Abstract
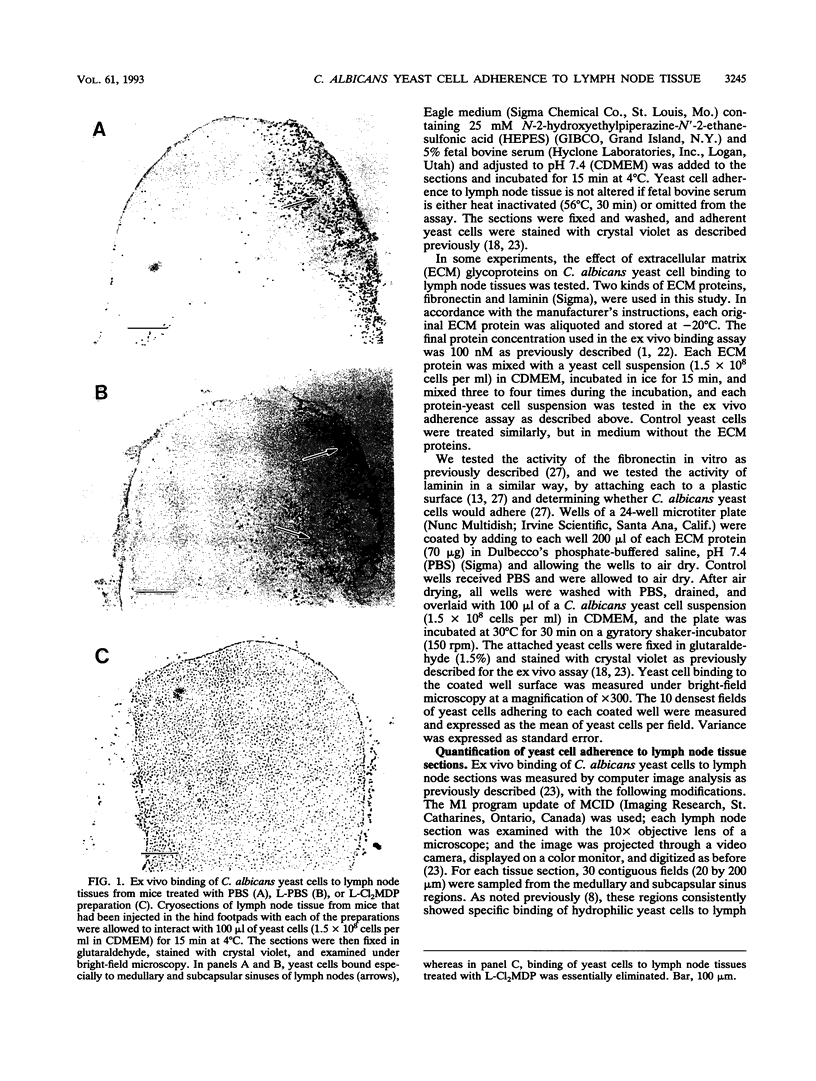
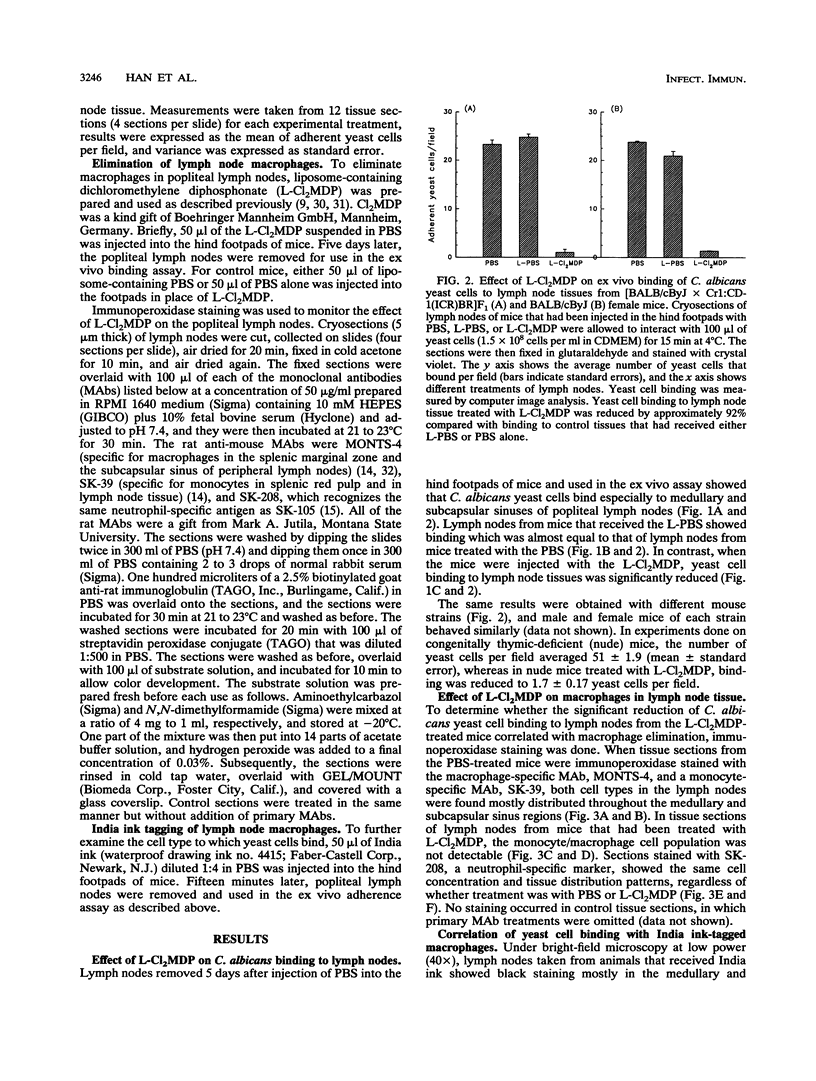
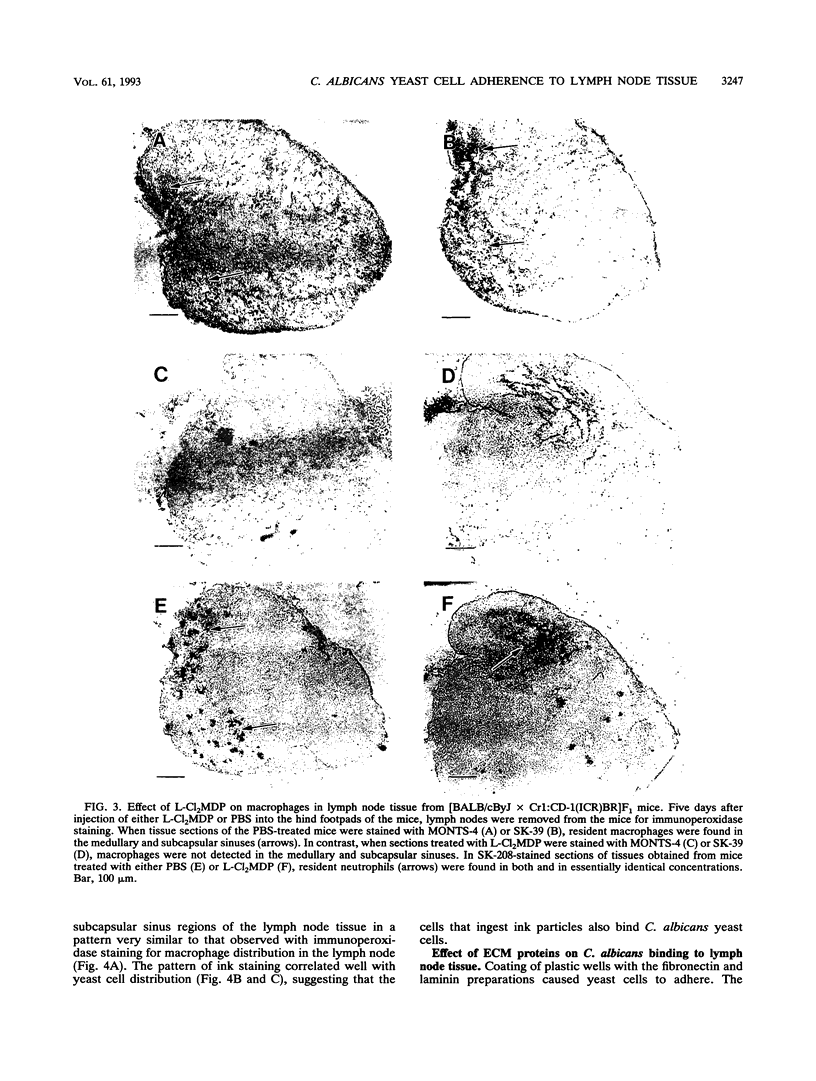
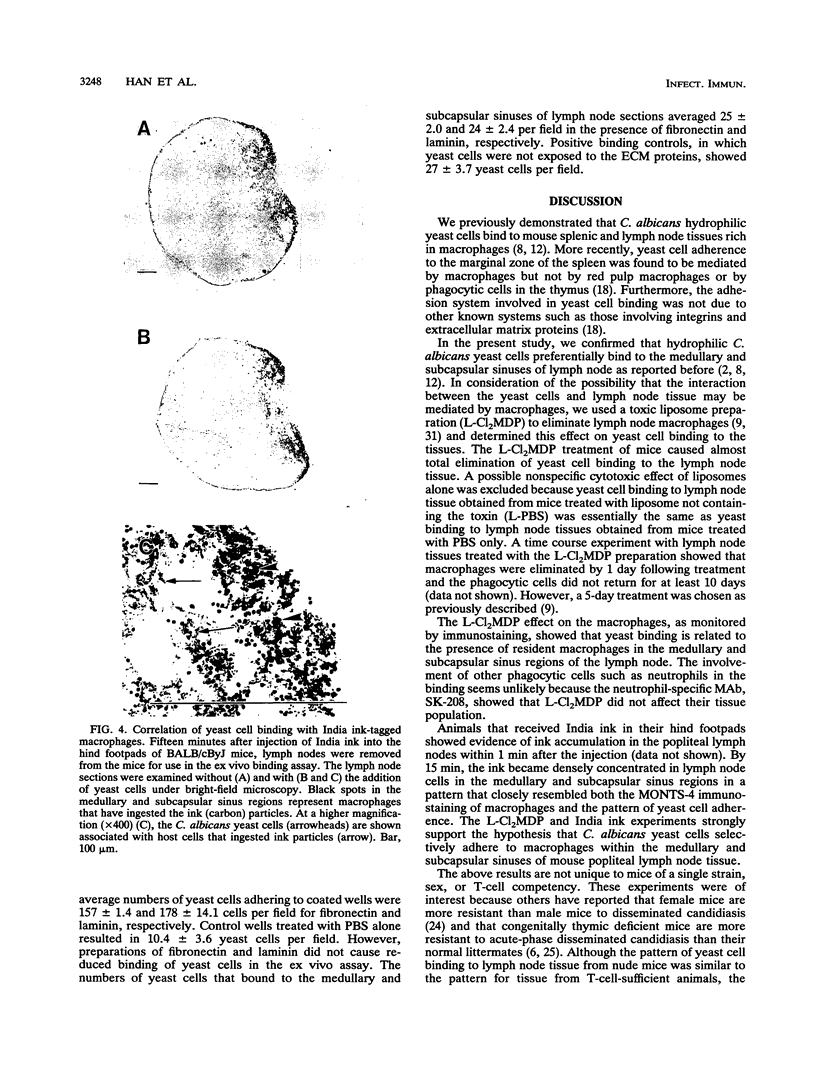
We previously reported that Candida albicans yeast cells adhere to the macrophage-rich medullary and subcapsular sinus areas of mouse lymph node tissue. To determine whether the yeast cell-lymph node interaction is mediated by macrophages, the effect of specific elimination of macrophages on yeast cell binding was studied, and yeast cell adherence was correlated with the ingestion of India ink by lymph node cells. Macrophage elimination was done by use of liposome-containing dichloromethylene diphosphonate (L-Cl2MDP). Mice were injected in the hind footpads with the L-Cl2MDP preparation, popliteal lymph nodes were removed 5 days later, and yeast cell adherence was determined by an ex vivo binding assay. As controls, lymph nodes from mice that received footpad injections of either phosphate-buffered saline (PBS) alone or liposome-containing PBS were used. Use of macrophage- and neutrophil-specific monoclonal antibodies in tissue immunostaining showed that the L-Cl2MDP treatment eliminated macrophages but not neutrophils from the medullary and subcapsular sinus areas of the popliteal lymph nodes. A striking reduction of yeast cell adherence occurred with lymph nodes from L-Cl2MDP-treated mice compared with lymph nodes from control animals. The lymph node-yeast cell binding patterns of L-Cl2MDP-treated and control mice were the same regardless of mouse strain, sex, or T-cell competency. Results of India ink