tissue due to chemical structure or potency (see also Martin et al. 2006; Chaijale et al. 2008). It is noteworthy that the binding affinity of opioid antagonists to mu- and kappa-opioid receptors have been well-characterized for mammals, but not yet for teleost fishes. A lack of strict dose-dependence for these opioid antagonist treatments on fictive calling makes non-specific effects of microinjection (such as concentration-dependent changes in pH or other interfering substances) unlikely. However, we cannot formally rule out the possibility of immediate and non-specific artifacts of microinjection on vocal motor network properties given that all of the antagonists, in general, suppress fictive call duration.
Opioid peptide and receptor expression in the midshipman central nervous system is currently un-described. Opioids and their receptors are highly conserved among vertebrates (Khalap et al. 2005), and neuroanatomical evidence for opioid networks exist for other teleost fishes (Brooks et al. 1994; Ebbesson et al. 1996). In coho salmon, mu-opioid binding sites are reported throughout the telencephalon, diencephalon and mesencephalon (Ebbesson et al. 1996), but the distribution of mu-opioid binding sites in the hindbrain are currently unknown in any teleost (delta-opioid receptors are found in the hindbrain of zebrafish, specifically in the periventricular zone and abutting the nucleus of the medial longitudinal fasciculus, Porteros et al. 1999). The indication that a mu-opioidergic network is critical for rapid actions of estrogens in midshipman fish is intriguing in light of the observation that brain mu-opioid systems are the most highly conserved among vertebrates (Li et al. 1996; Darlison et al. 1997).
Opioidergic neurons that modulate midshipman vocal patterning could have multiple neuroanatomical origins. One of the major descending opioid projections in vertebrates is the midbrain periaqueductal gray (PAG, Behbehani 1995), a critical node linking forebrain to hindbrain vocal neurons in midshipman fish (see Kittelberger et al. 2006, PAG is part of the mVAC in Fig 1 top) as well as in mammals (Jurgens 2008). The current findings are consistent with the hypothesis that descending opioidergic neurons from the PAG have terminals in the hindbrain–spinal VPG, and that their activity contributes to the rapid modulation of vocalizations in midshipman. This hypothesis predicts that a subset of the projections from the PAG to the VPG consist of opioidergic processes that are rapid modulated by estrogens (directly or indirectly, see below).
Opioids and rapid estrogen mechanisms
We report that mu-opioidergic antagonists block the rapid actions of E2 during a 15–60 min period after microinjection into the hindbrain ventricle adjacent to the VPG. This indicates that opioid blockers exert long-lasting effects on the VPG comprised of mechanisms that could be substantially different from the immediate (0–5 min) inhibitory effects of opioid blockers on fictive calls. Opioid agonists and antagonists are known to have delayed (minutes-to-hours) modulatory actions on network function (Brown et al. 1998), which consist of mechanisms distinct from rapid opioidergic (seconds-to-minutes) actions on neural activity (Heinricher 2003; Nugent et al. 2007). The current results suggest that similarly divergent acute versus delayed opioid mechanisms can each exert modulatory influences on the VPG circuit in midshipman.
One mechanism to account for the current findings is that opioid antagonists broadly suppress neuronal activity, including activity within the VPG, and that this suppression precludes the rapid actions of E2 on the VPG. Consistent with this hypothesis, opioid agonists and antagonists exert long-lasting suppression of activity within neural circuits in a variety of contexts (Bennett and Mayer 1979; Cheng et al. 1986; Charpak et al. 1988). However, in the current experiments, the midshipman VPG recovered from suppression (i.e., returned to 100% of baseline fictive calling) within 5–10 min following treatment with all three opioid receptor antagonists. Secondly, mu-opioid blockade suppressed baseline VPG activity (Fig. 2b) and subsequent rapid E2 actions (Fig. 3b), yet kappa-opioid blockade only rapidly suppressed VPG activity (Fig. 2c), but did not subsequently influence E2’s rapid actions (Fig. 3c). These findings together indicate that there are specific modulatory interactions between E2 and a candidate mu-opioidergic network in the midshipman VPG region.
The candidate cellular mechanism(s) for combined E2/mu-opioid regulation of the VPG include either E2 binding directly to opioid receptors (Gutierrez et al. 1998), or to nonclassical binding sites on opioidergic neurons (Lagrange et al. 1997). These nonclassical actions of E2 could occur either directly on modulatory mu-opioidergic neurons, or indirectly via an as yet unidentified modulatory input afferent to mu-opioidergic neurons. The acute nature of our findings indicate that the site of rapid E2 action within the VPG region is a nonclassical estrogen-binding site. Although the selective estrogen-receptor modulator ICI 182,780 potently blocks long-term estrogen-receptor mechanisms in other teleosts (e.g., Menuet et al. 2005; Pinto et al. 2006a, 2006b), pilot studies indicate that ICI is ineffective at modulating E2’s rapid actions on vocal patterning in midshipman fish (Remage-Healey and Bass, unpublished observations). The binding site which mediates rapid E2 effects in midshipman may therefore be structurally dissimilar to the nuclear estrogen receptor (i.e., a membrane receptor/ion channel); alternatively, the rapid actions of E2 could be mediated by nonclassical mechanisms associated with the canonical cytosolic estrogen receptor(s) (e.g., Vasudevan et al. 2005). Previous evidence from rats indicates that E2 increases the availability of mu-opioid receptors in hypothalamic inhibitory neurons (Torres-Reveron et al. 2009), suggesting that the rapid effects of E2 observed here could be via similarly nonclassical interactions with hindbrain/spinal GABAergic neurons that are mu-opioid responsive. Although more neuroanatomical evidence is needed for teleosts, E2 can modulate opioidergic function in the brainstem of rats (Tashiro et al. 2008) and opioid receptor mRNA is co-localized with estrogen-receptor positive cells in brainstem circuits (Flores et al. 2003).
The rapid E2/opioidergic modulation reported here is a part of a growing number of reports indicating that steroids interact with opioidergic systems to achieve rapid actions on neuronal activity. Steroids and steroidal drugs interact directly with opioid receptors, including mu-opioid receptors, to alter binding affinities for endogenous opioids and subsequent neuronal activity (Maggi et al. 1996; SanMartin et al. 1999; Sajadi et al. 2007; Brown et al. 2008). A membrane glucocorticoid receptor recently isolated in newts exhibits kappa-opioid binding properties, and has been proposed to mediate the rapid actions of corticosterone on reproductive clasping behavior (Evans et al. 2000). In guinea pig hypothalamus, E2 rapidly inhibits the mu-opioid effector system (Kelly et al. 1992), although not via a direct interaction with opioid receptors per se (Qiu et al. 2003). In contrast, in rat hypothalamus, E2 causes mu-opioid receptor trafficking inward from the membrane (internalization) within 30 min, and this effect can be blocked by treatment with the broad-spectrum opioid blocker naltrexone (Eckersell et al. 1998; Sinchak and Micevych 2001). Micevych and colleagues have proposed that E2 causes rapid release of opioid peptides, which then bind to mu-opioid receptors, leading to their internalization. The results of the current study are consistent with this model from rat hypothalamus, and support the hypothesis that the rapid effects of E2 cause the release of mu-opioids within the VPG and thereby increase fictive call duration. This model for the midshipman VPG generates two specific predictions: (1) opioidergic modulatory neurons express estrogen-binding sites, most likely presynaptically within the VPG itself, and (2) estrogen treatment will lead to rapid increases in local mu-opioid concentrations accompanied by rapid internalization of mu-opioid receptors in the VPG region of midshipman.
This study demonstrates that steroids and neuropeptides can interact in the minute-by-minute control of motor network patterning, suggesting that acute shifts in midshipman calling behavior in nature involve combined rapid actions of estrogens and opioids (Fig. 1). Our findings are consistent with a mechanism of second-order, or “meta”-modulation, in which the actions of one neuromodulator (in this case E2) influence the actions of an existing neuromodulatory circuit (in this case mu-opioid neurons). Metamodulation provides an important mechanism for generating a broad range of behavioral states from a discrete network of neurons (Katz and Edwards 1999; Mesce 2002; McLean and Sillar 2004). Metamodulation is likely an important mechanism for shaping vocal output in midshipman, since the neuropeptides arginine vasotocin and isotocin also exert rapid actions on fictive calling in this species (Goodson and Bass 2000).