experiments, in which India ink was injected into footpads of mice and was rapidly taken up by popliteal lymph node macrophages, showed a strong correlation between yeast adherence and India ink staining of cells. In addition, the interaction of yeast cells with lymph node tissue from normal mice was not significantly affected by the addition of two extracellular matrix proteins, fibronectin and laminin, during the ex vivo adherence assay. These data indicate that medullary and subcapsular sinus lymph node macrophages express an adhesion system similar to that described for mouse splenic marginal zone macrophages.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouchara J. P., Tronchin G., Annaix V., Robert R., Senet J. M. Laminin receptors on Candida albicans germ tubes. Infect Immun. 1990 Jan;58(1):48–54. doi: 10.1128/iai.58.1.48-54.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner D. L., Mori M. Adherence of Candida albicans to tissues from mice with drug- or radiation-induced immunodeficiencies. J Infect Dis. 1992 Sep;166(3):587–597. doi: 10.1093/infdis/166.3.587. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Scollay R. G., Weissman I. L. Lymphocyte adherence to high endothelial venules: characterization of a modified in vitro assay, and examination of the binding of syngeneic and allogeneic lymphocyte populations. J Immunol. 1979 Nov;123(5):1996–2003. [PubMed] [Google Scholar]
- Calderone R. A., Braun P. C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991 Mar;55(1):1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley I. A., Douglas L. J. Isolation and partial characterization of an adhesin from Candida albicans. J Gen Microbiol. 1987 Mar;133(3):629–636. doi: 10.1099/00221287-133-3-629. [DOI] [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Cutler J. E., Brawner D. L., Hazen K. C., Jutila M. A. Characteristics of Candida albicans adherence to mouse tissues. Infect Immun. 1990 Jun;58(6):1902–1908. doi: 10.1128/iai.58.6.1902-1908.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- Delemarre F. G., Kors N., Kraal G., van Rooijen N. Repopulation of macrophages in popliteal lymph nodes of mice after liposome-mediated depletion. J Leukoc Biol. 1990 Mar;47(3):251–257. doi: 10.1002/jlb.47.3.251. [DOI] [PubMed] [Google Scholar]
- Hazen K. C., Brawner D. L., Riesselman M. H., Jutila M. A., Cutler J. E. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect Immun. 1991 Mar;59(3):907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C. Participation of yeast cell surface hydrophobicity in adherence of Candida albicans to human epithelial cells. Infect Immun. 1989 Jul;57(7):1894–1900. doi: 10.1128/iai.57.7.1894-1900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P. E., Pittet D., Auckenthaler R., Lew P. D., Schumacher-Perdreau F., Peters G., Waldvogel F. A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988 Oct;158(4):693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- Jutila M. A., Kishimoto T. K., Finken M. Low-dose chymotrypsin treatment inhibits neutrophil migration into sites of inflammation in vivo: effects on Mac-1 and MEL-14 adhesion protein expression and function. Cell Immunol. 1991 Jan;132(1):201–214. doi: 10.1016/0008-8749(91)90019-8. [DOI] [PubMed] [Google Scholar]