The current results suggest that the steroid-sensitive neural circuitry that patterns vocal production in other vertebrates (Schlinger and Brenowitz 2002; Bass and Remage-Healey 2008; Jurgens 2008), could be regulated by acute steroid/opioid interactions similar to those in this study. Thus far, evidence has been suggestive in favor of this hypothesis. Masculinization of “crow” vocalizations in quail involves a testosterone-dependent upregulation of opioid binding sites in the brainstem (Bernroider et al. 1996). In addition, peripheral injections of opioid receptor antagonists increase male song production in starlings (Riters et al. 2005). However, to our knowledge neurophysiological evidence that opioids and steroids interact to regulate vocal patterning does not exist for birds and mammals. The mammalian hypothalamus exhibits sexually dimorphic expression of opioid peptides, and some neurons co-express mRNA for steroid receptors and opioids (Gu and Simerly 1994; Roepke et al. 2007). Therefore, rapid interactions between opioid- and steroid-dependent mechanisms, such as those outlined in this study, could be especially important for sex differences in brain function and behavioral plasticity in vertebrates.
Acknowledgments
The authors thank Sue Moenter and Catherine Woolley for helpful suggestions about experimental treatments, and two anonymous referees for comments on the manuscript. All experimental protocols were approved by the Cornell University Institutional Animal Care and Use Committee. The research was supported by National Institutes of Health NINDS grants F32NS058009 and 1K99NS066179 (L.R.-H.), NIMH institutional predoctoral training grant 2T32MH015793 (L.R.-H.) and National Science Foundation grant IOB-0516748 (A.H.B.).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Luke Remage-Healey, FAX: +1-310-2069184, Email: healey@ucla.edu.
Andrew H. Bass, Email: ahb3@cornell.edu
References
- Acosta-Martinez M, Etgen AM. Activation of mu-opioid receptors inhibits lordosis behavior in estrogen and progesterone-primed female rats. Horm Behav. 2002;41:88–100. doi: 10.1006/hbeh.2001.1741. [DOI] [PubMed] [Google Scholar]
- Bass AH, Baker R. Sexual dimorphisms in the vocal control-system of a teleost fish—morphology of physiologically identified neurons. J Neurobiol. 1990;21:1155–1168. doi: 10.1002/neu.480210802. [DOI] [PubMed] [Google Scholar]
- Bass AH, McKibben JR. Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog Neurobiol. 2003;69:1–26. doi: 10.1016/S0301-0082(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Bass AH, Remage-Healey L. Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm Behav. 2008;53:659–672. doi: 10.1016/j.yhbeh.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Marchaterre MA, Baker R. Vocal–acoustic pathways in a teleost fish. J Neurosci. 1994;14:4025–4039. doi: 10.1523/JNEUROSCI.14-07-04025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Gilland EH, Baker R. Evolutionary origins for social vocalization in a vertebrate hindbrain–spinal compartment. Science. 2008;321:417–421. doi: 10.1126/science.1157632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbehani MM. Functional-characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-K. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Mayer DJ. Inhibition of spinal-cord interneurons by narcotic micro-injection and focal electrical-stimulation in the periaqueductal central gray-matter. Brain Res. 1979;172:243–257. doi: 10.1016/0006-8993(79)90536-5. [DOI] [PubMed] [Google Scholar]
- Bernroider G, Holztrattner M, Rottner K. Sex steroid–opioid interactions associated with the temporal component of avian calling patterns. Horm Behav. 1996;30:583–589. doi: 10.1006/hbeh.1996.0061. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Klein GE. Endogenous opiates and behavior: 2005. Peptides. 2006;27:3391–3478. doi: 10.1016/j.peptides.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Bradford CS, Walthers EA, Searcy BT, Moore FL. Cloning, heterologous expression and pharmacological characterization of a kappa opioid receptor from the brain of the rough-skinned newt, Taricha granulosa. J Mol Endocrinol. 2005;34:809–823. doi: 10.1677/jme.1.01711. [DOI] [PubMed] [Google Scholar]
- Brantley RK, Bass AH. Alternative male spawning tactics and acoustic-signals in the plainfin midshipman fish Porichthys-notatus girard (teleostei, batrachoididae) Ethology. 1994;96:213–232. [Google Scholar]
- Brooks AI, Standifer KM, Cheng J, Ciszewska G, Pasternak GW. Opioid binding in giant toad and goldfish brain. Receptor. 1994;4:55–62. [PubMed] [Google Scholar]
- Brown CH, Ludwig M, Leng G. Kappa-opioid regulation of neuronal activity in the rat supraoptic nucleus in vivo. J Neurosci. 1998;18:9480–9488. doi: 10.1523/JNEUROSCI.18-22-09480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Brunton PJ, Russell JA. Rapid estradiol-17-beta modulation of opioid actions on the electrical and secretory activity of rat oxytocin neurons in vivo. Neurochem Res. 2008;33:614–623. doi: 10.1007/s11064-007-9506-7. [DOI] [PubMed] [Google Scholar]
- Ceccarelli I, Fiorenzani P, Grasso G, Lariviere WR, Massafra C, Massai L, Muscettola M, Aloisi AM. Estrogen and mu-opioid receptor antagonists counteract the 17 beta-estradiol-induced licking increase and interferon-gamma reduction occurring during the formalin test in male rats. Pain. 2004;111:181–190. doi: 10.1016/j.pain.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Chaijale NN, Aloyo VJ, Simansky KJ. A naloxonazine sensitive ([mu]1 receptor) mechanism in the parabrachial nucleus modulates eating. Brain Res. 2008;1240:111–118. doi: 10.1016/j.brainres.2008.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpak S, Duboisdauphin M, Raggenbass M, Dreifuss JJ. Direct inhibition by opioid-peptides of neurons located in the ventromedial nucleus of the guinea-pig hypothalamus. Brain Res. 1988;450:124–130. doi: 10.1016/0006-8993(88)91551-X. [DOI] [PubMed] [Google Scholar]
- Cheng ZF, Fields HL, Heinricher MM. Morphine microinjected into the periaqueductal gray has differential-effects on 3 classes of medullary neurons. Brain Res. 1986;375:57–65. doi: 10.1016/0006-8993(86)90958-3. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006;166:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Cross E, Roselli CE. 17 Beta-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. Am J Physiol-Reg I. 1999;276:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- Darlison MG, Greten FR, Harvey RJ, Kreienkamp HJ, Stuhmer T, Zwiers H, Lederis K, Richter D. Opioid receptors from a lower vertebrate (Catostomus commersoni): sequence, pharmacology, coupling to a g-protein-gated inward-rectifying potassium channel (girk1), and evolution. Proc Natl Acad Sci USA. 1997;94:8214–8219. doi: 10.1073/pnas.94.15.8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deviche P, Cotter P, Gulledge CC. Identification, partial characterization, and hypothalamic distribution of kappa-opioid, mu-opioid, and delta-opioid receptors in a passerine songbird (Junco hyemalis) Brain Res. 1993;614:220–226. doi: 10.1016/0006-8993(93)91038-T. [DOI] [PubMed] [Google Scholar]
- Durand SE, Liang WR, Brauth SE. Methionine enkephalin immunoreactivity in the brain of the budgerigar (Melopsittacus undulatus): similarities and differences with respect to oscine songbirds. J Comp Neurol. 1998;393:145–168. doi: 10.1002/(SICI)1096-9861(19980406)393:2<145::AID-CNE2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ebbesson LOE, Deviche P, Ebbesson SOE. Distribution and changes in mu- and kappa-opiate receptors during the midlife neurodevelopmental period of coho salmon, Oncorhynchus kisutch. J Comp Neurol. 1996;366:448–464. doi: 10.1002/(SICI)1096-9861(19960311)366:3<448::AID-CNE6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SJ, Searcy BT, Moore FL. A subset of kappa opioid ligands bind to the membrane glucocorticoid receptor in an amphibian brain. Endocrinology. 2000;141:2294–2300. doi: 10.1210/en.141.7.2294. [DOI] [PubMed] [Google Scholar]
- Flores CA, Shughrue P, Petersen SL, Mokha SS. Sex-related differences in the distribution of opioid receptor-like 1 receptor mRNA and colocalization with estrogen receptor mRNA in neurons of the spinal trigeminal nucleus caudalis in the rat. Neuroscience. 2003;118:769–778. doi: 10.1016/S0306-4522(02)01000-X. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J Neurosci. 2001;21:8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogas KR, Levine JD, Basbaum AI. Differential contribution of descending controls to the antinociceptive actions of kappa and mu opioids: an analysis of formalin-evoked c-fos expression. J Pharmacol Exp Ther. 1996;276:801–809. [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH (2002) Vocal-acoustic circuitry and descending vocal pathways in teleost fish: convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol 448:298–322 [DOI] [PubMed]