- Jutila M. A., Rott L., Berg E. L., Butcher E. C. Function and regulation of the neutrophil MEL-14 antigen in vivo: comparison with LFA-1 and MAC-1. J Immunol. 1989 Nov 15;143(10):3318–3324. [PubMed] [Google Scholar]
- Kanbe T., Han Y., Redgrave B., Riesselman M. H., Cutler J. E. Evidence that mannans of Candida albicans are responsible for adherence of yeast forms to spleen and lymph node tissue. Infect Immun. 1993 Jun;61(6):2578–2584. doi: 10.1128/iai.61.6.2578-2584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbe T., Jutila M. A., Cutler J. E. Evidence that Candida albicans binds via a unique adhesion system on phagocytic cells in the marginal zone of the mouse spleen. Infect Immun. 1992 May;60(5):1972–1978. doi: 10.1128/iai.60.5.1972-1978.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A. Fungal adherence to the vascular compartment: a critical step in the pathogenesis of disseminated candidiasis. Clin Infect Dis. 1992 Jan;14(1):340–347. doi: 10.1093/clinids/14.1.340. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Smith R. L. A fibronectin receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J Infect Dis. 1991 Mar;163(3):604–610. doi: 10.1093/infdis/163.3.604. [DOI] [PubMed] [Google Scholar]
- Riesselman M. H., Kanbe T., Cutler J. E. Improvements and important considerations of an ex vivo assay to study Candida albicans-splenic tissue interactions. J Immunol Methods. 1991 Dec 15;145(1-2):153–160. doi: 10.1016/0022-1759(91)90321-6. [DOI] [PubMed] [Google Scholar]