- Gu GB, Simerly RB. Hormonal-regulation of opioid peptide neurons in the anteroventral periventricular nucleus. Horm Behav. 1994;28:503–511. doi: 10.1006/hbeh.1994.1048. [DOI] [PubMed] [Google Scholar]
- Gutierrez M, Menendez L, Brieva R, Hidalgo A, Baamonde A. Different types of steroids inhibit [h-3]diprenorphine binding in mouse brain membranes. Gen Pharmacol. 1998;31:747–751. doi: 10.1016/s0306-3623(98)00110-4. [DOI] [PubMed] [Google Scholar]
- Heinricher MM. Orphanin fq/nociceptin: from neural circuitry to behavior. Life Sci. 2003;73:813–822. doi: 10.1016/S0024-3205(03)00412-0. [DOI] [PubMed] [Google Scholar]
- Jurgens U. The neural control of vocalization in mammals: a review. J Voice. 2008;23(1):1–10. doi: 10.1016/j.jvoice.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Katz PS, Edwards DH (1999) Metamodulation: the control and modulation of neuromodulation. In: Katz PS (ed) Beyond neurotransmission: neuromodulation and its importance for information processing. Oxford University Press, New York, pp 349–381
- Kelly MJ, Ronnekleiv OK (2002) Rapid membrane effects of estrogen in the central nervous system. In Pfaff D (ed) Hormones, brain and behavior. Elsevier, pp 361–380
- Kelly MJ, Loose MD, Ronnekleiv OK. Estrogen suppresses mu-opioid-mediated and GABA(b)-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci. 1992;12:2745–2750. doi: 10.1523/JNEUROSCI.12-07-02745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalap A, Bagrosky B, Le Caude S, Youson J, Danielson P, Dores RM. Trends in the evolution of the proenkephalin and prodynorphin genes in gnathostomes. Trends Comp Endocrinol Neurobiol. 2005;1040:22–37. doi: 10.1196/annals.1327.003. [DOI] [PubMed] [Google Scholar]
- Kittelberger JM, Land BR, Bass AH. The midbrain periaqueductal gray and vocal patterning in a teleost fish. J Neurophysiol. 2006;96:71–85. doi: 10.1152/jn.00067.2006. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Ronnekleiv OK, Kelly MJ. Modulation of g protein-coupled receptors by an estrogen receptor that activates protein kinase a. Mol Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- Li X, Keith DE, Evans CJ. Mu opioid receptor-like sequences are present throughout vertebrate evolution. J Mol Evol. 1996;43:179–184. doi: 10.1007/BF02338825. [DOI] [PubMed] [Google Scholar]
- Maggi R, Pimpinelli F, Casulari LA, Piva F, Martini L. Antiprogestins inhibit the binding of opioids to mu-opioid receptors in nervous membrane preparations. Eur J Pharmacol. 1996;301:169–177. doi: 10.1016/0014-2999(96)00003-9. [DOI] [PubMed] [Google Scholar]
- Martin TJ, McIntosh S, Smith JE. Alkylation of opioid receptors by 5′-naltrindole-isothiocyanate injected into the nucleus accumbens of rats: receptor selectivity and anatomical diffusion. Synapse. 2006;60:384–391. doi: 10.1002/syn.20310. [DOI] [PubMed] [Google Scholar]
- McLean DL, Sillar KT. Metamodulation of a spinal locomotor network by nitric oxide. J Neurosci. 2004;24:9561–9571. doi: 10.1523/JNEUROSCI.1817-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuet A, Pellegrini E, Brion F, Gueguen MM, Anglade I, Pakdel F, Kah O. Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J Comp Neurol. 2005;485:304–320. doi: 10.1002/cne.20497. [DOI] [PubMed] [Google Scholar]
- Mesce KA. Metamodulation of the biogenic amines: second-order modulation by steroid hormones and amine cocktails. Brain Behav Evol. 2002;60:339–349. doi: 10.1159/000067793. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berl) 2005;182:384–392. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Pascoe JE, Williams KL, Mukhopadhyay P, Rice KC, Woods JH, Ko M-C. Effects of mu, kappa, and delta opioid receptor agonists on the function of hypothalamic–pituitary–adrenal axis in monkeys. Psychoneuroendocrinology. 2008;33:478–486. doi: 10.1016/j.psyneuen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto PIS, Passos AL, Martins RS, Power DM, Canario AVM. Characterization of estrogen receptor beta b in sea bream (Sparus auratus): phylogeny, ligand-binding, and comparative analysis of expression. Gen Comp Endocrinol. 2006;145:197–207. doi: 10.1016/j.ygcen.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pinto PIS, Singh PB, Condeca JB, Teodosio HR, Power DM, Canario AVM. ICI 182, 780 has agonistic effects and synergizes with estradiol-17 beta in fish liver, but not in testis. Reprod Biol Endocrinol. 2006;4:67–78. doi: 10.1186/1477-7827-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteros A, Garcia-Isidoro M, Barrallo A, Gonzalez-Sarmiento R, Rodriguez RE. Expression of zfor1, a delta-opioid receptor, in the central nervous system of the zebrafish (Danio rerio) J Comp Neurol. 1999;412:429–438. doi: 10.1002/(SICI)1096-9861(19990927)412:3<429::AID-CNE4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel g-protein-coupled estrogen receptor that activates protein kinase c. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J Neurosci. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Rapid elevations in both steroid hormones and vocal signaling during playback challenge: a field experiment in gulf toadfish. Horm Behav. 2005;47:297–305. doi: 10.1016/j.yhbeh.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. From social behavior to neural circuitry: steroid hormones rapidly modulate advertisement calling via a vocal pattern generator. Horm Behav. 2006;50:432–441. doi: 10.1016/j.yhbeh.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J Neurosci. 2007;27:1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Schroeder MB, Auger CJ, Eens M, Pinxten R, Ball GF. Evidence for opioid involvement in the regulation of song production in male European starlings (Sturnus vulgaris) Behav Neurosci. 2005;119:245–255. doi: 10.1037/0735-7044.119.1.245. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Malyala A, Bosch MA, Kelly MJ, Ronnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148:4937–4951. doi: 10.1210/en.2007-0605. [DOI] [PubMed] [Google Scholar]
- Sajadi AA, Samaei SA, Rashidy-Pour A. Blocking effects of intra-hippocampal naltrexone microinjections on glucocorticoid-induced impairment of spatial memory retrieval in rats. Neuropharmacology. 2007;52:347–354. doi: 10.1016/j.neuropharm.2006.08.021. [DOI] [PubMed] [Google Scholar]
- SanMartin S, Gutierrez M, Menendez L, Hidalgo A, Baamonde A. Effects of diethylstilbestrol on mouse hippocampal evoked potentials in vitro. Cell Mol Neurobiol. 1999;19:691–703. doi: 10.1023/A:1006996805017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger B, Brenowitz EA (2002) Neural and hormonal control of birdsong. In: Pfaff DW (ed) Hormones, brain and behavior. Elsevier, pp 799–838
- Schmitt PM, Kaufman MP. Estrogen’s attenuating effect on the exercise pressor reflex is more opioid dependent in gonadally intact than in ovariectomized female. J Appl Physiol. 2005;98:633–639. doi: 10.1152/japplphysiol.00788.2004. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Knapp R, Bass AH. Seasonal variation of steroid hormone levels in an intertidal-nesting fish, the vocal plainfin midshipman. Gen Comp Endocrinol. 2004;136:101–116. doi: 10.1016/j.ygcen.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Bereiter DA. Morphine modulation of temporomandibular joint-responsive units in superficial laminae at the spinomedullary junction in female rats depends on estrogen status. Eur J Neurosci. 2008;28:2065–2074. doi: 10.1111/j.1460-9568.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- Torres-Reveron A, Williams TJ, Chapleau JD, Waters EM, McEwen BS, Drake CT, Milner TA. Ovarian steroids alter mu opioid receptor trafficking in hippocampal parvalbumin gabaergic interneurons. Exp Neurol. 2009;219:319–327. doi: 10.1016/j.expneurol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff D. Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids. 2005;70:388–396. doi: 10.1016/j.steroids.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Weeg MS, Land BR, Bass AH. Vocal pathways modulate efferent neurons to the inner ear and lateral line. J Neurosci. 2005;25:5967–5974. doi: 10.1523/JNEUROSCI.0019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]