- Rifkind D., Frey J. A. Influence of gonadectomy on Candida albicans urinary tract infection in CFW mice. Infect Immun. 1972 Mar;5(3):332–336. doi: 10.1128/iai.5.3.332-336.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Rotrosen D., Calderone R. A., Edwards J. E., Jr Adherence of Candida species to host tissues and plastic surfaces. Rev Infect Dis. 1986 Jan-Feb;8(1):73–85. doi: 10.1093/clinids/8.1.73. [DOI] [PubMed] [Google Scholar]
- Skerl K. G., Calderone R. A., Segal E., Sreevalsan T., Scheld W. M. In vitro binding of Candida albicans yeast cells to human fibronectin. Can J Microbiol. 1984 Feb;30(2):221–227. doi: 10.1139/m84-033. [DOI] [PubMed] [Google Scholar]
- Stamper H. B., Jr, Woodruff J. J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976 Sep 1;144(3):828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooijen N. The liposome-mediated macrophage 'suicide' technique. J Immunol Methods. 1989 Nov 13;124(1):1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- Webster E. L., Tracey D. E., Jutila M. A., Wolfe S. A., Jr, De Souza E. B. Corticotropin-releasing factor receptors in mouse spleen: identification of receptor-bearing cells as resident macrophages. Endocrinology. 1990 Jul;127(1):440–452. doi: 10.1210/endo-127-1-440. [DOI] [PubMed] [Google Scholar]
- van den Berg T. K., Brevé J. J., Damoiseaux J. G., Döpp E. A., Kelm S., Crocker P. R., Dijkstra C. D., Kraal G. Sialoadhesin on macrophages: its identification as a lymphocyte adhesion molecule. J Exp Med. 1992 Sep 1;176(3):647–655. doi: 10.1084/jem.176.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]